Abstract
The treatment of high-grade gliomas remains difficult despite recent advances in surgery, radiotherapy, and chemotherapy. True advances may emerge from the increasing understanding in molecular biology and discovery of novel mechanisms for the delivery of tumoricidal agents. In an attempt to overcome this formidable neoplasm, molecular approaches using gene therapy have been investigated clinically since 1992. The clinical trials have mainly been classified into three approaches: suicide gene therapy, immune gene therapy and oncolytic viral therapy. In this article, we review these approaches, which have been studied in previous and ongoing clinical trials.
Introduction
Gliomas are the most common primary tumors of the central nervous system, accounting for 30% of adult primary brain tumors. The treatment of brain tumors remains difficult despite recent advances in surgery, radiotherapy and chemotherapy. Currently, no optimal treatment option is available for glioblastoma multiforme, and the patients typically survive for less than a year.Citation1 The poor prognosis is partially because of the inability to deliver chemotherapeutic agents across the blood-brain barrier (BBB) and the low tumor response to radiation. Therefore, novel and more effective strategies are warranted and true advances may emerge from the increasing understanding in molecular biology and discovery of novel mechanisms for the delivery of tumoricidal agents. In an attempt to overcome this formidable neoplasm, molecular approaches using gene therapy have been investigated clinically since 1992.Citation2 The clinical trials have mainly studied three approaches: (1) suicide gene therapy, (2) cytokine gene therapy and (3) oncolytic viral therapy (; www.wiley.co.uk/genetherapy/clinical/). In this article, we review these approaches, which have been studied in previous and ongoing clinical trials.
Suicide Gene Therapy
The suicide gene therapy strategy involves the activation of prodrugs in tumor cells after transfecting the cells with a gene to induce the expression of an activating enzyme. The most extensively investigated model of this strategy is the herpes simplex virus-thymidine kinase (HSV-tk) gene system used along with the systemic administration of ganciclovir (GCV) (). This well-studied system was first reported by Moolten in 1986.Citation3 In brief, HSV-tk, unlike human-tk, monophosphorylates the nucleoside analog GCV; the monophosphorylated GCV is then converted to triphosphate GCV in the host cells. The triphosphate form is incorporated into the DNA and it consequently blocks DNA replication and inhibits cell division. Preclinical studies using the HSV-tk/GCV system have reported inspiring results in various cancer models, including brain tumors. In one of these studies, the experimental brain tumors disappeared completely in 11 of 14 animals following transduction with the HSV-tk-expressing retrovirus and administration of GCV.Citation2 As indicated in , most clinical studies have utilized a retroviral vector, but the therapeutic effect was limited.Citation4 In a randomized phase III clinical trial, which was completed in the year 2000, patients were randomized to receive either surgical resection followed by radiotherapy with or without HSV-tk/GCV gene therapy.Citation5 There was no difference between both the treatment arms in terms of median survival, which was the primary endpoint; this result was probably due to low transduction efficiency of the retroviral vectors. Eventually, they indicated the need to develop better techniques for the delivery and distribution of therapeutic genes to achieve effectiveness.
Adenoviral vectors with better transduction efficiency and potential to infect proliferating and non-proliferating cells may prove more effective. A recent dose-escalating phase I trial reported the safety of an adenoviral vector. In this trial, 10 of 11 patients survived 52 weeks from the time of diagnosis, with an average survival of 112.3 weeks;Citation6 this is more than twice the expected survival with conventional treatments, and at the time of publication, one patient had survived for 248 weeks from the time of diagnosis. However, further studies in this area are warranted.
