Abstract
Adherens junctions (AJs) are essential for the maintenance of epithelial homeostasis and a key factor in the regulation of cell migration and tumor progression. AJs maintain cell-cell adhesion by linking transmembrane proteins to the actin cytoskeleton. Additionally, they participate in recruitment of signaling receptors and cytoplasmic proteins to the membrane. During cellular invasion or migration, AJs are dynamically regulated and their composition modified to initiate changes in signaling pathways and cytoskeleton organization involved in cellular motility. Loss of E-cadherin, a key component of AJs, is characteristic of epithelial-mesenchymal-transition (EMT) and is associated with tumor cell invasion. We will review recent findings describing novel mechanisms involved in E-cadherin transcription regulation, endocytosis of E-cadherin and signaling associated with loss of AJs as well as reorganization of the AJ during EMT.
Introduction
Epithelial cell-cell adhesion is maintained by three basic structures connecting adjacent cells: tight junctions (zonula occludens), adherens junctions (zonula adherens) and desmosomes (macula adherens). AJs are established via calcium-dependent homophilic binding of the extracellular cadherin domains. The cytoplasmic domains of these cadherins recruit the catenins, β-catenin and p120 catenin, which in turn connect the AJs to the actin cytoskeleton. Linking catenins with the cytoskeleton may be mediated by the clustering of cadherin/catenin complexes to recruit high levels of α-cateninCitation1-Citation3 or by other cytoplasmic factors, such as epithelial protein lost in neoplasm (EPLIN or LIMA1).Citation4 EPLIN, a mechanosensitive biomarker, directly binds α-catenin and is absent in many cancer cell lines.Citation5,Citation6 Other major components of AJs are the nectins and afadins. Unlike cadherins, nectins are capable of establishing adhesive contacts via either heterophilic or homophilic binding.Citation7 The nectin-like protein NECL-5, better known as the poliovirus receptor (CD155), plays a role in cell motility, associating with integrin αvβ3 and platelet-derived growth factor receptor (PDGFR) at the leading edge of migrating cells.Citation8 Other adhesion molecules have been shown to have dual function as a cell adhesion and motility promoter. In this special focus, Kiefer et al. show that L1 cell adhesion molecule (L1CAM), which plays a major role in the development of the nervous system, also functions to promote the malignancy of human tumors. While it acts as a glue between cells, L1CAM can also drive motility during neural development and supports metastasis of human cancers. Important factors that contribute to the switch in the functional mode of L1CAM are cleavage from the cell surface by membrane proximal proteolysis and the ability to change binding partners and engage in L1CAM-integrin binding. Cleavage of E-cadherin is also a mechanism by which the function of L1CAM can be disrupted.
One of the key steps in tumor progression is the loss of cell-cell adhesion. As single cells invade into the underlying stroma, they express mesenchymal markers and proteins involved in matrix degradation and motility. The induction of the EMT phenotype during cancer is reminiscent of the EMT observed during development and neural cell crest migration. EMT involves the suppression of E-cadherin and a switch to the expression of mesenchymal cadherins such as N-cadherinCitation9 or cadherin 11.Citation10 At the center of this process is the inhibition of E-cadherin, the major mediator of cell adhesion in AJs.Citation11,Citation12 The regulation of E-cadherin involves both transcriptional and post-translational mechanisms. Whether by direct gene repression or as a consequence of weaker bonds between cells, loss of AJs is concomitant to the loss of the other junction complexes during EMT.Citation13,Citation14 Although E-cadherin is the most studied target of EMT inducers, the transcription factor SNAIL (SNAI1) is also known to inhibit tight junction components.Citation15,Citation16 In this review we will consider new aspects of the regulation of AJs, particularly cadherins, focusing on their role in EMT and tumor progression.
