Abstract
We compared the in vitro effect of boric acid (BA) versus phenylboronic acid (PBA) on the migration of prostate and breast cancer cell lines and non-tumorigenic cells from the same tissues. Treatment at 24 hours with BA (≤ 500 µM) did not inhibit chemotaxis on fibronectin in any cell line. However, treatment over the same time course with concentrations of PBA as low as 1 µM significantly inhibited cancer cell migration without effecting non-tumorigenic cell lines. The compounds did not affect cell adhesion or viability at 24 hours but did alter morphology; both decreased cancer cell viability at 8 days. These results suggest that PBA is more potent than BA in targeting the metastatic and proliferative properties of cancer cells.
Introduction
As cancer cells colonize other organs, they become increasingly difficult to treat and can cause widespread organ failure.Citation1 The malignant phenotype is driven by complex interactions between tumor cells and their microenvironment. This metastatic cascade begins when transformed cells invade the host stroma surrounding the primary neoplasm. For this to occur, tumor cells must first convert from a stationary to a migratory phenotype, which is often associated with a downregulation of intercellular and extracellular adhesion.Citation2 The subsequent migration from the primary site is mediated by both chemoattractants as well as interactions with extracellular matrix components, such as fibronectin.Citation3 Once the migrating cells invade the circulatory system, they must revert to an adhesive phenotype in order to attach to the endothelium and migrate out of circulation to form secondary tumors.Citation4 Malignant tumor cells must complete every step of the metastatic cascade in order to successfully form secondary tumors. Therefore, an inhibitor at any stage of this process could delay or prevent the progression of metastasis.
The majority of current cancer treatments control the rapid proliferation of primary tumor cells but are not highly selective for tumorigenic cells.Citation5,Citation6 This damage to normal tissues causes serious side effects such as nausea, severe weight loss, fatigue and a depressed immune system.Citation7–Citation9 Additionally, intended targets often develop methods of evading drugs over course of the treatment.Citation10,Citation11 Current cancer therapeutics could be significantly improved if new compounds were developed targeting the actively migrating tumor cells, while leaving normal cells unaffected.
Naturally occurring compounds are a potentially rich source for novel anticancer agents.Citation12,Citation13 A promising new class of candidates is boron and its derivatives.Citation14,Citation15 Boron is found primarily in fruits, vegetables and drinking water and therefore is a common component of most diets.Citation14 It is estimated that the average dietary intake of boric acid in humans is approximately 15 µM.Citation26,Citation31 There are reported links between low intake of these foods and increased risk of prostate cancer.Citation16,Citation32–Citation34 Recent epidemiological, in vitro, and animal studies reveal a possible role for boric acid (BA), the most abundant physiological form of boron in the plasma, as a chemotherapeutic agent.Citation16–Citation21 In a nude mouse model of prostate cancer, BA decreases tumor size, IGF-1 serum levels and PSA levels and proteolytic activity.Citation20,Citation21 Barranco et al. also demonstrate that 1 mM of boric acid significantly decreases migration and proliferation of the prostate cancer cell line DU-145 in vitro.Citation17,Citation18
These studies motivate our search for additional derivatives of BA that may be effective at selectively inhibiting the metastatic properties of tumor cells. In addition, we are also interested in expanding the possible targets of these compounds to other cancer types. Our results reveal that phenylboronic acid (PBA) is a more potent and selective inhibitor of cancer cell migration and viability than its parent compound, boric acid. The ability of PBA to elicit a selective anti-migratory response short term, while decreasing cancer cell viability long term, makes it a promising candidate for a novel anti-cancer treatment.
Results
PBA is a potent inhibitor of cancer cell migration.
