Abstract
Genetically engineered, conditionally replicating herpes simplex viruses type 1 (HSV-1) are promising therapeutic agents for brain tumors and other solid cancers. They can replicate in situ, spread, and exhibit oncolytic activity via a direct cytocidal effect. One of the advantages of HSV-1 is the capacity to incorporate large and/or multiple transgenes within the viral genome. Oncolytic HSV-1 can therefore be “armed” to add certain functions. Recently, the field of armed oncolytic HSV-1 has drastically advanced, due to development of recombinant HSV-1 generation systems that utilize bacterial artificial chromosome and multiple DNA recombinases. Because antitumor immunity is induced in the course of oncolytic activities of HSV-1, transgenes encoding immunomodulatory molecules have been most frequently used for arming. Other armed oncolytic HSV-1 include those that express antiangiogenic factors, fusogenic membrane glycoproteins, suicide gene products, and proapoptotic proteins. Provided that the transgene product does not interfere with viral replication, such arming of oncolytic HSV-1 results in augmentation of antitumor efficacy. Immediate-early viral promoters are often used to control the arming transgenes, but strict-late viral promoters have been shown useful to restrict the expression in the late stage of viral replication when desirable. Some armed oncolytic HSV-1 have been created for the purpose of noninvasive in vivo imaging of viral infection and replication. Development of a wide variety of armed oncolytic HSV-1 will lead to an establishment of a new genre of therapy for brain tumors as well as other cancers.
Introduction
Oncolytic virus therapy is an attractive and rapidly developing means for treating cancer.Citation1 Genetically engineered viruses, such as herpes simplex virus type 1 (HSV-1) and adenovirus, are designed so that virus replication is restricted to tumor cells and therefore infection causes no harm to normal tissues. In principle, infected tumor cells are destroyed by a direct oncolytic activity of the viruses. Importantly, oncolytic viruses can also act as vectors that provide amplified transgene delivery.
HSV-1, especially in comparison with adenovirus, has suitable features for cancer therapy: (1) HSV-1 infects most tumor cell types. (2) A relatively low multiplicity of infection is needed for total cell killing. (3) Anti-viral drugs are available. (4) A large genome (∼152 kb) allows the insertion of large and/or multiple transgenes. (5) The host immune reactions enhance antitumor effects. (6) Circulating anti-HSV-1 antibodies do not affect cell-to-cell spread of the virus. (7) There are HSV-1 sensitive mouse and nonhuman primate models for preclinical evaluation. (8) Viral DNA is not integrated into the host genome. HSV-1 is neurotropic and the genes necessary for neuropathogenicity have been identified and can be mutated. Therefore, the use of HSV-1 is especially advantageous for brain tumor therapy.
In order to target HSV-1 replication to tumor cells, viral genes that are essential for viral replication in normal cells but dispensable in tumor cells are inactivated or deleted.Citation2 This principle uses features common for all types of cancer, therefore the application of oncolytic HSV-1 is not restricted to brain tumors, but also includes a wide variety of cancer. The key for successful and practical development of oncolytic HSV-1 is to achieve a wide therapeutic window by the use of genetic engineering technology.
Second-Generation Oncolytic HSV-1
G207 was the first oncolytic HSV-1 used in a clinical trial in the United States.Citation3 This second-generation oncolytic HSV-1 has double mutations created in the HSV-1 genome.Citation4 G207 has deletions in both copies of the γ34.5 gene, the major determinant of HSV-1 neurovirulence.Citation5 γ34.5-deficient HSV-1 vectors are considerably attenuated in normal cells, but retain their ability to replicate within neoplastic cells. In normal cells, HSV-1 infection induces activation of double-stranded RNA-dependent protein kinase (PKR), which in turn leads to phosphorylation of the α-subunit of eukaryotic initiation factor 2 and a subsequent shutdown of host and viral protein synthesis.Citation6 The product of the γ34.5 gene antagonizes this PKR activity. However, tumor cells have low PKR activities, thereby allowing γ34.5-deficient HSV-1 vectors to replicate.Citation7,Citation8 G207 also has an insertion of the E. coli lacZ gene in the infected-cell protein 6 (ICP6) coding region (UL39), inactivating ribonucleotide reductase, a key enzyme for viral DNA synthesis in non-dividing cells but not in dividing cell.Citation9
In preclinical studies using immunocompetent animals, the most remarkable finding with G207 was that it induced systemic antitumor immunity in the course of oncolytic activity.Citation10,Citation11 For example, in A/J mice bearing bilateral subcutaneous N18 (syngeneic neuroblastoma) tumors, intraneoplastic G207 inoculation into the left tumor alone caused growth reduction not only of the inoculated tumors but also of the non-inoculated contralateral tumors. The antitumor immunity was associated with an elevated cytotoxic T lymphocyte activity specific to N18 tumor cells that persisted for at least 13 months.