Suicide gene therapy via the introduction of apoptosis-inducing genes is another promising strategy. The tumor protein TP53 performs numerous functions, including regulation of progression via the cell cycle, DNA repair after damage and induction of apoptosis. p53 mutations have been reported in 30–60% of malignant gliomas.Citation7 The replacement of the wild-type p53 gene function may reduce tumor growth or induce apoptosis. In 2003, a group of researchers at the MD Anderson Medical Center performed a phase I clinical trial of adenovirus-mediated p53 gene therapy in 15 patients with recurrent glioma; their protocol involved intratumoral injection of p53-expressing adenoviral vector followed by tumor resection.Citation8 It was demonstrated that exogenous p53 activated downstream effectors and induced apoptosis, and clinical toxicity was minimal. However, the transfected cells were on an average only 5 mm away from the injection site. A more widespread distribution of a vector should be achieved to gain maximum therapeutic benefit. The currently known limitations of adenoviral vectors are as follows: (1) the immunogenicity of the vector may limit the efficacy of this strategy,Citation9,Citation10 and (2) the cell entry of an adenovirus depends on the coxsackie-adenovirus receptor (CAR) that is scarcely expressed in malignant gliomas.Citation11 Several attempts have been made to resolve this issue by engineering adenoviruses with tropism for glioma-specific receptors.Citation12
Immune Gene Therapy
The central nervous system in mammals is often regarded as immunologically privileged and may have a disadvantage with regard to augmenting the immune response. It is generally believed that brain tumors have low immunogenicity and suppress the immune response. However, recent advances in the understanding of cytokine action and gene delivery techniques have revived the interest in the concept of immune gene therapy using a variety of cytokine genes that increase tumor immunogenicity and/or activate the host immune response. To achieve regression of infiltrative intracranial neoplasms that form multiple foci, two approaches have been employed: (1) administration of tumor vaccines using genetically engineered cells that express cytokines such as interleukin (IL)-2 and IL-4 and (2) intratumoral secretion in situ by transfecting tumor cells with cytokine genes. With regard to the former, previous reports have suggested that peripheral tumor vaccination can definitely initiate a systemic immune response against intracranial tumors.Citation13,Citation14 However, large amounts of tumor tissues or autologous tumor cell lines are required to generate gene-modified tumor vaccines of a clinical grade; this may limit the feasibility of whole tumor vaccination strategies. With regard to the latter, IL-4 expression in situ was reported to induce an inflammatory response, leading to tumor regression,Citation15,Citation16 while severe CNS toxicity caused by brain edema has been documented when IL-2 or interferon-gamma (IFN)γ was secreted intracranially by tumor cells. IFNβ is another potential cytokine that exerts pleiotropic biological effects.Citation17 Although identified for and named due to its ability to interfere with viral replication in treated cells, IFNβ also has immunomodulatory and antiproliferative effects. In 2000, our group at Nagoya University, Japan, started using the cytokine gene therapy: the IFNβ gene was delivered via cationic liposomes based on the following preclinical and experimental studies. In vitro experiments demonstrated that the cationic liposome-mediated human IFNβ gene transfer to cultured human glioma cells induced a cytocidal but not a cytostatic response even in IFN-resistant human glioma cell lines, probably by inducing apoptosis.Citation17 In vivo experiments using nude mice implanted with human glioma cells intracranially or subcutaneously revealed that the local administration of cationic liposomes containing the human IFNβ gene induced apparent tumor growth reduction, prolonged survival and natural killer (NK) cell activation.Citation18,Citation19 In addition, a similar growth-inhibitory effect was also observed in a syngeneic intracranial mouse glioma model treated with the liposome-mediated murine IFNβ gene. This gene therapy system induced specific cytotoxic T-cell immunity against mouse glioma and the NK cells.Citation20,Citation21 Based on these observations, a phase I clinical trial of IFNβ gene therapy was performed in five patients with recurrent malignant glioma.Citation22 This was a two-stage trial in which the initial treatment comprised tumor removal and injection of liposomes containing the human IFNβ gene into the margin of the resulting defect and subsequent delivery of subsequent injections via an implanted catheter. The clinical toxicity was found to be minimal. At ten weeks after treatment initiation, two patients showed more than 50% reduction while others had stable disease. The median survival was longer in the treated subjects than in the matched historical control from our institution. After the gene therapy, significant changes were observed in histology and gene expression related to immunoresponse, apoptosis and neovascularizationCitation23 (). This study provides the foundation for a phase II trial of IFNβ gene therapy. Very recently, Chiocca et al. reported a phase I clinical trial (a dose-escalating cohort) of stereotactic injection of an IFNβ-expressing adenoviral vector in 11 patients with malignant glioma. Direct injection of the vector into the tumor and the surrounding normal brain areas after surgical tumor removal was feasible. A reproducible increase in tumor cell apoptosis was observed after the treatment.Citation24
Oncolytic Viral (Gene) Therapy
While gene therapy strategies typically employ replication-incompetent viruses as the gene delivery tool, oncolytic viral therapy utilizes replication-competent viruses to infect and lyse the cells with or without employing gene transfer (). Numerous viruses have been proposed and investigated for their therapeutic application as oncolytic agents. Three human pathogenic viruses—herpes simplex virus (HSV), adenovirus and poliovirus—have been extensively studied and demonstrated to have clinical potential.
