Abstract
Lens development and differentiation are intricate and complex processes characterized by distinct molecular and morphological changes. The growth of a transparent lens involves proliferation of the epithelial cells and their subsequent differentiation into secondary fiber cells. Prior to differentiation, epithelial cells at the lens equator exit from the cell cycle and elongate into long, ribbon-like cells. Fiber cell elongation takes place bidirectionally as fiber tips migrate both anteriorly and posteriorly along the apical surface of the epithelium and inner surface of the capsule, respectively. The differentiating fiber cells move inward from the periphery to the center of the lens on a continuous basis as the lens grows throughout life. Finally, when fiber cells reach the center or suture line, their basal and apical tips detach from the epithelium and capsule, respectively, and interlock with cells from the opposite direction of the lens and form the suture line. Further, symmetric packing of fiber cells and degradation of most of the cellular organelle during fiber cell terminal differentiation are crucial for lens transparency. These sequential events are presumed to depend on cytoskeletal dynamics and cell adhesive interactions; however, our knowledge of regulation of lens fiber cell cytosketal reorganization, cell adhesive interactions and mechanotransduction, and their role in lens morphogenesis and function is limited at present. Recent biochemical and molecular studies have targeted cytoskeletal signaling proteins, including Rho GTPases, Abl kinase interacting proteins, cell adhesion molecules, myosin II, Src kinase and phosphoinositide 3-kinase in the developing chicken and mouse lens and characterized components of the fiber cell basal membrane complex. These studies have begun to unravel the vital role of cytoskeletal proteins and their regulatory pathways in control of lens morphogenesis, fiber cell elongation, migration, differentiation, survival and mechanical properties.
Lens morphogenesis involves a complex network of regulatory genes and interplay between growth factor, mitogenic, cell adhesive and cytoskeletal signaling pathways. The lens originates from surface ectoderm near the optic vesicle and lens vesicle that is formed via invagination of lens placode differentiates into primary fibers (the posterior half ) and epithelial cells (the anterior half ). These changes in embryonic cells control the lens distinctive anterior-posterior polarity. Subsequently, the lens grows through the proliferation of epithelial cells and the differentiation of their progeny into secondary fiber cells.Citation1,Citation2 The continuous addition of new fiber cells at the lens periphery leads to a gradual inward movement of older cells to the center of the lens. The ectodermal basement membrane that surrounds the lens vesicle thickens to form the lens capsule and is composed of mainly proteins of extracellular matrix.Citation2,Citation3 Since the lens does not shed cells, they are retained throughout the lens's life and are packed symmetrically within the lensCitation4 ().
Lens fiber cell elongation and differentiation is associated with a remarkable change in cell morphology, with the length of fiber cells increasing on the order of several hundredfold. These morphological changes are associated with extensive membrane and cortical cytoskeletal remodeling, actomyosin reorganization and cell adhesion turnover.Citation5–Citation17 Additionally, the tips of the elongating fiber cells at both the anterior and posterior terminals slide along the lens epithelium and capsule, respectively, as these cells migrate inward, and finally detach at the suture, where they form contacts with their counterparts from the opposite side of the lens.Citation4,Citation12 These cell movements are fundamental for maintaining distinct lens fiber cell polarity and are temporally and spatially regulated as the lens grows continuously throughout life.Citation1,Citation2,Citation12 Another unique feature of the lens is that during fiber cell terminal differentiation, all the cellular organelles, including nuclei, endoplasmic reticulum and mitochondria, are degraded in a programmed manner.Citation18 It has been well documented that lens epithelial cell elongation and differentiation is associated with reorganization of actin cytoskeleton, increased ratio of G-actin to F-actin, integrin switching, formation of N-cadherin linked cell adhesions, and expression of actin capping protein tropomodulin.Citation5,Citation6,Citation9,Citation10,Citation13,Citation15,Citation17,Citation19–Citation21 Importantly, disruption of actin cytoskeletal organization has been shown to impair lens epithelial differentiation and induce cataract formation, indicating the significance of actin cytoskeleton in lens differentiation and maintenance of lens optical quality.Citation14,Citation22 Further, during accommodation, lens shape is changed in a reversible manner. Therefore, the tensional homeostasis between actomyosin inside the fiber cell and fiber cell adhesion on the inner side of the lens capsule is considered to be crucial for accommodation.Citation12