After an extensive in vivo safety evaluation using HSV-1-susceptible mice and non-human primates, the G207 phase I clinical trial was performed between 1998 and 2000 at two institutions.Citation3 Twenty-one patients with recurrent malignant glioma were treated, and G207 was administered directly into the tumor via stereotactic inoculation. This dose escalation study started from 106 plaque-forming units (pfu) and increased to 3 × 109 pfu, with three patients at each dose. As a result, no acute, moderate to severe adverse events attributable to G207 were observed. Eight of 20 patients that had serial MRI evaluations had a decrease in tumor volume between four days and one month post-inoculation and two patients survived for more than five years.
Third-Generation Oncolytic HSV-1
The phase I clinical trial proved the safety of G207 and hinted its efficacy for human brain tumors. However, in order to further improve the efficacy without compromising its safety, a third-generation oncolytic HSV-1 termed G47Δ was newly created from G207 by introducing another genetic alteration, i.e., the deletion of the α47 gene and the overlapping US11 promoter region, in the G207 genome.Citation12 Because the α47 gene product inhibits transporter associated with antigen presentation, which translocates peptides across the endoplasmic reticulum, the downregulation of MHC class I that normally occurs in human cells after infection with HSV-1 does not occur when the α47 gene is deleted.Citation13 G47Δ-infected human cells in fact presented higher levels of MHC class I expression than cells infected with other HSV-1 vectors.Citation12 Further, human melanoma cells infected with G47Δ were better at stimulating their matched tumor-infiltrating lymphocytes in vitro than those infected with G207. The deletion also places the late US11 gene under control of the immediate-early α47 promoter, which results in suppression of the reduced growth phenotype of γ34.5-deficient HSV-1 mutants including G207.Citation14 In the majority of cell lines tested, G47Δ replicated better than G207, resulting in the generation of higher virus titers, and exhibiting greater cytopathic effect.Citation12 In athymic mice bearing subcutaneous U87MG human glioma and A/J mice bearing subcutaneous Neuro2a neuroblastoma, G47Δ was significantly more efficacious than G207 at inhibiting the tumor growth when inoculated intraneoplastically.Citation12 G47Δ was also more efficacious than G207 in athymic mice bearing intracerebral U87MG tumors (Ino Y et al., manuscript in preparation). Nevertheless, the safety of G47Δ remained unchanged from G207 following injection into the brain of HSV-1-sensitive A/J mice.Citation12 In Japan, a clinical trial of G47Δ in recurrent glioblastoma patients is underway. G47Δ has been shown efficacious in animal tumor models of a variety of cancers including brain tumors, prostate cancer, breast cancer and neurofibroma.Citation12,Citation15–Citation17
Construction of “Armed” Oncolytic HSV-1
One of the advantages of HSV-1 is the capacity to incorporate large and/or multiple transgenes within the viral genome. Certain antitumor functions may be added to oncolytic activities of HSV-1. The use of replication-competent HSV-1 for transgene expression has multiple attractive advantages over replication-incompetent or defective HSV-1 vectors: (1) A continuous generation of a high-titer, homogenous vector stock is possible, which allows manufacturing of a large amount with a better quality control. (2) An amplified gene delivery can be obtained in vivo. And, (3) transgene expression may lower administering doses required, therefore decrease toxicity. On the other hand, potential demerits of using replication-competent viruses for expressing foreign proteins are that the transgene expression may increase the toxicity of the vector, and may also interfere with viral replication. Transgene expression by armed oncolytic HSV-1 could be shorter in duration than replication-incompetent vectors due to destruction of the host cell by viral replication. However, we have observed that, because continuous viral spread and infection occur within the tumor, a larger number of tumor cells consequently express the transgene, therefore a much higher total amount of transgene product is achieved compared with non-replicating vectors.