Transcriptional Regulation and Epigenetic Silencing of E-Cadherin (CDH1)
Regulation of E-cadherin transcription during EMT is dependent upon many known transcription factors capable of binding the E-box sequence in the promoter region of CDH1. Two of these transcription factors, SNAI1 and the basic helix-loop-helix family member TWIST1, have been considered to be master regulators of EMT.Citation17-Citation19 Repression of E-cadherin by SNAI1/TWIST1 involves the recruitment of histone remodeling proteins to the promoter, where SNAI1 interacts with histone deacetylase (HDAC) 1 and 2.Citation20 TWIST1 interacts with several other components, the Mi2/nucleosome remodeling and deacetylases (Mi2/NuRD) complex.Citation21 Deacetylation of histones by HDAC prevents the opening of chromatin and the binding of the transcription machinery. SNAI1/TWIST1 can induce repression of the CDH1 promoter by phosphorylation of histone H3 at Thr45 through Akt2.Citation22,Citation23
The genetic program induced in putative cancer stem cells, similarly to embryonic stem cells, can be regulated by global epigenetic changes.Citation24 EMT has been shown to induce stem cell properties in tumor cells suggesting that both EMT-inducing transcription factors and epigenetic regulators do cooperate.Citation25,Citation26 A review by Hale et al. in this special issue highlights how cell-cell adhesion mechanisms provide molecular cues that are used by cancer stem cells. Such EMT-inducing factors have recently been found to interact with the epigenetic regulators DNA methyl transferases (DNMT), polycomb proteins and H3K9 methyl transferase () to regulate stemness. These data have allowed for a much better understanding of how CDH1 is silenced in cancer cells.
Table 1. Targets of Histone methylases and demethylases
Promoter methylation
The silencing of E-cadherin expression by hypermethylation is a common event in cancer.Citation27,Citation28 DNMTs target cytosine residues in CpG dinucleotides for methylation and have recently been identified in the repression of E-cadherin in normalCitation29-Citation32 and pathological contexts,Citation33-Citation36 such as colorectal cancer, gastric cancer and hepatocellular carcinomas.Citation37-Citation42 Multiple signaling pathways involved in EMT and tumorigenesis activate DNMTs, e.g., rasCitation43 and TGF-β.Citation44,Citation45
DNMTs bind several histone remodeling enzymes, such as MPP8,Citation46 Sirtuin 1Citation47 and G9aCitation48 (). However, SNAI1 has been shown to be linked to DNMT1,Citation49 notably in association with G9a and Suv39H1Citation50,Citation51 (see also ). As polycomb proteins act as a platform to recruit DNMT, the two epigenetic mechanisms could intersect.Citation52
Table 2. Histone remodeling enzymes interaction with EMT-inducing transcription factors and involved in methylation and demethylation
Cooperation between Polycomb proteins and EMT-inducing transcription factors
The polycomb proteins are part of repressor complexes that inhibit gene expression through chromatin remodeling. Polycomb-mediated gene expression is essential for the maintenance of embryonic stem cells and is involved in development as well as tumor suppression.Citation53,Citation54 The polycomb repressive complex 2 (PRC2) recruits PRC1 after chromatin methylation at H3K27 through enhancer of Zeste Homolog 2 (EZH2), a histone H3 lysine-27-specific methyltransferase.Citation55 Both, PRC1 and PRC2 have been shown to interact with SNAI1 and TWIST1 to promote EMT.
SNAI1 is stabilized through its interaction with the PRC1 component BMI-1Citation56,Citation57 and interacts with Suppressor of Zeste 12 (Suz12) and EZH2 to repress CDH1 expression.Citation58,Citation59 Interestingly, EZH2 also participates in transforming growth factor β 1 (TGF-β1) signaling,Citation60 a potent inducer of EMT. BMI-1 can also interact with TWIST to induce EMT.Citation61
The intricate interactions of EMT-inducing transcription factors and chromatin remodeling complexes PRC1 and PRC2 may offer novel approaches to control EMT and thus cell adhesion in cancer cells via a plethora of new drug, such as HDACs and DNMT inhibitors.