To date, the only published study examining the effect of BA on cancer cell migration was performed on uncoated polycarbonate filters.Citation18 To better represent the extracellular environment surrounding migrating tumors, we used fibronectin as an adhesive substrate. Untreated DU-145 and PC-3 highly metastatic prostate cancer cell lines, RWPE-1 non-tumorigenic prostate epithelial, ZR-75-1 highly metastatic breast cancer cells, and MCF-10A non-tumorigenic breast epithelial cells exhibited significant chemotactic migration towards 10% fetal bovine serum compared to negative controls (). Cells plated on nd-blotto coated filters migrating towards 10% serum or cells plated on fibronectin coated filters migrating towards serum-free media served as the negative controls. BA did not significantly inhibit chemotactic migration in any of the cell lines tested when used at concentrations as high as 500 µM (). BA only inhibited migration in one of these cell lines, DU-145, at a concentration of 1,000 µM, which is ∼100 times above average serum levels of BA in humans.Citation15,Citation26
In contrast, PBA significantly inhibited chemotaxis of all three cancer cell lines in a dose dependent manner. The cell lines also exhibited different sensitivities to varying PBA doses. Overall, the prostate cancer cell lines were more sensitive to PBA than the breast cancer line. DU-145 cell migration was significantly inhibited by concentrations starting at 1 µM PBA (). Chemotaxis of PC-3 cells, which were less sensitive to PBA than DU-145 cells, was significantly decreased at a concentration ≥10 µM compared to untreated cells (). ZR-75-1 cells, which were inhibited from migrating at concentrations ≥100 µM (), were the least sensitive cancer line. None of the effective doses significantly reduced migration in the corresponding non-tumorigenic cell lines ( and E). The MCF-10A cell line was refractive to all concentrations tested. PBA treatment inhibited migration of the RWPE-1 cell line at 50 µM, which is at least five times higher than the effective doses for the corresponding prostate tumor cells. Addition of BA to the effective PBA dose had no effect.
BA and PBA do not affect cancer cell adhesion to fibronectin.
BA or PBA treatments did not affect adhesion of DU-145, PC-3, RWPE-1, ZR-75-1 or MCF-10A cells to fibronectin. In one hour adhesion assays, there was no significant difference in the adhesion of any cell line on fibronectin between the untreated positive control and samples treated with up to 1000 µM of BA or PBA (). Adhesion in the positive control was significantly increased over the nd-blotto negative control in all cell lines. The data supports the hypothesis that the decrease in cell migration is not due to an inability of cells to adhere to the fibronectin substrate.
PBA is more potent than BA at decreasing cancer cell viability at eight days.
The highest dose used in all migration and adhesion assays (1,000 µM) had no impact on the viability of any cell line as measured by the MTT assay over the course of one day (). After an eight day exposure, this dose of PBA did significantly decrease viability of all cancer lines. BA also decreased cancer cell viability in DU-145 and ZR-75-1 cells, but this dose induced a significantly lower response than PBA (, B and D). Neither compound had any significant effect on the viability of the corresponding normal cell lines ( and E).
BA and PBA do not impact cell cycle progression.
MTT measures the reductive capacity of mitochondria, which can vary in otherwise healthy cells. In order to assess the growth characteristics of these cells, we measured DNA content using propidium idodide and flow cytometry. While the distribution of cells in G1, S and G2/M varied slightly between cell types, we observed no change in cell cycle distribution in any cell type treated with BA or PBA that would indicate cycle arrest ().
BA and PBA induce cell spreading on fibronectin.
The fact that BA or PBA treatment of all cell lines does not significantly decrease adhesion to fibronectin at one hour () while inhibiting migration at 24 hours () led us to examine cell morphology at these time points. Cells were stained for F-Actin (green) and nuclei (Blue), and morphology was examined by fluorescent microscopy. We saw no observable difference in cell morphology after 1 hour in any cell with the highest concentration of BA or PBA tested (1,000 µM) (data not shown). At 24 hours, the time that corresponds to the length of the migration assays, cells treated with BA or PBA at 1 mM showed an increase in cell spreading on fibronectin (). This increased spreading was quantified and found to be statistically significant with BA treatment in DU-145 cells and with PBA treatment of all cancer cells (). No significant increase in cell area was seen in the RWPE-1 or MCF-10A non-tumorigenic cell lines with BA or PBA treatment.