In the past, a recombinant HSV-1 was constructed by conventional homologous recombination techniques that required selection of a correctly structured clone from millions of candidates. It often took a few years until the intended HSV-1 was obtained. In order to circumvent the time-consuming processes, we have developed an innovative “armed” oncolytic HSV-1 construction system using G47Δ as the backbone.Citation18 Besides its favorable features for human cancer therapy, including the safety, high yields of virus, improved oncolytic activity and potent stimulation of antitumor immune cells, G47Δ is especially suited as a replication-competent backbone for expressing any foreign protein molecules, because of the wide therapeutic window and preclusion of the shutoff of protein synthesis in the infected host cells. The system, termed T-BAC system, utilizes bacterial artificial chromosome and two DNA recombinase systems (Cre/loxP and FLP/FRT) (). It allows (1) a construction of armed oncolytic HSV-1 in a short period (usually 3–4 months), (2) a simultaneous construction of multiple vectors, (3) an accurate insertion of a desired transgene into the deleted ICP6 locus, (4) an insertion of multiple transgenes using the same effort as inserting a single transgene, and (5) a direct comparison of multiple “armed” oncolytic HSV-1 with the same backbone. A similar system, termed HSVQuik system, has been also developed using a G207-like backbone.Citation19,Citation20
Oncolytic HSV-1 Armed with Immunostimulatory Genes
Aside from the extent of replication capability within the tumor, the efficacy of an oncolytic HSV-1 depends on the extent of antitumor immunity induction.Citation10,Citation11 Therefore, while any transgene that does not interfere with HSV-1 replication may be used, the genes encoding immunomodulatory molecules would be reasonable candidates for arming oncolytic HSV-1. Immunostimulatory functions should augment the antitumor immunity induction that adds to direct oncolytic activity of the virus, resulting in enhanced antitumor activities ().Citation2 γ34.5 -deficient HSV-1 containing the murine interleukin 4 (IL-4) gene displayed a significantly higher antitumor activity and prolonged survival of mice with intracranial tumors compared with its parental virus or the one expressing IL-10.Citation21 First-generation oncolytic HSV-1 expressing IL-12 (M002 and NV1042) showed improved in vivo efficacy against 4C8 glioma in syngeneic B6D2F miceCitation22 and brain tumors of Neuro2a neuroblastoma in syngeneic A/J mice,Citation23 and also against murine squamous cell carcinomaCitation24 and murine colorectal tumor.Citation25 Immunohistochemical analyses of tumors treated with these IL-12-expressing HSV-1 revealed a significant influx of CD4+, CD8+ T cells and macrophages. The oncolytic HSV-1 expressing IL-12 (NV1042) was more efficacious than the one expressing granulocyte macrophage colony-stimulating factor (GM-CSF) in the same backbone (NV1034) in mice with subcutaneous squamous cell carcinoma.Citation24 The mice cured by NV1042 had a higher rate of rejecting rechallenged tumor cells than those cured by NV1034.Citation24
The HSVQuik was used to create G207-like second-generation oncolytic HSV-1 armed with murine IL-4, CD40 ligand or 6CK ().Citation20 In BALB/c mice bearing 4T1 breast cancer in the brain, all of these armed HSV-1 showed better antitumor efficacy than the control virus. Using the HSVQuik system, we also created oncolytic HSV-1 armed with IL-12, IL-18 or soluble B7-1.Citation19 All of these armed HSV-1 demonstrated replicative capabilities similar to the parental virus in vitro. The in vivo efficacy was tested in A/J mice harboring subcutaneous tumors of syngeneic and poorly immunogenic Neuro2a neuroblastoma. IL-12 was the most efficacious among the immunostimulatory molecules investigated when expressed by the G207-like HSV-1. The triple combination of the three armed viruses exhibited the highest efficacy amongst all single viruses or combinations of two viruses. Combining 1 × 105 pfu each of the three armed viruses showed stronger antitumor activities than any single armed virus at 3 × 105 pfu in inoculated tumors as well as non-inoculated remote tumors.
Using the Neuro2a subcutaneous tumor model, another research group demonstrated that the antitumor efficacy of M002, a first-generation γ34.5-deficient HSV-1 that expresses IL-12, could be augmented when used in combination with M010, the same backbone HSV-1 that expresses chemokine CCL2.Citation26 The group also demonstrated that the virus selected after in vivo serial passage of M002 in tumors of a D54-MG human malignant glioma cell line improved survival in two independent murine brain tumor models compared to the parent M002.Citation27 This enhanced antitumor efficacy was not due to restoration of protein synthesis or early US11 expression.
Recently, using the T-BAC system, we generated a G47Δ-backbone oncolytic HSV-1 armed with mouse fusion-type IL-12, termed T-mfIL12 (). In A/J mice bearing bilateral subcutaneous Neuro2a tumors, intraneoplastic inoculation with T-mfIL12 into the left tumor alone led to a significantly better antitumor activity than the unarmed control virus, T-01, not only in the inoculated left tumors but also in the non-inoculated remote tumors (Miyamoto S, et al., manuscript in preparation). We also created a G47Δ-backbone HSV-1 armed with both IL-18 and soluble B7-1.Citation18 This double-armed oncolytic HSV-1 showed a significant enhancement of antitumor efficacy via T-cell mediated immune responses in A/J mice with subcutaneous Neuro2a tumors as well as in C57BL/6 mice bearing subcutaneous TRAMP-C2 prostate cancer.