Endocytosis of AJ Components
AJs are highly dynamic structures.Citation62 Remodeling of AJs and associated proteins occurs through endocytosis and recycling of the complex components.Citation63 Endocytic pathways are often misregulated in cancers and a shift in the balance between recycling and degradationCitation64 can result, for example, in the degradation of E-cadherin and increased cell migration. Endocytic signaling pathways enclose a vast number of mechanisms and associated proteins some of them recently described such as CD2AP.Citation65,Citation66 Others such as Rab, Rap and Rho GTPases, endocytosis proteins like dynamin and other associated proteins like Bar proteins etc have been extensively reviewed.Citation64,Citation67-Citation69 Here we will here focus on some recent findings involving E-cadherin endocytosis, particularly during EMT.
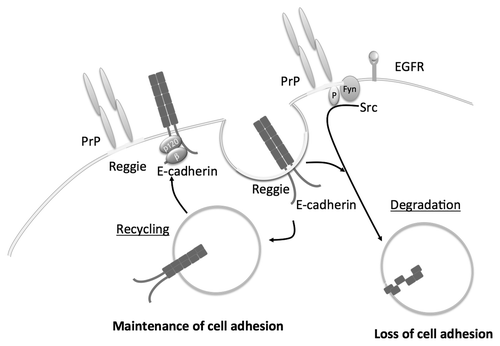
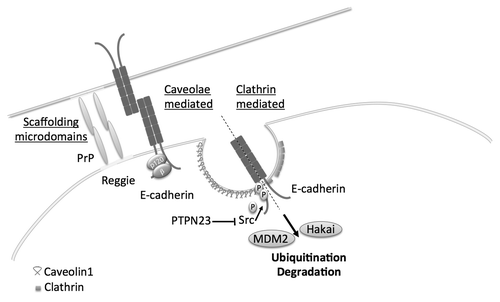
E-cadherin endocytosis can be mediated by clathrin-mediated vesicles or non-clathrin pathways: caveolae- (lipid raft-mediated) endocytosis and macropinocytosisCitation67 (). The degradation or recycling of E-cadherin is dependent on its phosphorylation state. Src targets the tyrosine residues, Tyr755 and 756, at the juxtamembranous domain of E-cadherin displacing p120 catenin,Citation70,Citation71 thereby destabilizing AJs. E-cadherin is then degraded by the ubiquitin ligases Hakai, or MDM2Citation72-Citation74 ().
Figure 1. Endocytosis pathways involved in the regulation of E-cadherin. Non-clathrin mediated endocytosis can be regulated by scaffolding microdomains, PrP and Reggie/Flotillin or caveolae compared with clathrin-mediated endocytosis. Phosphorylation of E-cadherin at Tyr755 and 756, e.g., by Src, induces its endocytosis and degradation by the proteasome complex after ubiquitination by Hakai or Mdm2.
Src is a known oncogene and activates different signaling pathways that can ultimately inhibit E-cadherin to promote tumor progression.Citation75 The proto-oncogenes epidermal growth factor receptor (EGFR)Citation76 and hepatocyte growth factor receptor (HGFR) target Src but also physically interact with E-cadherin.Citation77-Citation80
Protein tyrosine phosphatases (PTPs) have been considered tumor suppressors as they are the functional opposites of tyrosine kinases. PTPs (PTP-μ, DEP1, PTP-LAR, PTP1B, PTB-PEST and others) activate kinases by removing inhibitory phosphates and are involved in the regulation of cell-cell junction (for a review see refs. Citation81 and Citation82). Rather than acting as pleiotropic suppressors of tyrosine kinases, PTPs recently have been described to act as specific regulators of signaling pathways.Citation83 In mammary epithelial cells, loss of the PTPs PTPRG, PTPRR and PTPN23 was associated with enhanced cell motility. However only PTPN23 was been shown to directly induce E-cadherin internalization and was associated with increased invasion. Src, E-cadherin and β-catenin appear to be direct substrates of PTPN23. An increase in Src activity with the subsequent loss of PTPN23 was responsible for the expression of mesenchymal markers and the observed increased invasion in mammary cell lines ().