Discussion
Boric acid has a number of distinctive features that make it a promising pharmaceutical agent for cancer treatments.Citation27 It is a mild organic Lewis acid with structural features similar to carbon, allowing it to act as a competitive inhibitor for many carbon containing substrates.Citation27,Citation28 This characteristic of BA makes it an effective inhibitor of enzymes such as peptidases, proteases, proteasomes, arginase, nitric oxide synthase and transpeptidases.Citation29 The pharmacological properties of BA, including ease of handling, low toxicity, an almost complete clearance via urine within 24 hours and the fact that it is not metabolized make it an attractive cancer treatment candidate.Citation28,Citation35–Citation39
To date, five published studies have focused on the potential of BA to control progression of cancer at the cellular level.Citation17,Citation18,Citation20,Citation21,Citation40 Of these, only one study examined the ability of BA to inhibit prostate cancer cell migration.Citation18 This previous study revealed that an eight day treatment of 1 mM BA inhibited migration of one prostate cancer cell line, DU-145, on a polycarbonate substrate in vitro. To further elucidate the potential impact of BA on tumor metastasis, we expanded our study to include multiple cell lines, multiple organ sites, healthy and tumorigenic samples and fibronectin, a physiological substrate of migrating cells. The results are consistent with the observation that an eight day treatment with 1 mM of BA inhibits migration of DU-145 cells.Citation18 After refining the experimental procedure, it was noted that a single 24 hour exposure of BA or PBA can effectively inhibit the migration of cancer cells. The fact that non-tumorigenic cells are at least five-fold less sensitive to PBA at the effective dose for cancer cells suggests it may be a promising cancer treatment and raises the possibility that it could be used prophylactically. Despite our findings, the lack of understanding of mechanisms governing the action of BA on cancer cells limits its potential usefulness as an anticancer agent. Similarly, it is not known if or how metabolic products of PBA, phenol and catechol, contribute to its effectiveness.
Phenylboronic acid compares favorably with the in vitro properties of other compounds (e.g. migrastatin, carboxyamido-triazole) shown to selectively inhibit breast and prostate cancer migration in vivo and tumor metastasis in mice.Citation41 For example, it was discovered that carboxyamido-triazole (CAI) is a selective inhibitor of breast and prostate cancer migration in vitro, and this compound has progressed to phase III clinical trials in cancer patients.Citation22,Citation42 The properties that phenylboronic acid shares with these other anti-cancer drugs suggest it may be more effective than boric acid for cancer treatment.Citation12,Citation13,Citation43,Citation44
Materials and Methods
Materials.
All cell culture reagents were purchased from Invitrogen, Inc. (Carlsbad, CA) including RPMI-1640 medium, Dulbecco's modified Eagle Medium (DMEM)/Ham's F12, medium, Fetal Bovine Serum (FBS), horse serum, Keratinocyte Serum Free Media (cat. #10724) with Defined Keratinocyte growth supplement (cat. #10784), Phosphate Buffered Saline (PBS), penicillin, streptomycin and trypsin-EDTA. HEPES buffer, cholera toxin, hydrocortisone, epidermal growth factor, BA (cat. #B7660-500G), and PBA (cat. # P20009-10G) were purchased from Sigma-Aldrich Corporation (St. Louis, MO). Purified human plasma fibronectin was purchased from Chemicon International (Temecula, CA). Unless otherwise specified, other standard reagents were obtained from Fisher Scientific (Fair Lawn, NJ).
Tissue culture.