An armed oncolytic HSV-1 has not been tested in patients with brain tumors, however a phase I clinical trial with a second-generation oncolytic HSV-1 expressing GM-CSF was conducted in patients with cutaneous or subcutaneous deposits of breast, head and neck and gastrointestinal cancers and recurrent malignant melanoma.Citation28 OncoVEXGM-CSF has a deletion in the α47 gene and the γ34.5 gene replaced with the GM-CSF gene driven by a CMV promoter (). A single dose (13 patients) or multiple doses (17 patients), ranging from 106 to 108 pfu/ml/dose, were injected intratumorally. Local inflammation, erythema and febrile responses were the main side effects, and the local reaction to injection was dose limiting in HSV-1-seronegative patients at 107 pfu/ml. Some of biopsy specimens after treatment showed areas of necrosis that strongly stained for HSV-1. Three patients had stable disease, six patients showed flattened injected and/or uninjected tumors, and four patients showed inflammation of uninjected tumors.
Armed Oncolytic HSV-1 with Other Antitumor Functions
Various types of transgenes other than immunomodulatory genes have been used to arm oncolytic HSV-1. Theoretically, antiangiogenic factors can augment the antitumor activities of oncolytic HSV-1 without compromising the viral replication and antitumor immunity induction. Early generation oncolytic HSV-1, such as G207, was shown to retain the ability of wild type HSV-1 to increase infected tissue vascularity, whereas third-generation G47Δ showed suppressed vascularity in infected tumors.Citation29 By using the G47Δ-BAC system, a preliminary version of the T-BAC system, G47Δ-backbone oncolytic HSV-1 armed with Platelet Factor 4 or dominant negative fibroblast growth factor receptor have been created.Citation30,Citation31 Both of these armed oncolytic HSV-1 were more efficacious in inhibiting the tumor growth and angiogenesis than the control virus in both human U87MG glioma and mouse 37-3-18-4 malignant peripheral nerve sheath tumor models. By using the HSVQuik system, an oncolytic HSV-1 armed with tissue inhibitor of metalloproteinases 3, termed rQT3, has been created.Citation32 In athymic mice bearing human neuroblastoma or malignant peripheral nerve sheath tumor, treatment with rQT3 caused delayed tumor growth, increased peak levels of infectious virus, and immature collagen extracellular matrix. Remarkably, rQT3 treatment caused reduced tumor vascular density, which was associated with reduced circulating endothelial progenitors.
Another approach for arming oncolytic HSV-1 is the use of fusogenic membrane glycoproteins. Expression of fusogenic proteins by infected tumor cells could cause involvement of surrounding uninfected cells to form syncytium and facilitate tumor cell killing, but might also increase toxicity in the normal tissue. Fu et al. constructed an oncolytic HSV-1 armed with a truncated form of the gibbon ape leukemia virus envelope fusogenic membrane glycoprotein (GALV.fus).Citation33 In athymic mice bearing human Hep 3B hepatocellular carcinoma xenografts, the expression of GALV.fus significantly enhanced the antitumor effect of the virus. Furthermore, by using a strict late viral promoter instead of a CMV promoter, GALV.fus glycoprotein could be expressed only in tumor cells and not in normal non-dividing cells.
So-called suicide genes have been used from early stages of armed oncolytic HSV-1 development. Expression of a suicide gene by an infected tumor cell should elicit bystander killing of surrounding uninfected tumor cells via extracellular diffusion of activated prodrug, but premature killing of the host cell could also suppress viral replication. HSV-1 naturally expresses thymidine kinase that activates the prodrug ganciclovir. However, a combination with systemic ganciclovir administration did not significantly enhance the efficacy of G207 in A/J mice with intracerebral N18 neuroblastoma.Citation34 rRp450 was engineered by replacing the lacZ gene within the ICP6 locus of the first-generation oncolytic HSV-1, hrR3, with the gene encoding rat cytochrome P450 2B1 (CYP2B1), a member of the cytochrome P450 family responsible for activating the prodrug cyclophosphamide.Citation35 In rat 9L and human U87ΔEGFR glioma models, systemic administration of both cyclophosphamide and ganciclovir in combination with rRp450 showed the most efficacy compared with any other combinations.Citation36 By using the HSVQuik system, an oncolytic HSV-1, termed MGH2, was created that expressed both CYP2B1 and secreted human intestinal carboxylesterase.Citation37 The latter enzyme converts irinotecan into an active metabolite. In athymic mice bearing Gli36ΔEFGR glioma in the brain, MGH2 displayed increased antitumor efficacy when combined with cyclophosphamide and irinotecan. The researchers found that, unlike ganciclovir, cyclophosphamide, irinotecan or the combination of both did not significantly affect virus replication. HSV1yCD was created by replacing the ICP6 gene of HSV-1 with the gene encoding yeast cytosine deaminase (yCD).Citation38 yCD converts the prodrug 5-fluorocytosine (5-FC) to a cytotoxic agent, 5-fluorouracil. This research group also observed that the approach enhanced cytotoxicity without significantly reducing viral replication and oncolysis. In BALB/c mice bearing subcutaneous tumors or diffuse liver metastases of MC26 colon cancer, anti-neoplastic activity of HSV1yCD combined with systemic 5-FC administration was greater than HSV1yCD alone. By utilizing the same backbone as OncoVEXGM-CSF (), an oncolytic HSV-1 termed OncoVEXGALV/CD double-armed with yCD/uracil phosphoribosyltransferase fusion and GALV.fus has been created.Citation39 In Fischer f344 rats bearing subcutaneous 9L glioma, OncoVEXGALV/CD proved most efficacious compared with the control viruses (OncoVEX, OncoVEXGALV or OncoVEXCD) when combined with systemic 5-FC administration.