In the developing mouse and chicken lens, the tips of the fiber cells (both apical and basal) have been reported to cluster with different cytoskeletal proteins, including actin, myosin II, actin capping protein tropomodulin, and N-cadherins.Citation10,Citation19,Citation21 Similarly, adhesion regulating signaling molecules including integrins, focal adhesion kinase, Cdk5, abl kinase interacting protein (Abi-2), and Rho GTPases have been shown to localize to the fiber cell apical and basal tips.Citation20,Citation23–Citation26 Moreover, isolation and characterization of the fiber cell basal membrane complexes (BMCs) had revealed a symmetric organization of N-cadherin, myosin II, actin in association with myosin light chain kinase, focal adhesion kinase, β1 integrin and caldesmon.Citation12 The signaling activity, tensional property and dynamics of BMCs are thought to control the coordinated migration of fiber cells along the lens capsule, formation of lens suture line, and lens accommodation.Citation12 Additionally, the BMCs have been shown to undergo a characteristic regional rearrangement (including size and shape) during lens elongation and migration along the lens capsule.Citation27 Therefore, impaired fiber cell migration on the lens capsule is expected to induce cataractogenesis.Citation27 Taken together, these different observations convincingly indicate the importance of cytoskeleton and cell adhesion regulatory mechanisms in lens fiber cell elongation and migration.
Although important insights have emerged regarding external cues controlling lens epithelial cell proliferation, elongation and differentiation, little is known regarding the specific signaling pathways that drive the processes culminating in fiber cell formation, migration, packing and maturation.Citation1,Citation7,Citation28 For example, growth factors are known to play key roles in influencing cell fates during development. Some of the major growth factor families, including FGFs and TGFβ/BMPs, have been shown to be involved in the regulation of lens developmental processes and primary fiber cell differentiation via ERK kinase activation.Citation1,Citation28,Citation29 However, the identity and role of signaling pathways acting downstream to growth factors regulating lens secondary fiber cell elongation, migration, adhesion, membrane remodeling and survival are poorly understood.Citation1,Citation12,Citation21,Citation30 In particular, regulatory mechanisms involved in cytoskeletal reorganization, tensional force and cell adhesive interactions during these cellular processes have yet be identified and characterized.Citation7,Citation9,Citation12,Citation21,Citation30–Citation32
Our laboratory has been working on a broad hypothesis that the actin cytoskeletal and cell adhesive signaling mechanisms composed of Rho GTPases (Rho, Rac and Cdc42) and their effector molecules play a critical role in controlling lens growth and differentiation, and in maintaining lens integrity.Citation7 The Rho family of small GTPases regulates morphogenesis, polarity, migration and cell adhesion.Citation33 These proteins bind GTP, exhibit GTPase activity, and cycle between an inactive GDP-bound form and an active GTP-bound form. This cycling is regulated by three groups of proteins: guanine-nucleotide exchange factors, which facilitate the exchange of GDP for GTP, thus rendering Rho GTPases active; GTPase-activating proteins, which regulate the inactivation of Rho by accelerating intrinsic GTPase activity and converting Rho GTPases back to their GDP-bound form; and GDP dissociation inhibitors (GDIs), which inhibit the dissociation of GDP bound to Rho GTPases.Citation33,Citation34 The GTP-bound form of the Rho GTPases interact with downstream effectors, which include protein kinases (e.g., ROCK and PAK), regulators of actin polymerization (e.g., N-WASP/WAVE, PI3-kinase and mDia), and other proteins with adaptor functions.Citation33 The selective interaction of the different Rho GTPases with a variety of effectors determines the final outcome of their activation.Citation33 For example, during cell movement, Rac and Cdc42 stimulate formation of protrusions at the leading edges of cells, and RhoA induces retraction at the tail ends of cells. This coordinated cytoskeletal reorganization permits cells to move toward a target.Citation35 PI3-kinase and PI (3, 4, 5) P3 have also been widely implicated in controlling cell migration and polarity in a Rac GTPase-dependent manner.Citation35 Members of the Wiskott-Aldrich syndrome protein (WASP) and WASP-family verprolin homologous protein (WAVE) families serve to link Rho GTPases signals to the ARP2/3 complex, leading to actin polymerization that is crucial for the reorganization of the actin cytoskeleton at the leading edge for processes such as cell movement and protrusions.Citation36 Importantly, all three Rho GTPases also regulate microtubule polymerization and assembly of adherens junctions to influence polarity and cell adhesion, respectively.Citation33,Citation37