All cultures were maintained at 37°C in a humidified atmosphere of 5% CO2. The cells were harvested during the exponential growth phase using phosphate buffered saline (PBS) and 0.25% trypsin-EDTA (Invitrogen Corporation, Carlsbad, CA). DU-145 cells are a highly metastatic, androgen-independent, human prostate carcinoma obtained from American Type Culture Collection (ATCC) (Manassas, VA), number HTB-81. PC-3 cells are a highly metastatic, androgen-independent, human prostate adenocarcinoma obtained from ATCC, number CRL-1435. RWPE-1 cells are non-tumorigenic human prostate epithelial, ATCC number CRL-11609. ZR-75-1 cells are a highly metastatic, estrogen-independent, human breast ductal carcinoma obtained from ATCC, number CRL-1500. MCF-10A cells are a non-tumorigenic breast epithelial cell line obtained from ATCC, number CRL-10317. DU-145, PC-3 and ZR-75-1 cells were grown in RPMI-1640 Medium supplemented with 10% FBS, 15 mM HEPES buffer (pH 7.4), 50,000 units Penicillin and 50 mg Streptomycin. RWPE-1 cells were grown according to manufacturer's instructions in Keratinocyte Serum Free Media (K-SFM), Kit Catalog Number 17005-042 (Invitrogen Corporation, Carlsbad, CA). MCF-10A cells were grown in DMEM/Ham's F12 medium supplemented with 5% horse serum, 100 units/mL penicillin-streptomycin (Invitrogen), 1 mg/ml hydrocortisone, 10 µg/ml insulin, 100 ng/ml cholera toxin and 20 ng/ml epidermal growth factor (Sigma Aldrich).
Migration assay.
Cell migration assays were performed according to Rust et al.Citation22 with modifications described by Klees et al.Citation23 Corning Costar 8 µm MIC transwell plates (Corning, Corning NY) were used for the assay. Well filters were coated with purified fibronectin at a concentration of 20 µg/mL in PBS or nd-blotto (5% nondairy creamer in PBS + 0.2% Tween 20) in PBST for one hour at 37°C prior to assay and then rinsed with PBS before use. Cells were harvested and resuspended in migration media (DMEM + 1% sodium pyruvate + 1X GPS) at a density of 5 × 105 cells per well. One lane of cells was left untreated while the rest were treated with BA or PBA in varying concentrations. Cells were allowed to migrate toward serum free or medium containing 10% FBS in basal wells for 24 hours at 37°C. At this time, 5 µM calcein AM from Alexis Biochemicals (San Diego, CA) was added to the migration wells and incubated for 30 minutes at 37°C. To quantitate migration, cells were removed from the top of the migration filter with cotton-tipped applicators and fluorescence of the incorporated calcein AM was read from the bottom of the filter with a TECAN SPECTRAFluor spectrometer at 484Ex/535Em.
Adhesion assay.
Cell adhesion assays were performed as previously described by Plopper et al.Citation24 Sarstedt 96-well suspension cell culture plates were coated with purified fibronectin at a concentration of 20 µg/mL for 1 hour at room temperature. Wells were washed twice with PBS and incubated with nd-blotto for 30 minutes prior to the assay. Cells were allowed to attach for 30 minutes at 37°C in the presence of various concentrations of BA or PBA or left untreated as a control. To remove unbound cells following treatment, wells were filled with PBS and then plates were inverted in a tank of PBS and allowed to gently shake for 15 minutes. Adhered cells were subsequently fixed with 3% paraformaldehyde, washed twice in PBS and incubated in 0.5% crystal violet dye in 20% methanol/80% H2O for 15 minutes. Wells were washed thoroughly with dH2O and the violet dye was extracted with 1% SDS solution. Absorbance was measured using a TECAN SPECTAFluor spectrophotometer at 595 nm and relative adhesion was compared to untreated cells attached to fibronectin or to nd-blotto.
MTT viability assay.
Cancerous and non-tumorigenic cell viability was examined by the colorimetric methyl thiazolyl tetrazolium (MTT) assay in the presence of BA or PBA.Citation25 DU-145, PC-3, RWPE-1, ZR-75-1 and MCF-10A cells were grown in the absence or presence of 1mM BA or PBA for 1 or 8 days. Cells were seeded into 96-well microtiter plates coated with 20 µg/mL fibronectin in their respective growth media. Cells were left untreated as a control or grown in 1 mM BA or PBA. After 24 hours or 8 days, MTT solution (5 mg MTT/ml PBS) was added to each well, and each plate was incubated for four hours at 37°C/5% CO2, allowing viable cells to metabolize the yellow MTT and form the insoluble purple formazan product. The medium was aspirated, and the formazan was dissolved in 200 µl of DMSO. The absorbance was then read at 570 nm using a SPECTAFluor spectrophotometer reader.