HSV is an enveloped double-strand DNA virus with natural neurotropism and the ability to replicate in dividing and nondividing cells. Martuza et al. developed a conditionally replicating HSV, G207; this virus exhibits mutations in both copies of the neurovirulence gene γ134.5 and disrupts the gene encoding ribonucleotide reductase. Preclinical studies have demonstrated that G207 decreased the growth of experimental glioma and that it was well tolerated in nonhuman primates.Citation25 In 2000, a dose-escalated phase I clinical trial was published, and it was the first report on the use of a replication-competent HSV mutant in a human brain tumor trial.Citation26 Twenty-one patients with recurrent malignant glioma were enrolled in the study, and no dose-limiting toxicities (DLTs) were observed. Of the 21 patients, eight demonstrated reduced tumor enhancement volumes, and two patients were long-term survivors. Another HSV mutant 1716 exhibited deletion only in the γ134.5 genes. The phase I trial for recurrent glioma has been completed. No DLTs were encountered in the nine patients enrolled in the trial, and viral replication was demonstrated in tumor; the amount of recovered virus exceeded the input dose in some patients, and this result further confirmed the validity of the principle.Citation27 In a phase II clinical trial of this mutant HSV, a response was noted in 2 of 12 patients.Citation28 Both G207 and 1716 are currently under further clinical investigation.
Adenoviruses are nonenveloped DNA viruses capable of infecting both the dividing and nondividing cells. Bischoff et al. developed a conditionally replicative adenovirus, ONYX-015.Citation29–Citation31 ONYX-015 contains a deletion in the viral protein E1B-55K, which normally binds to and inactivates the host cell p53 protein. Therefore, it is assumed that cells with functional p53 cannot support viral replication in the absence of this protein, whereas tumor cells with a nonfunctional p53 can support viral replication. Preclinical studies in human malignant glioma xenografts demonstrated cell lysis and impaired tumor growth in response to ONYX-015, but the response was independent of the p53 status.Citation32 Although the exact mechanism is not completely understood and may not be related to p53, ONYX-015 is only the third oncolytic virus to be tested in human clinical trials for malignant glioma (following G207 and 1716). In the initial phase I clinical trial of intratumoral delivery, none of the 24 patients with glioma exhibited a significant response, with 96% experiencing disease progression. This was a dose-escalated study wherein no patient demonstrated any serious adverse events and the maximum tolerated dose was not reached.Citation33 Of the 12 patients who received the highest doses of the virus, three survived until the end of the study and were available for follow-up for more than 19 months.
Poliovirus is a nonenveloped RNA virus with natural neurotropism. The cellular receptor responsible for viral entry, CD155 is ectopically upregulated in malignant glioma,Citation34–Citation36 and the virulence of poliovirus can be manipulated in a cell-type specific manner at the level of translation control.Citation34,Citation37 Viral protein translation is mediated by the internal ribosome entry site (IRES) element in the 5′ nontranslated region of the viral genome. The IRES function depends on cell-specific constraints that can be exploited to drive the poliovirus gene expression and cytotoxicity, preferably in malignant cells.Citation38,Citation39 These findings led to the development of a recombinant poliovirus for the treatment of malignant gliomas. By exchanging the IRES element of the poliovirus with that of human rhinovirus type 2 (HRV2), Gromeier et al. created a recombinant virus (PV-RIPO) with a greatly diminished viral propagation in normal neuronal cells while retaining excellent lytic growth in malignant glioma cells.Citation34 Preclinical studies of PV-RIPO provide principle data for a planned phase I clinical trial. When delivered directly into the spinal cord, PV-RIPO significantly attenuated the in vitro growth of neuronal cells and failed to cause poliomyelitis in both mice transgenic for the CD155 receptorCitation35 and cynomolgus monkeys.Citation34 On the other hand, eight different glioma cell lines tested in vitro were highly susceptible to PV-RIPO infection, and the intratumoral injection into HTB-14 astrocytic intracerebral tumors resulted in complete regression in 18 of 25 mice. A phase I clinical trial of PV-RIPO is currently underway for application in recurrent malignant glioma.