Likewise, a tensional balance between cell adhesion on the outside and myosin II-based contractility on the inside of the cells is regulated by Rho GTPases.Citation38
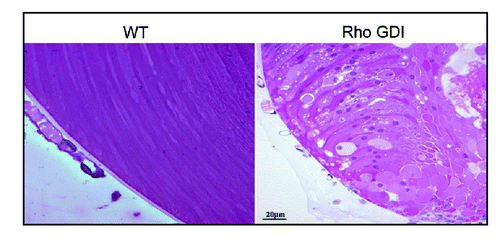
To explore the role of the Rho GTPases in lens morphogenesis and differentiation, we have targeted the lens Rho GTPases by overexpressing either the C3 exoenzyme (inactivator of RhoA and RhoB) or RhoGDIα (Rho GDP dissociation inhibitor) in a lens-specific manner in transgenic mice and followed their effects developmentally. These two transgenic mouse models exhibited ocular phenotype, including lens opacity (cataract) and microphthalmic eyes. Importantly, various histological, immunofluorescence and biochemical analyses performed in these developing transgenic mice have revealed defective lens morphogenesis, abnormal fiber cell migration, elongation, disrupted cytoskeletal organization and adhesive interactions, along with changes in proteins of the fiber cell gap junctions and water channels.Citation32,Citation39 These lenses have also shown decreased ERM (ezrin, radixin, moesin) protein phosphorylation,Citation40 proteins that are involved in crosslinking of the plasma membrane with actin cytoskeleton,Citation41 and increased apoptosis.Citation32 Defective fiber cell migration has been found to be more notable in the Rho GDI overexpressing lenses than in the C3 exoenzyme expressing lenses (). The Rho GDI overexpressing lenses have shown a defective membrane localization of Rho, Rac and Cdc42 confirming their inactivation. These data, together with mechanistic studies performed using the lens epithelial cells and the noted effects on cell shape, actin polymerization, myosin phosphorylation and cell adhesive interactions, reveal the importance of Rho GTPase-dependent signaling pathways in processes underlying fiber cell migration, elongation, cytoskeletal and membrane organization and survival in the developing lens.Citation7 Lens fiber cell BMC has been found to be localized intensely with Rac GTPase involved in cell migration (our unpublished work). Additionally, the Rho GDI transgenic lenses showed an impaired apical-apical cell-cell interactions between the fiber cells and epithelial cells.Citation32 Moreover, the ruptured posterior capsule and disrupted suture lines in these lenses are indicative of defective BMC organization and activity.Citation32
Further support for involvement of Rho GTPases in lens fiber cell differentiation and survival has come from studies conducted with chick lens epithelial explants and cultured epithelial cells. Inactivation of Rho kinase or Rac activation by PI3 kinase in chick lens epithelial cells has been reported to induce fiber cell differentiation and survival in association with distinct cortical actin cytoskeletal reorganization, indicating the significance of Rho GTPases in lens fiber cell differentiation and survival.Citation9,Citation42 Additionally, lens fiber cell elongation and differentiation has been found to be associated with increased myosin light chain (MLC) phosphorylation, and inhibition of MLC phosphorylation regulated by MLC kinase and Rho kinase has induced lens opacity and disruption of cytoskeletal integrity, supporting the importance of myosin II activity in maintaining lens architecture and transparency.Citation10 Importantly, various growth factors that regulate lens morphogenesis, fiber cell differentiation, and survival have been found to activate Rho and Rac GTPases and to induce MLC phosphorylation, actin cytoskeletal reorganization, and focal adhesion formation in lens epithelial cells.Citation7,Citation30 In addition to Rho GTPases, inhibition of Src kinase has been shown to induce fiber cell differentiation in association with actin cytoskeletal reorganization and cell adhesive interactions.Citation43 Also, the expression and activation of focal adhesion kinase has been reported to increase in differentiating and migrating lens epithelial cells.Citation44 Both these molecules are well recognized to regulate cell migration by participating in the disassembly of cell adhesions at the front of migrating cells.Citation35