Propidium iodide cell cycle analysis.
Cells were collected, centrifuged at 2,000 rpm (800 × g) for 5 min, and rinsed with PBS. Cells were fixed with 1 ml ice-cold 70% ethanol for 30 min and then centrifuged at 2,000 rpm (800 × g) for 10 min and washed twice in ice-cold PBS. The cell pellets were resuspended in 0.5 ml PBS containing 50 µg/ml propidium iodide (Sigma-Aldrich Chemical Co., St. Louis, MO) and 100 µg/ml RNase (Invitrogen, Carlsbad, CA), incubated at 37°C for 30 min and analyzed by fluorescence-activated cells sorting (FACS) using a BD Sciences LSRII flow cytometer (Becton Dickinson, San Jose, CA).
Morphological imaging and analysis.
Cells were plated on glass slides coated with 20 µg/mL fibronectin and grown for 24 hours. Cells were then fixed with 3% formaldehyde for one hour and incubated in PBS solution with 0.25% Tween and 1% BSA to block non-specific binding. F-Actin was stained with a 1:500 dilution of Texas Red Phalloidin 488 (Molecular Probes, Inc., Eugene, OR) in PBS solution containing 0.25% Tween20 and 1% BSA for one hour. The nuclear dye 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes) was used to visualize the nucleus. Cells were washed in PBS containing 0.25% Triton X-100, rinsed with water and mounted on microscope slides using Prolong Gold Antifade Reagent (Invitrogen). Samples were imaged with a 40x objective using a Nikon Eclipse TE2000S inverted fluorescence microscope equipped with a SPOT camera and SPOT Advanced software (Diagnostics Instruments, Sterling Heights, MI). Cell area was calculated using the National Institutes of Health ImageJ image processing program. The area was calculated as an average of ten cells with clearly defined perimeters and values are presented as square pixels.
Statistical analysis.
All experiments were repeated a minimum of three times. The representative data is presented as mean ± SEM. Statistical analyses were performed using Student's unpaired t-test, and a p value of less than 0.05, was considered significant.
Abbreviations
| BA | = | boric acid |
| ECM | = | extracellular matrix |
| FN | = | fibronectin |
| PBA | = | phenylboronic acid |
Figures and Tables
Figure 1 PBA inhibits cancer cell chemotaxis but BA does not. DU-145, PC-3, RWPE-1, ZR-75-1 and MCF-10A cells were assayed for chemotactic migration in the presence of indicated concentrations of Boric Acid (BA) or Phenol Boric Acid (PBA). Migration assays were performed on fibronectin coated filters with 10% FBS in the lower chambers as a chemoattractant. As a positive control, cells were plated on fibronectin coated filters in the presence of serum containing medium, without BA or PBA (Untreated). For a negative control, cells were plated on filters coated with fibronectin in the presence of serum containing medium (Blotto), or they were plated on fibronectin coated filters in medium lacking serum (Serum Free). A single asterisk (*) signifies that serum induces a significant increase in migration compared to negative controls. A double asterisk (**) indicates a significant increase in migration compared to untreated cells. Results expressed as mean ± SEM, n = 8 for each of three replicates.
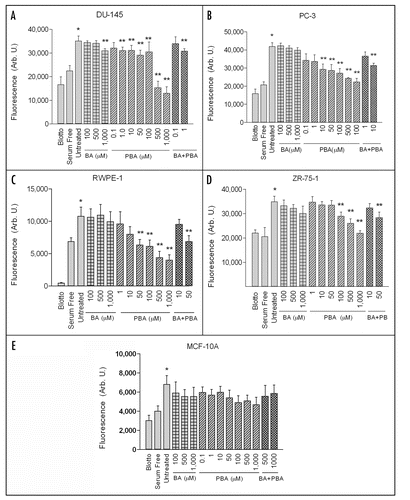
Figure 2 BA and PBA do not affect cancer or non-tumorigenic cell adhesion to fibronectin. DU-145 and PC-3 prostate cancer, RWPE-1 non-tumorigenic prostate, ZR-75-1 breast cancer and MCF-10A non-tumorigenic mammary cell lines were assayed for adhesion to purified fibronectin for 1 hour at 37°C. Cells were treated with various concentrations of either BA or PBA. As a control, cells were plated on blotto or fibronectin without treatment. Adherent cells were stained with crystal violet and then solubilized in SDS. Absorbance was determined at 595 nm. A single asterisk (*) indicates a significant increase in adhesion of untreated cells on fibronectin compared to untreated cells on fibronectin. In all cell lines tested, no BA or PBA concentration caused a significant decrease in adhesion compared to untreated control on fibronectin. Results expressed as mean ± SEM (n = 3).