Future Directions
Each of the strategies described herein have their own distinct advantages and limitations inherent to the technology employed. Despite the relatively rapid advancement in our scientific understanding and technological capabilities, all the major molecular approaches currently being investigated are significantly deficient in one or more of the following categories.
Delivery—the ability to reach the tumor efficiently and spread throughout the entire population of malignant cells.
Effectiveness—the ability to control or eradicate malignant cells. i.e., to halt the growth or induce regression of malignant gliomas.
Adverse Events (Safety)—the ability to target tumor cells without causing significant toxicity to the surrounding normal brain.
With increase in our understanding of molecular biology and technical advances, a strategy that is not limited by these factors may become a tenable goal.
A rational combination of therapeutic strategies with different modes of action has the potential to deliver synergistic benefit by using one agent to sensitize treatment by the other. The oncolytic viruses G207 and ONYX-015 have been studied in the context of glioma therapy combined with radiotherapy. Other interesting molecular therapeutic approaches include anti-angiogenic molecules such as endostatin; anti-receptor antibodies for EGFR, VEGF and PDGFR; and inhibitors of downstream signaling such as mTOR inhibitors. These approaches have provided encouraging results in glioma models. Although preclinical data are intriguingly promising, all these approaches are yet to demonstrate a significant benefit in a phase II or III clinical trial. In the near future, malignant gliomas will probably be treated through synergistic effects of a multipronged attack.
Figures and Tables
Figure 1 HSV-tk/GCV Suicide gene therapy. In tumor cells transduced with the HSV-tk gene, HSV-tk monophosphorylates the nucleoside analog GCV that is administered systemically. The monophosphorylated GCV is then converted to triphosphate GCV in the host cells. The triphosphate form is incorporated into the DNA, and it consequently blocks DNA replication and induced apoptosis (A). The toxic triphosphate is eventually transported to neighboring cells, and provides “bystander effects” (B) leading to apoptosis (C).
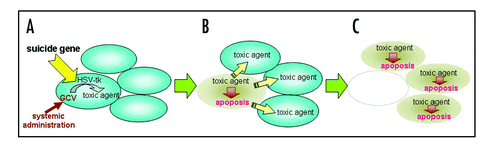
Figure 2 Mechanisms of liposome-mediated IFNβ gene therapy. Histology and cDNA expression microarray analyses revealed significant inductions in apoptosis (A) and antitumor immunoresponse (B), and inhibition of neovascularization (C).
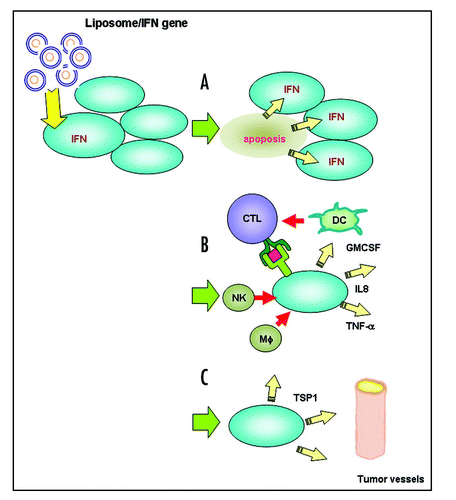
Figure 3 Oncolytic therapy. Oncolytic viral therapy utilizes replication-competent viruses which ideally can infect and replicate in tumor cells (A). The viruses specifically lyse tumor cells (B) and sequentially infect neighboring cells (C).