Han et al. recently created an oncolytic HSV-1, with double deletions in the γ34.5 and α47 genes, armed with tumor necrosis factor alpha (TNFα).Citation40 TNFα is a cytokine with a potent antitumor activity, but a local delivery of TNFα is known to cause toxicity, and its ability to induce tumor cell apoptosis could interfere with viral replication. To avoid these problems, they used the US11 true late HSV-1 promoter to drive the TNFα gene. Whereas the virus armed with US11-driven TNFα expressed lower amounts of TNFα, it exhibited higher antitumor effects and less toxicity than the virus that used the immediate-early CMV promoter.
Armed Oncolytic HSV-1 for in vivo Imaging
With the advancement of oncolytic virus therapy development, there has been an increasing need for non-invasive methods of imaging or monitoring of viral infection and replication. Oncolytic HSV-1 can be armed not only for the purpose of augmenting the therapeutic efficacy but also for realizing such non-invasive in vivo imaging. In preclinical settings, one approach is to utilize a luciferase-based bioluminescent system. Two HSVQuik-based oncolytic HSV-1 were generated that express firefly luciferase under the control of the immediate-early (IE) 4/5 promoter or gC promoter.Citation41 The IE4/5 promoter acts immediately after viral infection, whereas the strict late gC promoter acts in the late stage of the replication cycle. When athymic mice bearing subcutaneous tumors of Gli36ΔEGFR glioma were observed under a supersensitive charged coupled device camera, the expression of luciferase controlled by the IE4/5 promoter correlated with viral infection and that by the gC promoter with viral replication.
Systemic Delivery of Armed Oncolytic HSV-1
Whereas the most common route of delivery of oncolytic HSV-1 has been a direct intratumoral inoculation, an intravenous delivery would further broaden the clinical application of oncolytic HSV-1 if proven effective. The main hurdle for intravenous delivery is that only a small percentage of the administered virus reaches the tumor. By arming of oncolytic HSV-1, a large antitumor effect can be induced from a small number of virus that initiates replication at the tumor. We observed that intravenous delivery of IL-12-expressing T-mfIL12 caused a significant inhibition of tumor growth compared with mock and the unarmed control virus (T-01) treatments in A/J mice bearing subcutaneous Neuro2a tumors (Guan et al., manuscript in preparation). When A/J mice bearing intracerebral tumors were treated by repeated intravenous injections, T-mfIL12, but not T-01, significantly prolonged the survival compared with mock. Also, in a renal cancer lung metastases model using BALB/c mice and syngeneic RenCa cells, intravenous administrations of T-mfIL12 significantly inhibited the number of metastases compared with mock and T-01 treatments (Tsurumaki et al., manuscript in preparation).
Summary
In summary, “arming” of oncolytic HSV-1 with transgenes leads to development of a variety of oncolytic HSV-1 with certain functions resulting in enhancement of antitumor efficacy and/or in vivo imaging capability. In the future, a series of armed oncolytic HSV-1 suited for certain tumor types or certain administration routes may be used differentially or in combination according to conditions of patients. Armed oncolytic HSV-1 has high potential as a new genre of therapy for brain tumors as well as other cancers.