Non-clathrin-mediated endocytic pathways
The suppression of PTPN23 is also associated with caveolin-1 mediated internalization and blocking of early endosome vesicle trafficking.Citation83 Caveolin-1 plays an important role in EGFR signalingCitation84,Citation85 as downregulation of caveolin-1 in response to EGF results in the expression of SNAI1, EMT and loss of E-cadherin.Citation86 In cancer cells, EGF can induce caveolin-1-mediated degradation of E-cadherin and tumor cell dissociation.Citation87,Citation88
Reggie/flotillins are microdomain scaffolding proteins (lipid raft components) that play an important role in Wnt and Shh signalingCitation89 and are involved in regulating membrane trafficking and turnover.Citation90,Citation91 In association with prion protein (PrP), reggies/flotillins control activation of Src kinases and the presence of receptor tyrosine kinases such as EGFR at the membrane.Citation92 Reggies contribute to the internalization and turnover of E-cadherin. Together with PrP they target the recycling of E-cadherin to the AJs and its binding to catenins for the maintenance of cell adhesion ().Citation93 In the context of EGFR-dependent loss of cell adhesion, reggies are necessary for EGFR endocytosis to prevent sustained activity and concurrently cell migration.
Clathrin-mediated E-cadherin recycling
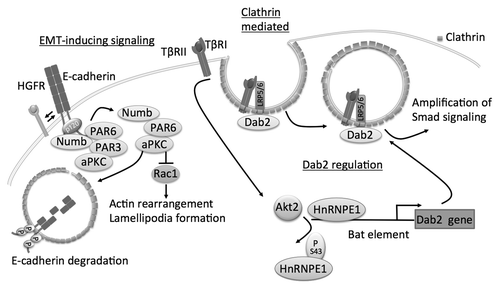
Endocytosis of E-cadherin can be mediated by clathrin-coated vesicles upon calcium depletion.Citation94 Several adaptor proteins in this pathway, notably Numb and Disabled-2 (Dab2), participate in the regulation of members of adherens junctions during EMT ().
Figure 3. Schematics of clathrin-mediated endocytosis of E-cadherin and role of adaptator proteins Dab2 and Numb. Numb interacts with E-cadherin and binds to the polarity complex protein aPKC/PAR3/PAR6. Upon EMT-induction by HGFR signaling the complex shifts to Numb aPKC/PAR3 promoting endocytosis of E-cadherin. aPKC can decrease binding of Numb to p120 by phosphorylation of E-cadherin and reduce endocytosis of E-cadherin. Numb prevents the activation of Rac1. Dab2 participates in the endocytosis of TβRII and promotes signaling of TGFβ through canonical Smad signaling. TGFβ signaling itself can induce expression of Dab2 by inducing phosphorylation of hnRNPE1, displacing it from the Bat element in Dab2 promoter, through Akt2.
Numb is often misregulated in cancers and plays many roles in the cell from endocytosis, to cell migration, cell adhesion, signaling pathways and asymmetric cell division.Citation95 However, Numb’s function during tumorigenesis remains controversial: Much of the work done on Numb highlights its role as a tumor suppressor. Not only does it inhibit ubiquitination and subsequent destruction of p53, but it also has been shown to inhibit Rac1-GTP accumulation. Rac1 is initially increased by cell-cell contacts and E-cadherinCitation96 but is rapidly downregulated after the establishment of adherens junctions.Citation97,Citation98 Indeed, Rac1-GTP accumulation is associated with loss of cell-cell adhesion and lamellipodia formation.Citation99 With respect to AJs more specifically, Numb knockdown was recently shown to sensitize cells to HGF-mediated EMT.Citation100 The proposed mechanism for Numb’s role in maintaining cell adhesion and apicobasal polarity involves its binding and sub-membranous localization, via its phosphotyrosine-binding domain (PTB), to both the PAR3-aPKC-PAR6 complexCitation101 and E-cadherin (). Upon phosphorylation by aPKC, Numb is endocytosed. Wang et al. propose that Numb binds the same NVYY motif that Hakai binds to target E-cadherin for degradation. Numb would therefore be an important regulator of cell adhesion and maintenance of cell polarity. In a similar fashion, signaling induced by TGF-β1 destabilizes PAR6 and affects both E-cadherin expression and localization of the PAR complex.Citation102,Citation103 Because Numb establishes a dynamic link between AJs and tight junctions, loss of Numb upon HGF stimulation could affect both structures simultaneously during EMT.