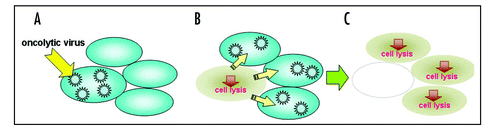
Table 1 Clinical gene therapy trials for glioma
References
- Fine HA, Dear KB, Loeffler JS, Black PM, Canellos GP. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer 1993; 71:2585 - 2597
- Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science (New York, NY) 1992; 256:1550 - 1552
- Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res 1986; 46:5276 - 5281
- Ram Z, Culver KW, Oshiro EM, Viola JJ, DeVroom HL, Otto E, Long Z, Chiang Y, McGarrity GJ, Muul LM, Katz D, Blaese RM, Oldfield EH. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med 1997; 3:1354 - 1361
- Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther 2000; 11:2389 - 2401
- Germano IM, Fable J, Gultekin SH, Silvers A. Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: preliminary results of a phase I trial in patients with recurrent malignant gliomas. J Neuro-Oncol 2003; 65:279 - 289
- Ware ML, Berger MS, Binder DK. Molecular biology of glioma tumorigenesis. Histol Histopathol 2003; 18:207 - 216
- Lang FF, Bruner JM, Fuller GN, Aldape K, Prados MD, Chang S, Berger MS, McDermott MW, Kunwar SM, Junck LR, Chandler W, Zwiebel JA, Kaplan RS, Yung WK. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol 2003; 21:2508 - 2518
- Morral N, O'Neal W, Zhou H, Langston C, Beaudet A. Immune responses to reporter proteins and high viral dose limit duration of expression with adenoviral vectors: comparison of E2a wild type and E2a deleted vectors. Hum Gene Ther 1997; 8:1275 - 1286
- Tripathy SK, Black HB, Goldwasser E, Leiden JM. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med 1996; 2:545 - 550
- Asaoka K, Tada M, Sawamura Y, Ikeda J, Abe H. Dependence of efficient adenoviral gene delivery in malignant glioma cells on the expression levels of the Coxsackievirus and adenovirus receptor. J Neurosurg 2000; 92:1002 - 1008
- Shinoura N, Sakurai S, Asai A, Kirino T, Hamada H. Transduction of a fiber-mutant adenovirus for the HSVtk gene highly augments the cytopathic effect towards gliomas. Jpn J Cancer Res 2000; 91:1028 - 1034
- Sobol RE, Fakhrai H, Shawler D, Gjerset R, Dorigo O, Carson C, Khaleghi T, Koziol J, Shiftan TA, Royston I. Interleukin-2 gene therapy in a patient with glioblastoma. Gene Ther 1995; 2:164 - 167
- Okada H, Giezeman-Smits KM, Tahara H, Attanucci J, Fellows WK, Lotze MT, Chambers WH, Bozik ME. Effective cytokine gene therapy against an intracranial glioma using a retrovirally transduced IL-4 plus HSVtk tumor vaccine. Gene Ther 1999; 6:219 - 226
- Yu JS, Wei MX, Chiocca EA, Martuza RL, Tepper RI. Treatment of glioma by engineered interleukin 4-secreting cells. Cancer Res 1993; 53:3125 - 3128
- Wei MX, Tamiya T, Hurford RK Jr, Boviatsis EJ, Tepper RI, Chiocca EA. Enhancement of interleukin-4-mediated tumor regression in athymic mice by in situ retroviral gene transfer. Hum Gene Ther 1995; 6:437 - 443
- Borden EC, Lindner D, Dreicer R, Hussein M, Peereboom D. Second-generation interferons for cancer: clinical targets. Semin Cancer Biol 2000; 10:125 - 144
- Yagi K, Hayashi Y, Ishida N, Ohbayashi M, Ohishi N, Mizuno M, Yoshida J. Interferon-beta endogenously produced by intratumoral injection of cationic liposome-encapsulated gene: cytocidal effect on glioma transplanted into nude mouse brain. Biochemistry and molecular biology international 1994; 32:167 - 171
- Mizuno M, Yoshida J. Effect of human interferon beta gene transfer upon human glioma, transplanted into nude mouse brain, involves induced natural killer cells. Cancer Immunol Immunother 1998; 47:227 - 232
- Natsume A, Mizuno M, Ryuke Y, Yoshida J. Antitumor effect and cellular immunity activation by murine interferon-beta gene transfer against intracerebral glioma in mouse. Gene Ther 1999; 6:1626 - 1633