Abbreviations
| HSV-1 | = | herpes simplex virus type 1 |
| PKR | = | double-stranded RNA-dependent protein kinase |
| ICP6 | = | infected-cell protein 6 |
| pfu | = | plaque-forming units |
| IL | = | interleukin |
| GM-CSF | = | granulocyte macrophage colony-stimulating factor |
| GALV.fus | = | gibbon ape leukemia virus envelope fusogenic membrane glycoprotein |
| yCD | = | yeast cytosine deaminase |
| TNFα | = | tumor necrosis factor alpha |
Figures and Tables
Figure 1 A schema describing the T-BAC system for constructing “armed” oncolytic HSV-1 with the G47Δ backbone. The desired transgene for “arming” is inserted into the multiple cloning site of the shuttle vector (SV-01). The first step is to insert the entire sequence of the shuttle vector into the loxP site of T-BAC by a Cre-mediated recombination, followed by an electroporation into E. coli. The second step is to co-transfect the co-integrate with a plasmid expressing FLP onto Vero cells to excise the BAC sequence flanked by the FRT sites. The objective armed oncolytic HSV-1 appear as GFP-negative and lacZ-positive virus plaques. Non-recombined viruses do not appear, due to the presence of the lambda stuffer sequence (lmd) causing an oversize of the genome.Citation18
Figure 2 Concept of antitumor efficacy augmentation using oncolytic HSV-1 armed with an immunostimulatory gene. When oncolytic HSV-1 armed with the IL-12 gene infects tumor cells, IL-12 is secreted in the course of viral replication and stimulates the immune cells. In addition to direct tumor cell killing via viral replication and spread, tumor cells are destroyed by augmented antitumor immune responses, resulting in enhanced antitumor activities.
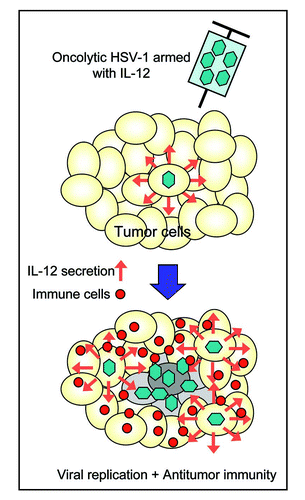
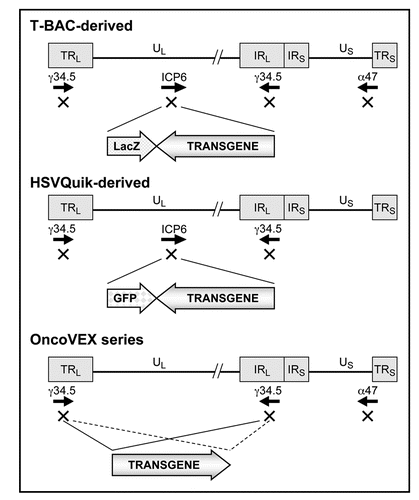
Figure 3 Structures of representative armed oncolytic HSV-1. The HSV-1 genome consists of long and short unique regions (UL and US) each bounded by terminal (T) and internal (I) repeat regions (RL and RS). Armed oncolytic HSV-1 created by using the T-BAC (or G47Δ-BAC) system has the backbone structure of G47Δ, a third-generation oncolytic HSV-1. It has triple deletions in the γ34.5, ICP6 and α47 genes. The transgene is inserted into the deleted ICP6 locus. As a marker, it also expresses the LacZ gene driven by the ICP6 promoter. Armed oncolytic HSV-1 created by using the HSVQuik system has the backbone structure similar to G207 or MGH1, second-generation oncolytic HSV-1. It has double deletions in the γ34.5 and ICP6 genes. The transgene is inserted into the deleted ICP6 locus. As a marker, it also expresses the GFP gene driven by the ICP6 promoter. The OncoVEX series has the backbone structure of a second-generation oncolytic HSV-1 with double deletions in the γ34.5 and α47 genes. The transgene is inserted into the deleted γ34.5 loci.