By contrast, Numb expression at the cell membrane has been shown to promote the loss of cell-cell adhesion. It was suggested that Numb causes E-cadherin endocytosis by binding directly to p120 catenin, which itself prevents E-cadherin internalization.Citation104 Sato et al. propose that phosphorylation of Numb by the atypical protein kinase aPKC prevents Numb binding to p120 and helps maintain apicobasal polarity. Overall, more work is needed to elucidate the roles of Numb in the regulation of E-cadherin endocytosis.
Dab2 is an endocytotic adaptor proteinCitation105 and is considered a tumor suppressor as Dab2 expression is frequently lost in different types of cancer.Citation106-Citation110 Interestingly, Dab2 is a central node to TGF-β1 mediated EMT-signaling. As a downstream target of TGF-β1,Citation111,Citation112 Dab2 gene expression is induced by Akt2, which phosphorylates hnRNP E1, thereby preventing suppression of the Dab2 promoter.Citation113,Citation114 However, more recent studies have found that the epigenetic silencing of Dab2 is responsible for the switch of TGF-β1 signaling from a tumor suppressor to an oncogenic factor.Citation115,Citation116 Furthermore, Dab2 participates in the endocytosis of TβRII and promotes signaling of TGFβ through canonical Smad signaling. Dab2 can decrease E-cadherin levels and upregulate the mesenchymal marker vimentinCitation117 demonstrating not only a role for Dab2 in EMT, but also in the regulation of E-cadherin. In addition, a study focusing on Dab2 function in embryogenesis, identified that Dab2 mediates directional trafficking and the polarized distribution of E-cadherin.Citation118 Taken together, it could be conceivable that Dab2 is affecting E-cadherin trafficking and degradation directly, but the mechanisms have yet to be identified.
The Fate of Cell-Cell Adhesion in Tumor Progression
In the absence of E-cadherin, individual tumor cells migrate by one of two modes: mesenchymal movement (directional migration with proteolysis at the leading edge) or amoeboid movement with lack of direction and of polarity.Citation119 In the presence of E-cadherin, tumor cells use collective migration.Citation119,Citation120
Yang et al. propose that the induced mesenchymal mode of migration will be associated with local invasion but limited metastasis. Interestingly, in head-and-neck squamous cell carcinoma individual as well as collective migration can be observed. This prompts the question of whether cell-cell contacts could still be preserved after EMT.
N-cadherin adherens junctions: collective migration after EMT
Recent work demonstrates that the transition between single cell migration and collective migration in breast cancer cell lines is reversible and depends upon TGF-β1 signaling.Citation121 In fact, alternating between single-cell and collective migration is conducive to a more metastatic disease phenotype. Collective cell migration requires mechanisms to maintain cell adhesion and is dependent on cell polarity.
One hallmark of EMT is the shift from E-cadherin to N-cadherin expression.Citation9 The “cadherin switch” does not necessarily imply that E-cadherin will always be replaced by N-cadherin. Rather, N-cadherin expression increases as cells undergo EMT. Ectopic expression of N-cadherin increases migration of breast cancer cell linesCitation122 as well as in the mouse mammary epithelial cell line NmuMG after treatment with TGF-β1.Citation123 Interestingly, in this last study N-cadherin upregulation preceded morphological changes induced by EMT. The precise mechanism controlling an increase in N-cadherin expression is unknown, although previous studies have suggested it to be TWIST1-dependent.Citation124,Citation125
It is important to note that although N-cadherin AJs are weaker than their E-cadherin counterparts,Citation126 they may still maintain cell-cell contacts during migration. In the collective migration of neural crest cells, N-cadherin inhibits formation of protrusions at cell-cell contacts helping the cells at the leading edge to progress.Citation127 The mechanism involved in the regulation of Rac1 activity to promote N-cadherin-mediated adhesion was also shown to be integrin-dependent.Citation128
N-cadherin can also play an essential role in collective migration.Citation129 Cells grown in a three-dimensional environment have been shown to establish focal adhesion differently compared with cells grown in a two-dimensional environment such as on plastic, which allows for the possibility that cell-cell adhesion could also be affected.Citation130,Citation131 A three-dimensional environment, in which the cells were embedded in an extracellular matrix rather than grown on plastic, was required for the establishment of N-cadherin-mediated cell-cell adhesion after EMT hinting at matrix-dependent cues, such as stiffness of the matrix, which have been demonstrated to affect cell migration and invasion (see below). Shih and Yamada also described a distinct role of the cytoplasmic domain of N-cadherin in promoting migration by an unknown mechanism. However, the potential role of AJs formed by N-cadherin with respect to the migration of epithelial cells undergoing EMT remains largely unexplored.