Additional evidence for the participation of actin cytoskeletal organization and Rho GTPases in lens fiber cell migration and elongation has been derived from the studies of Abi-2 deficient mouse. Abl-interactor adaptor proteins Abi-1 and Abi-2 are linked to the Rac-WAVE-Arp2/3 signaling pathway and regulate actin polymerization and cell-cell adhesive interactions.Citation45 Homozygous deletion of Abi-2 in mice has been shown to exhibit ocular phenotype including microphthalmia and lens opacity similar to the Rho GDI overexpressing transgenic mouse eyes noted in previous studies.Citation23,Citation32 In the absence of Abi-2, the secondary lens fiber orientation, migration and elongation were found to be defective, supporting the importance of Rac-WAVE-Arp2/3 signaling in lens fiber cell migration and cell adhesion.Citation23 Abi-2 has been shown to localize intensely to the both basal and apical regions of the fiber cells and adherens junctions, and suppression of Abi-2 expression in epithelial cells resulted in impaired adherens junctions and downregulation of actin nucleation promoting factors.Citation23 The significance of cytoskeletal signaling in lens has also been implicated in Lowe syndrome, a rare X-linked disorder characterized by congenital cataracts, results from mutations in the OCRL1 gene. The OCRL1 protein product (phosphatidylinositol 4, 5 bisphosphate 5-phosphatase) has been shown to participate in Rac GTPase regulated actin cytoskeletal organization, cell migration, and cell adhesion in various cell types.Citation46 Finally, Wnt/PCP signaling via activation of Rho GTPases has been suggested to control lens morphogenesis, fiber cell migration and differentiation.Citation26
Importantly, given how the activity of the Rho GTPases is regulated by external cues and various effector proteins, a detailed understanding of the regulation of Rho GTPase signaling is necessary for a better appreciation of their role in lens morphogenesis, fiber cell elongation and differentiation, and tensional homeostasis. Further mechanistic studies are critical to unravel the specific role(s) of Rho GTPases and other cytoskeletal regulatory mechanisms involved in regulating the formation and disassembly of fiber cell basal and apical membrane complexes, fiber cell lateral membrane remodeling, and fiber cell-cell adhesive interactions during lens differentiation. Very little is known in terms of the assembly of different cell adhesive molecules at the apical-apical interface between the lens fibers and epithelial cells. We are only beginning to glimpse the regulatory networks involved in the regulation of fiber cell elongation, polarity, migration and adhesion. Many challenging questions remain: for example, how are the pathways regulating migration, basal and apical membrane complexes, and tensional homeostasis controlled by extracellular signals, and how are they integrated during fiber cell migration, suture formation, and packing? Novel insights into the molecular mechanisms regulating these cellular processes are expected to advance our understanding of lens morphogenesis, function and cataractogenesis.
Figures and Tables
Figure 1 Diagram of organization of lens epithelial and differentiating fiber cells. The lens is enclosed by a thick capsule consisting of various extracellular matrix proteins. Lens epithelial cells at the equator divide and exit from the cell cycle, and as they exit from the cell cycle, they start to elongate bidirectionally by making apical (AMC) and basal (BMC) membrane complexes with epithelium and capsule, respectively. As fiber cells elongate, they are pushed down and migrate toward the center. As the fiber cells migrate toward the center, both the basal and apical membrane complexes are expected to undergo changes in a regulated manner to control fiber cell adhesive, protrusive and contractile activity. Finally, when the fiber cells reach the center or suture line, their basal and apical ends detach from the epithelium and capsule, respectively and interlock with cells from the opposite direction of the lens and form suture. During fiber cell elongation and differentiation, cell adhesive interactions are reorganized extensively, and terminally differentiated fiber cells exhibit loss of cellular organelle and extensive membrane remodeling with unique ball and socket interdigitations. Arrows indicate the direction of fiber cell movement. This schematic is a modified version of from Lovicu and McAvoy.Citation1
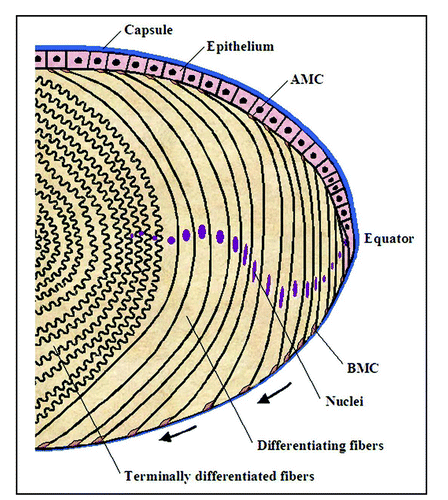
Figure 2 Abnormal lens phenotype in the neonatal Rho GDIα overexpressing transgenic mouse. Hematoxylin and eosin-stained sagittal sections of P1 RhoGDIα transgenic eyes reveal abnormal migration and morphology of the posterior lens fibers as compared with the symmetric organization of lens fibers and their migration toward the lens suture in the wild type mouse (reproduced with permission from Maddala et al.)Citation32.