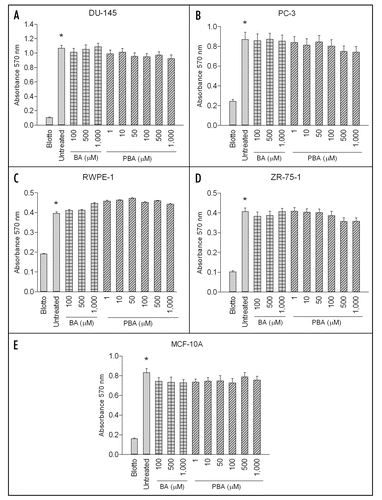
Figure 3 PBA decreases cell viability at eight days in cancer cells. DU-145 and PC-3 prostate cancer, RWPE-1 non-tumorigenic prostate, ZR-75-1 breast cancer and MCF-10A non-tumorigenic mammary cell lines were grown for either 24 hours or eight days in the presence of absence of 1 mM Boric Acid (BA) or Phenylboronic Acid (PBA). The MTT reagent was added to each well for four hours and then solubilized in DMSO and absorbance was read at 570 nm. Results were normalized to untreated cell line controls and expressed as mean ± SEM (n = 3). After one day, no cell line showed a significant decrease in viability when grown in the presence of either 1 mM BA or PBA. Following the eight day treatment, DU-145 and ZR-75-1 cell viability was significantly decreased by the addition of both 1 mM BA and PBA compared to untreated controls. PC-3 cell viability was not decreased by BA at eight days but was decreased by PBA at this timepoint. PBA treatment led to significantly lower cell viability staining than BA at eight days in all cancer cell lines as well. However, neither BA nor PBA had a significant effect on non-tumorigenic prostate or breast cancer cell line viability at either one or eight days.
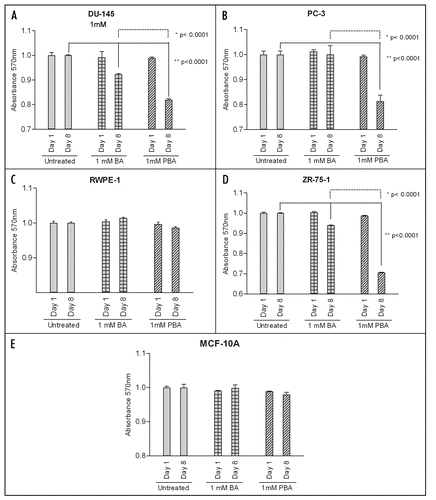
Figure 4 PBA induces spreading of cancer cells on fibronectin. Actin (green) and nuclei (blue) staining of fixed DU-145, PC-3 prostate cancer, RWPE-1 normal prostate, ZR-75-1 breast cancer, and MCF-10A non-tumorigenic breast cells grown on fibronectin for 24 h. Cells were imaged with a 40X objective.
Figure 5 Increased cell spreading induced by BA and PBA is statistically significant. Cell area from images in was quantified using ImageJ software. All images were taken at a 40x objective. Values represent mean ± standard deviation (n = 10).