The role of the matrix in collective invasion
Matrix rigidity and orientation promotes collective cell migration.Citation119 Matrix stiffening was recently shown to sensitize cells to EGF.Citation132 Moreover, as the matrix stiffens, TGF-β1 loses its anti-proliferative role to become pro-tumorigenic. This effect is not mediated by Smad signaling, but by the PI3K/Akt pathway.Citation133 Matrix stiffness is important in controlling control the strength of AJs in collective migration.Citation134 The composition of the matrix itself is regulated by the fibroblasts, which can be activated by paracrine factors secreted from the tumor. At the same time, secretion of growth factors from the activated fibroblasts contributes to tumor progression.Citation135 Carcinoma-associated fibroblasts can promote tumor invasion and generate a track in the matrix for the invading tumor cells to follow.Citation136
Interestingly, lysyl oxidase-like 2 (LOXL2), a tumor marker, increases collective cell migration.Citation137 LOXL2 interacts with and stabilizes SNAI1 at the invasion front in actively migrating cells. In addition, LOXL2 catalyzes the cross-linking of collagen fibrils and contributes to the matrix stiffening. Proteins such as LOXL2, which participate in mechanotransduction-mediated invasion are likely the regulators of temporal and spatial assembly and disassembly of cell junctions to facilitate single vs. collective migration.
Concluding Remarks
The regulation of cell adhesion is under control of multiple signaling pathways and mechanisms that allow fine-tuning of the process. Continuous formation and disassembly is necessary during embryogenesis and development. Some of the processes involved at this stage, such as EMT, are hijacked during carcinogenesis. While the structural importance of adherens junctions has been known for a long time, the impact of the complex components on cell signaling has been identified rather recently. The central role of E-cadherin is apparent by the many layers of regulation: promoter methylation, histone methylation, transcriptional repression and post-translational modifications leading to endocytosis. As our understanding of these machineries increases, we will discover novel players and, hopefully, therapeutics that could restore cell adhesion in the clinical setting.
| Abbreviations: | ||
| AJ | = | adherens junctions |
| EMT | = | epithelial-mesenchymal-transition |
| HDAC | = | histone deacetylase |
| DNMT | = | DNA methyl transferase |
Acknowledgments
C.D.A. is the recipient of a Research Scholar Award from the AGA/FDHN and supported by the National Institute of Health (DK075379). Due to the limitations in space the references cannot be all-inclusive, but we recognize other achievements to advance the ongoing research that are not cited.