Acknowledgements
I thank the current members of my laboratory, especially Drs. Yasushi Ino and Hiroshi Fukuhara. Our research has been supported in part by grants to T.T. from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Aghi M, Martuza RL. Oncolytic viral therapies—the clinical experience. Oncogene 2005; 24:7802 - 7816
- Todo T. Oncolytic virus therapy using genetically engineered herpes simplex viruses. Front Biosci 2008; 13:2060 - 2064
- Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, Palmer CA, Feigenbaum F, Tornatore C, Tufaro F, Martuza RL. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther 2000; 7:867 - 874 [see comments]
- Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med 1995; 1:938 - 943
- Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 34.5, a gene nonessential for growth in culture. Science 1990; 250:1262 - 1266
- He B, Chou J, Brandimarti R, Mohr I, Gluzman Y, Roizman B. Suppression of the phenotype of gamma(1)34.5-herpes simplex virus 1: failure of activated RNA-dependent protein kinase to shut off protein synthesis is associated with a deletion in the domain of the alpha47 gene. J Virol 1997; 71:6049 - 6054
- Leib DA, Machalek MA, Williams BR, Silverman RH, Virgin HW. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc Natl Acad Sci USA 2000; 97:6097 - 6101
- Farassati F, Yang AD, Lee PW. Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1. Nat Cell Biol 2001; 3:745 - 750
- Goldstein DJ, Weller SK. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol 1988; 62:196 - 205
- Todo T, Rabkin SD, Sundaresan P, Wu A, Meehan KR, Herscowitz HB, Martuza RL. Systemic antitumor immunity in experimental brain tumor therapy using a multimutated, replication-competent herpes simplex virus. Hum Gene Ther 1999; 10:2741 - 2755
- Todo T, Rabkin SD, Chahlavi A, Martuza RL. Corticosteroid administration does not affect viral oncolytic activity, but inhibits antitumor immunity in replication-competent herpes simplex virus tumor therapy. Hum Gene Ther 1999; 10:2869 - 2878
- Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci USA 2001; 98:6396 - 6401
- York IA, Roop C, Andrews DW, Riddell SR, Graham FL, Johnson DC. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 1994; 77:525 - 535
- Mohr I, Gluzman Y. A herpesvirus genetic element which affects translation in the absence of the viral GADD34 function. Embo J 1996; 15:4759 - 4766
- Fukuhara H, Martuza RL, Rabkin SD, Ito Y, Todo T. Oncolytic herpes simplex virus vector G47Δ in combination with androgen ablation for the treatment of human prostate adenocarcinoma. Clin Cancer Res 2005; 11:7886 - 7890
- Liu R, Varghese S, Rabkin SD. Oncolytic herpes simplex virus vector therapy of breast cancer in C3(1)/SV40 T-antigen transgenic mice. Cancer Res 2005; 65:1532 - 1540
- Messerli SM, Prabhakar S, Tang Y, Mahmood U, Giovannini M, Weissleder R, Bronson R, Martuza R, Rabkin S, Breakefield XO. Treatment of schwannomas with an oncolytic recombinant herpes simplex virus in murine models of neurofibromatosis type 2. Hum Gene Ther 2006; 17:20 - 30
- Fukuhara H, Ino Y, Kuroda T, Martuza RL, Todo T. Triple gene-deleted oncolytic herpes simplex virus vector double-armed with interleukin 18 and soluble B7-1 constructed by bacterial artificial chromosome-mediated system. Cancer Res 2005; 65:10663 - 10668
- Ino Y, Saeki Y, Fukuhara H, Todo T. Triple combination of oncolytic herpes simplex virus-1 vectors armed with interleukin-12, interleukin-18 or soluble B7-1 results in enhanced antitumor efficacy. Clin Cancer Res 2006; 12:643 - 652
- Terada K, Wakimoto H, Tyminski E, Chiocca EA, Saeki Y. Development of a rapid method to generate multiple oncolytic HSV vectors and their in vivo evaluation using syngeneic mouse tumor models. Gene Ther 2006; 13:705 - 714
- Andreansky S, He B, van Cott J, McGhee J, Markert JM, Gillespie GY, Roizman B, Whitley RJ. Treatment of intracranial gliomas in immunocompetent mice using herpes simplex viruses that express murine interleukins. Gene Ther 1998; 5:121 - 130
- Hellums EK, Markert JM, Parker JN, He B, Perbal B, Roizman B, Whitley RJ, Langford CP, Bharara S, Gillespie GY. Increased efficacy of an interleukin-12-secreting herpes simplex virus in a syngeneic intracranial murine glioma model. Neuro Oncol 2005; 7:213 - 224
- Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ, Markert JM. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci USA 2000; 97:2208 - 2213