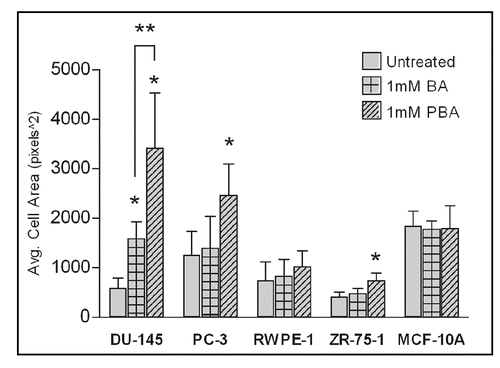
Table 1 Cell cycle analysis by flow cytometry
Acknowledgements
The research was supported in part by NIH Grant Number 2P20RR016464 from the INBRE Program of the National Center for Research Resources to the state of Nevada, Department of Defense contract W81XWH-06-2-0015 to SWC, and the NIGMS Biomolecular Science and Engineering Training, 1 T32 GM067545-01A1 (TMB).
References
- Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer 2004; 4:448 - 456
- Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev 2007; 28:297 - 321
- Varani J. Interaction of tumor cells with the extracellular matrix. Revis Biol Celular 1987; 12:1 - 113
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 2006; 12:895 - 904
- Devi GR. Prostate cancer: status of current treatments and emerging antisense-based therapies. Curr Opin Mol Ther 2002; 4:138 - 148
- Fallowfield LJ. Evolution of breast cancer treatments: current options and quality-of-life considerations. Eur J Oncol Nurs 2004; 8:75 - 82
- Theobald DE. Cancer pain, fatigue, distress and insomnia in cancer patients. Clin Cornerstone 2004; 6:15 - 21
- Amado F, Lourenco MT, Deheinzelin D. Metastatic breast cancer: do current treatments improve quality of life? A prospective study. Sao Paulo Med J 2006; 124:203
- Mattox TW. Treatment of unintentional weight loss in patients with cancer. Nutr Clin Pract 2005; 20:400 - 410
- Perez-Tomas R. Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Curr Med Chem 2006; 13:1859 - 1876
- Lehnert M. Multidrug resistance in human cancer. J Neurooncol 1994; 22:239 - 243
- Sinha S, Jain S. Natural products as anticancer agents. Prog Drug Res 1994; 42:53 - 132
- Parkinson DR, Arbuck SG, Moore T, Pluda JM, Christian MC. Clinical development of anticancer agents from natural products. Stem Cells 1994; 12:30 - 43
- Nielsen FH. The emergence of boron as nutritionally important throughout the life cycle. Nutrition 2000; 16:512 - 514
- Woods WG. An introduction to boron: history, sources, uses and chemistry. Environ Health Perspect 1994; 102:5 - 11
- Cui Y, Winton MI, Zhang ZF, Rainey C, Marshall J, De Kernion JB, Eckhert CD. Dietary boron intake and prostate cancer risk. Oncol Rep 2004; 11:887 - 892
- Barranco WT, Eckhert CD. Boric acid inhibits human prostate cancer cell proliferation. Cancer Lett 2004; 216:21 - 29
- Barranco WT, Eckhert CD. Cellular changes in boric acid-treated DU-145 prostate cancer cells. Br J Cancer 2006; 94:884 - 890
- Barranco WT, Hudak PF, Eckhert CD. Evaluation of ecological and in vitro effects of boron on prostate cancer risk (United States). Cancer Causes Control 2007; 18:71 - 77
- Gallardo-Williams MT, Chapin RE, King PE, Moser GJ, Goldsworthy TL, Morrison JP, Maronpot RR. Boron supplementation inhibits the growth and local expression of IGF-1 in human prostate adenocarcinoma (LNCaP) tumors in nude mice. Toxicol Pathol 2004; 32:73 - 78
- Gallardo-Williams MT, Maronpot RR, Wine RN, Brunssen SH, Chapin RE. Inhibition of the enzymatic activity of prostate-specific antigen by boric acid and 3-nitrophenyl boronic acid. Prostate 2003; 54:44 - 49
- Rust WL, Huff JL, Plopper GE. Screening assay for promigratory/antimigratory compounds. Anal Biochem 2000; 280:11 - 19