Acknowledgements
The author wishes to thank Dr. Rupalatha Maddala for her contribution to the work cited in this article and Dr. Toshihiro Inoue for his help in drawing . The author's group work, cited in this article, was supported by grants from the National Institutes of Health (EY12201 and EY018590).
References
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol 2005; 280:1 - 14
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation 1981; 19:134 - 153
- Cammarata PR, Cantu-Crouch D, Oakford L, Morrill A. Macromolecular organization of bovine lens capsule. Tissue Cell 1986; 18:83 - 97
- Taylor VL, al-Ghoul KJ, Lane CW, Davis VA, Kuszak JR, Costello MJ. Morphology of the normal human lens. Invest Ophthalmol Vis Sci 1996; 37:1396 - 1410
- Rafferty NS, Scholz DL. Actin in polygonal arrays of microfilaments and sequestered actin bundles (SABs) in lens epithelial cells of rabbits and mice. Curr Eye Res 1985; 4:713 - 718
- Blanquet PR, Courtois Y. Differential assemblage of the basal membrane-cytoskeleton complex in bovine epithelial lens cells. Exp Eye Res 1989; 48:187 - 207
- Rao PV, Maddala R. The role of the lens actin cytoskeleton in fiber cell elongation and differentiation. Semin Cell Dev Biol 2006; 17:698 - 711
- Beebe DC, Vasiliev O, Guo J, Shui YB, Bassnett S. Changes in adhesion complexes define stages in the differentiation of lens fiber cells. Invest Ophthalmol Vis Sci 2001; 42:727 - 734
- Weber GF, Menko AS. Actin filament organization regulates the induction of lens cell differentiation and survival. Dev Biol 2006; 295:714 - 729
- Maddala R, Skiba N, Vasantha Rao P. Lens fiber cell elongation and differentiation is associated with a robust increase in myosin light chain phosphorylation in the developing mouse. Differentiation 2007; 75:713 - 725
- Straub BK, Boda J, Kuhn C, et al. A novel cell-cell junction system: the cortex adhaerens mosaic of lens fiber cells. J Cell Sci 2003; 116:4985 - 4995
- Bassnett S, Missey H, Vucemilo I. Molecular architecture of the lens fiber cell basal membrane complex. J Cell Sci 1999; 112:2155 - 2165
- Courtois Y, Arruti C, Barritault D, Tassin J, Olivie M, Hughes RC. Modulation of the shape of epithelial lens cells in vitro directed by a retinal extract factor. A model of interconversions and the role of actin filaments and fibronectin. Differentiation 1981; 18:11 - 27
- Mousa GY, Trevithick JR. Differentiation of rat lens epithelial cells in tissue culture II. Effects of cytochalasins B and D on actin organization and differentiation. Dev Biol 1977; 60:14 - 25
- Ramaekers FC, Boomkens TR, Bloemendal H. Cytoskeletal and contractile structures in bovine lens cell differentiation. Exp Cell Res 1981; 135:454 - 461
- Ireland M, Maisel H. Actin filaments of lens fiber cells. Ophthalmic Res 1982; 14:428 - 435
- Lo WK, Shaw AP, Paulsen DF, Mills A. Spatiotemporal distribution of zonulae adherens and associated actin bundles in both epithelium and fiber cells during chicken lens development. Exp Eye Res 2000; 71:45 - 55
- Bassnett S. Lens organelle degradation. Exp Eye Res 2002; 74:1 - 6
- Lee A, Fischer RS, Fowler VM. Stabilization and remodeling of the membrane skeleton during lens fiber cell differentiation and maturation. Dev Dyn 2000; 217:257 - 270
- Menko S, Philp N, Veneziale B, Walker J. Integrins and development: how might these receptors regulate differentiation of the lens. Ann N Y Acad Sci 1998; 842:36 - 41
- Sue Menko A. Lens epithelial cell differentiation. Exp Eye Res 2002; 75:485 - 490