- Wong RJ, Patel SG, Kim S, DeMatteo RP, Malhotra S, Bennett JJ, St-Louis M, Shah JP, Johnson PA, Fong Y. Cytokine gene transfer enhances herpes oncolytic therapy in murine squamous cell carcinoma. Hum Gene Ther 2001; 12:253 - 265
- Bennett JJ, Malhotra S, Wong RJ, Delman K, Zager J, St-Louis M, Johnson P, Fong Y. Interleukin 12 secretion enhances antitumor efficacy of oncolytic herpes simplex viral therapy for colorectal cancer. Ann Surg 2001; 233:819 - 826
- Parker JN, Meleth S, Hughes KB, Gillespie GY, Whitley RJ, Markert JM. Enhanced inhibition of syngeneic murine tumors by combinatorial therapy with genetically engineered HSV-1 expressing CCL2 and IL-12. Cancer Gene Ther 2005; 12:359 - 368
- Shah AC, Price KH, Parker JN, Samuel SL, Meleth S, Cassady KA, Gillespie GY, Whitley RJ, Markert JM. Serial passage through human glioma xenografts selects for a Deltagamma134.5 herpes simplex virus type 1 mutant that exhibits decreased neurotoxicity and prolongs survival of mice with experimental brain tumors. J Virol 2006; 80:7308 - 7315
- Hu JC, Coffin RS, Davis CJ, Graham NJ, Groves N, Guest PJ, Harrington KJ, James ND, Love CA, McNeish I, Medley LC, Michael A, Nutting CM, Pandha HS, Shorrock CA, Simpson J, Steiner J, Steven NM, Wright D, Coombes RC. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res 2006; 12:6737 - 6747
- Aghi M, Rabkin SD, Martuza RL. Angiogenic response caused by oncolytic herpes simplex virus-induced reduced thrombospondin expression can be prevented by specific viral mutations or by administering a thrombospondin-derived peptide. Cancer Res 2007; 67:440 - 444
- Liu TC, Zhang T, Fukuhara H, Kuroda T, Todo T, Canron X, Bikfalvi A, Martuza RL, Kurtz A, Rabkin SD. Dominant-negative fibroblast growth factor receptor expression enhances antitumoral potency of oncolytic herpes simplex virus in neural tumors. Clin Cancer Res 2006; 12:6791 - 6799
- Liu TC, Zhang T, Fukuhara H, Kuroda T, Todo T, Martuza RL, Rabkin SD, Kurtz A. Oncolytic HSV armed with platelet factor 4, an antiangiogenic agent, shows enhanced efficacy. Mol Ther 2006; 14:789 - 797
- Mahller YY, Vaikunth SS, Ripberger MC, Baird WH, Saeki Y, Cancelas JA, Crombleholme TM, Cripe TP. Tissue inhibitor of metalloproteinase-3 via oncolytic herpesvirus inhibits tumor growth and vascular progenitors. Cancer Res 2008; 68:1170 - 1179
- Fu X, Tao L, Jin A, Vile R, Brenner MK, Zhang X. Expression of a fusogenic membrane glycoprotein by an oncolytic herpes simplex virus potentiates the viral antitumor effect. Mol Ther 2003; 7:748 - 754
- Todo T, Rabkin SD, Martuza RL. Evaluation of ganciclovir-mediated enhancement of the antitumoral effect in oncolytic, multimutated herpes simplex virus type 1 (G207) therapy of brain tumors. Cancer Gene Ther 2000; 7:939 - 946
- Chase M, Chung RY, Chiocca EA. An oncolytic viral mutant that delivers the CYP2B1 transgene and augments cyclophosphamide chemotherapy. Nature Biotechnol 1998; 16:444 - 448
- Aghi M, Chou TC, Suling K, Breakefield XO, Chiocca EA. Multimodal cancer treatment mediated by a replicating oncolytic virus that delivers the oxazaphosphorine/rat cytochrome P450 2B1 and ganciclovir/herpes simplex virus thymidine kinase gene therapies. Cancer Res 1999; 59:3861 - 3865
- Tyminski E, Leroy S, Terada K, Finkelstein DM, Hyatt JL, Danks MK, Potter PM, Saeki Y, Chiocca EA. Brain tumor oncolysis with replication-conditional herpes simplex virus type 1 expressing the prodrug-activating genes, CYP2B1 and secreted human intestinal carboxylesterase, in combination with cyclophosphamide and irinotecan. Cancer Res 2005; 65:6850 - 6857
- Nakamura H, Mullen JT, Chandrasekhar S, Pawlik TM, Yoon SS, Tanabe KK. Multimodality therapy with a replication-conditional herpes simplex virus 1 mutant that expresses yeast cytosine deaminase for intratumoral conversion of 5-fluorocytosine to 5-fluorouracil. Cancer Res 2001; 61:5447 - 5452
- Simpson GR, Han Z, Liu B, Wang Y, Campbell G, Coffin RS. Combination of a fusogenic glycoprotein, prodrug activation, and oncolytic herpes simplex virus for enhanced local tumor control. Cancer Res 2006; 66:4835 - 4842
- Han ZQ, Assenberg M, Liu BL, Wang YB, Simpson G, Thomas S, Coffin RS. Development of a second-generation oncolytic Herpes simplex virus expressing TNFalpha for cancer therapy. J Gene Med 2007; 9:99 - 106
- Yamamoto S, Deckter LA, Kasai K, Chiocca EA, Saeki Y. Imaging immediate-early and strict-late promoter activity during oncolytic herpes simplex virus type 1 infection and replication in tumors. Gene Ther 2006; 13:1731 - 1736