- Natsume A, Tsujimura K, Mizuno M, Takahashi T, Yoshida J. IFNbeta gene therapy induces systemic antitumor immunity against malignant glioma. J Neuro-oncol 2000; 47:117 - 124
- Yoshida J, Mizuno M, Fujii M, Kajita Y, Nakahara N, Hatano M, Saito R, Nobayashi M, Wakabayashi T. Human gene therapy for malignant gliomas (glioblastoma multiforme and anaplastic astrocytoma) by in vivo transduction with human interferon beta gene using cationic liposomes. Hum Gene Ther 2004; 15:77 - 86
- Wakabayashi T, Natsume A, Hashizume Y, Fujii M, Mizuno M, Yoshida J. A phase I clinical trial of interferon-beta gene therapy for high-grade glioma: novel findings from gene expression profiling and autopsy. J Gene Med 2008;
- Chiocca EA, Smith KM, McKinney B, Palmer CA, Rosenfeld S, Lillehei K, Hamilton A, Demasters BK, Judy K, Kirn D. A Phase I Trial of Ad.hIFNbeta Gene Therapy for Glioma. Mol Ther 2008;
- Hunter WD, Martuza RL, Feigenbaum F, Todo T, Mineta T, Yazaki T, Toda M, Newsome JT, Platenberg RC, Manz HJ, Rabkin SD. Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation of intracerebral injection in nonhuman primates. J Virol 1999; 73:6319 - 6326
- Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, Palmer CA, Feigenbaum F, Tornatore C, Tufaro F, Martuza RL. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther 2000; 7:867 - 874
- Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, Petty R, MacLean A, Harland J, McKie E, Mabbs R, Brown M. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther 2000; 7:859 - 866
- Papanastassiou V, Rampling R, Fraser M, Petty R, Hadley D, Nicoll J, Harland J, Mabbs R, Brown M. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther 2002; 9:398 - 406
- Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science (New York, NY) 1996; 274:373 - 376
- Rainov NG, Ren H. Oncolytic viruses for treatment of malignant brain tumours. Acta neurochirurgica 2003; 88:113 - 123
- Vecil GG, Lang FF. Clinical trials of adenoviruses in brain tumors: a review of Ad-p53 and oncolytic adenoviruses. J Neuro-oncol 2003; 65:237 - 246
- Geoerger B, Grill J, Opolon P, Morizet J, Aubert G, Lecluse Y, van Beusechem VW, Gerritsen WR, Kirn DH, Vassal G. Potentiation of radiation therapy by the oncolytic adenovirus dl1520 (ONYX-015) in human malignant glioma xenografts. Brit J Cancer 2003; 89:577 - 584
- Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, Kracher J, Grossman SA, Fisher JD, Carson K, Rosenblum M, Mikkelsen T, Olson J, Markert J, Rosenfeld S, Nabors LB, Brem S, Phuphanich S, Freeman S, Kaplan R, Zwiebel J. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther 2004; 10:958 - 966
- Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. P Natl Acad Sci-Biol 2000; 97:6803 - 6808
- Merrill MK, Bernhardt G, Sampson JH, Wikstrand CJ, Bigner DD, Gromeier M. Poliovirus receptor CD155-targeted oncolysis of glioma. Neuro-Oncol 2004; 6:208 - 217
- Sloan KE, Stewart JK, Treloar AF, Matthews RT, Jay DG. CD155/PVR enhances glioma cell dispersal by regulating adhesion signaling and focal adhesion dynamics. Cancer Res 2005; 65:10930 - 10937
- Gromeier M, Alexander L, Wimmer E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. P Natl Acad Sci-Biol 1996; 93:2370 - 2375
- Merrill MK, Gromeier M. The double-stranded RNA binding protein 76:NF45 heterodimer inhibits translation initiation at the rhinovirus type 2 internal ribosome entry site. J Virol 2006; 80:6936 - 6942
- Song Y, Friebe P, Tzima E, Junemann C, Bartenschlager R, Niepmann M. The hepatitis C virus RNA 3′-untranslated region strongly enhances translation directed by the internal ribosome entry site. J Virol 2006; 80:11579 - 11588