- Beebe DC, Cerrelli S. Cytochalasin prevents cell elongation and increases potassium efflux from embryonic lens epithelial cells: implications for the mechanism of lens fiber cell elongation. Lens Eye Toxic Res 1989; 6:589 - 601
- Grove M, Demyanenko G, Echarri A, et al. ABI2-deficient mice exhibit defective cell migration, aberrant dendritic spine morphogenesis, and deficits in learning and memory. Mol Cell Biol 2004; 24:10905 - 10922
- Gao CY, Zakeri Z, Zhu Y, He H, Zelenka PS. Expression of Cdk5, p35 and Cdk5-associated kinase activity in the developing rat lens. Dev Genet 1997; 20:267 - 275
- Maddala R, Peng YW, Rao PV. Selective expression of the small GTPase RhoB in the early developing mouse lens. Dev Dyn 2001; 222:534 - 537
- Chen Y, Stump RJ, Lovicu FJ, McAvoy JW. A role for Wnt/planar cell polarity signaling during lens fiber cell differentiation?. Semin Cell Dev Biol 2006; 17:712 - 725
- Al-Ghoul KJ, Kuszak JR, Lu JY, Owens MJ. Morphology and organization of posterior fiber ends during migration. Mol Vis 2003; 9:119 - 128
- Boswell BA, Lein PJ, Musil LS. Cross-Talk between FGF and BMPs Regulates Gap Junction-mediated Intercellular Communication in Lens Cells. Mol Biol Cell 2008;
- Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development 2002; 129:3795 - 3802
- Maddala R, Reddy VN, Epstein DL, Rao V. Growth factor induced activation of Rho and Rac GTPases and actin cytoskeletal reorganization in human lens epithelial cells. Mol Vis 2003; 9:329 - 336
- Zelenka PS. Regulation of cell adhesion and migration in lens development. Int J Dev Biol 2004; 48:857 - 865
- Maddala R, Reneker LW, Pendurthi B, Rao PV. Rho GDP dissociation inhibitor-mediated disruption of Rho GTPase activity impairs lens fiber cell migration, elongation and survival. Dev Biol 2008; 315:217 - 231
- Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans 2005; 33:891 - 895
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002; 420:629 - 635
- Ridley AJ, Schwartz MA, Burridge K, et al. Cell migration: integrating signals from front to back. Science 2003; 302:1704 - 1709
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol 2007; 8:37 - 48
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell 2004; 116:167 - 179
- Clark K, Langeslag M, Figdor CG, van Leeuwen FN. Myosin II and mechanotransduction: a balancing act. Trends Cell Biol 2007; 17:178 - 186
- Maddala R, Deng PF, Costello JM, Wawrousek EF, Zigler JS, Rao VP. Impaired cytoskeletal organization and membrane integrity in lens fibers of a Rho GTPase functional knockout transgenic mouse. Lab Invest 2004; 84:679 - 692
- Rao PV, Ho T, Skiba NP, Maddala R. Characterization of lens fiber cell triton insoluble fraction reveals ERM (ezrin, radixin, moesin) proteins as major cytoskeletal-associated proteins. Biochem Biophys Res Commun 2008; 368:508 - 514
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 2002; 3:586 - 599
- Weber GF, Menko AS. Phosphatidylinositol 3-kinase is necessary for lens fiber cell differentiation and survival. Invest Ophthalmol Vis Sci 2006; 47:4490 - 4499
- Walker JL, Zhang L, Menko AS. Transition between proliferation and differentiation for lens epithelial cells is regulated by Src family kinases. Dev Dyn 2002; 224:361 - 372
- Kokkinos MI, Brown HJ, de Iongh RU. Focal adhesion kinase (FAK) expression and activation during lens development. Mol Vis 2007; 13:418 - 430
- Innocenti M, Gerboth S, Rottner K, et al. Abi1 regulates the activity of N-WASP and WAVE in distinct actin-based processes. Nat Cell Biol 2005; 7:969 - 976
- Suchy SF, Nussbaum RL. The deficiency of PIP2 5-phosphatase in Lowe syndrome affects actin polymerization. Am J Hum Genet 2002; 71:1420 - 1427