- Klees RF, De Marco PC, Salasznyk RM, Ahuja D, Hogg M, Antoniotti S, Kamath L, Dordick JS, Plopper GE. Apocynin derivatives interrupt intracellular signaling resulting in decreased migration in breast cancer cells. J Biomed Biotechnol 2006; 2006:87246
- Plopper GE, Domanico SZ, Cirulli V, Kiosses WB, Quaranta V. Migration of breast epithelial cells on Laminin-5: differential role of integrins in normal and transformed cell types. Breast Cancer Res Treat 1998; 51:57 - 69
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65:55 - 63
- Meacham SL, Hunt CD. Dietary boron intakes of selected populations in the United States. Biol Trace Elem Res 1998; 66:65 - 78
- Groziak MP. Boron therapeutics on the horizon. Am J Ther 2001; 8:321 - 328
- Yang W, Gao X, Wang B. Boronic acid compounds as potential pharmaceutical agents. Med Res Rev 2003; 23:346 - 368
- Hunt CD. Regulation of enzymatic activity: one possible role of dietary boron in higher animals and humans. Biol Trace Elem Res 1998; 66:205 - 225
- Nielsen FH. The justification for providing dietary guidance for the nutritional intake of boron. Biol Trace Elem Res 1998; 66:319 - 330
- Rainey CJ, Nyquist LA, Christensen RE, Strong PL, Culver BD, Coughlin JR. Daily boron intake from the American diet. J Am Diet Assoc 1999; 99:335 - 340
- Clinton SK, Giovannucci E. Diet, nutrition and prostate cancer. Annu Rev Nutr 1998; 18:413 - 440
- Kirsh VA, Peters U, Mayne ST, Subar AF, Chatterjee N, Johnson CC, Hayes RB. Prospective study of fruit and vegetable intake and risk of prostate cancer. J Natl Cancer Inst 2007; 99:1200 - 1209
- Stacewicz-Sapuntzakis M, Borthakur G, Burns JL, Bowen PE. Correlations of dietary patterns with prostate health. Mol Nutr Food Res 2008; 52:114 - 130
- Jansen JA, Andersen J, Schou JS. Boric acid single dose pharmacokinetics after intravenous administration to man. Arch Toxicol 1984; 55:64 - 67
- Schou JS, Jansen JA, Aggerbeck B. Human pharmacokinetics and safety of boric acid. Arch Toxicol Suppl 1984; 7:232 - 235
- Linden CH, Hall AH, Kulig KW, Rumack BH. Acute ingestions of boric acid. J Toxicol Clin Toxicol 1986; 24:269 - 279
- Litovitz TL, Klein-Schwartz W, Oderda GM, Schmitz BF. Clinical manifestations of toxicity in a series of 784 boric acid ingestions. Am J Emerg Med 1988; 6:209 - 213
- Moseman RF. Chemical disposition of boron in animals and humans. Environ Health Perspect 1994; 102:113 - 117
- Scorei R, Ciubar R, Ciofrangeanu CM, Mitran V, Cimpean A, Iordachescu D. Comparative Effects of Boric Acid and Calcium Fructoborate on Breast Cancer Cells. Biol Trace Elem Res 2008;
- Shan D, Chen L, Njardarson JT, Gaul C, Ma X, Danishefsky SJ, Huang XY. Synthetic analogues of migrastatin that inhibit mammary tumor metastasis in mice. Proc Natl Acad Sci USA 2005; 102:3772 - 3776
- Johnson EA, Marks RS, Mandrekar SJ, Hillman SL, Hauge MD, Bauman MD, et al. Phase III randomized, double-blind study of maintenance CAI or placebo in patients with advanced non-small cell lung cancer (NSCLC) after completion of initial therapy (NCCTG 97-24-51). Lung Cancer 2007; 60:200 - 207
- Ram VJ, Kumari S. Natural products of plant origin as anticancer agents. Drug News Perspect 2001; 14:465 - 482
- Gordaliza M. Natural products as leads to anticancer drugs. Clin Transl Oncol 2007; 9:767 - 776