References
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 2005; 123:903 - 15; http://dx.doi.org/10.1016/j.cell.2005.09.021; PMID: 16325583
- Pokutta S, Drees F, Yamada S, Nelson WJ, Weis WI. Biochemical and structural analysis of alpha-catenin in cell-cell contacts. Biochem Soc Trans 2008; 36:141 - 7; http://dx.doi.org/10.1042/BST0360141; PMID: 18363554
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell 2005; 123:889 - 901; http://dx.doi.org/10.1016/j.cell.2005.09.020; PMID: 16325582
- Abe K, Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U S A 2008; 105:13 - 9; http://dx.doi.org/10.1073/pnas.0710504105; PMID: 18093941
- Chervin-Pétinot A, Courçon M, Almagro S, Nicolas A, Grichine A, Grunwald D, et al. Epithelial protein lost in neoplasm (EPLIN) interacts with α-catenin and actin filaments in endothelial cells and stabilizes vascular capillary network in vitro. J Biol Chem 2012; 287:7556 - 72; http://dx.doi.org/10.1074/jbc.M111.328682; PMID: 22194609
- Taguchi K, Ishiuchi T, Takeichi M. Mechanosensitive EPLIN-dependent remodeling of adherens junctions regulates epithelial reshaping. J Cell Biol 2011; 194:643 - 56; http://dx.doi.org/10.1083/jcb.201104124; PMID: 21844208
- Sakisaka T, Ikeda W, Ogita H, Fujita N, Takai Y. The roles of nectins in cell adhesions: cooperation with other cell adhesion molecules and growth factor receptors. Curr Opin Cell Biol 2007; 19:593 - 602; http://dx.doi.org/10.1016/j.ceb.2007.09.007; PMID: 17942295
- Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol 2008; 9:603 - 15; http://dx.doi.org/10.1038/nrm2457; PMID: 18648374
- Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci 2008; 121:727 - 35; http://dx.doi.org/10.1242/jcs.000455; PMID: 18322269
- Feltes CM, Kudo A, Blaschuk O, Byers SW. An alternatively spliced cadherin-11 enhances human breast cancer cell invasion. Cancer Res 2002; 62:6688 - 97; PMID: 12438268
- Capaldo CT, Macara IG. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell 2007; 18:189 - 200; http://dx.doi.org/10.1091/mbc.E06-05-0471; PMID: 17093058
- Martinez-Rico C, Pincet F, Perez E, Thiery JP, Shimizu K, Takai Y, et al. Separation force measurements reveal different types of modulation of E-cadherin-based adhesion by nectin-1 and -3. J Biol Chem 2005; 280:4753 - 60; http://dx.doi.org/10.1074/jbc.M412544200; PMID: 15550395
- Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol 1997; 137:1403 - 19; http://dx.doi.org/10.1083/jcb.137.6.1403; PMID: 9182671
- Vandewalle C, Comijn J, De Craene B, Vermassen P, Bruyneel E, Andersen H, et al. SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. Nucleic Acids Res 2005; 33:6566 - 78; http://dx.doi.org/10.1093/nar/gki965; PMID: 16314317
- Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci 2003; 116:1959 - 67; http://dx.doi.org/10.1242/jcs.00389; PMID: 12668723
- Ohkubo T, Ozawa M. The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. J Cell Sci 2004; 117:1675 - 85; http://dx.doi.org/10.1242/jcs.01004; PMID: 15075229
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype?. Nat Rev Cancer 2007; 7:415 - 28; http://dx.doi.org/10.1038/nrc2131; PMID: 17508028
- Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139:871 - 90; http://dx.doi.org/10.1016/j.cell.2009.11.007; PMID: 19945376
- Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol 2011; 27:347 - 76; http://dx.doi.org/10.1146/annurev-cellbio-092910-154036; PMID: 21740232
- Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol 2004; 24:306 - 19; http://dx.doi.org/10.1128/MCB.24.1.306-319.2004; PMID: 14673164
- Fu J, Qin L, He T, Qin J, Hong J, Wong J, et al. The TWIST/Mi2/NuRD protein complex and its essential role in cancer metastasis. Cell Res 2011; 21:275 - 89; http://dx.doi.org/10.1038/cr.2010.118; PMID: 20714342
- Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang L-H. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res 2007; 67:1979 - 87; http://dx.doi.org/10.1158/0008-5472.CAN-06-1479; PMID: 17332325
- Villagrasa P, Díaz VM, Viñas-Castells R, Peiró S, Del Valle-Pérez B, Dave N, et al. Akt2 interacts with Snail1 in the E-cadherin promoter. Oncogene 2011; In press http://dx.doi.org/10.1038/onc.2011.562; PMID: 22158034
- Vincent A, Van Seuningen I. On the epigenetic origin of cancer stem cells. Biochim Biophys Acta 2012; 1826:83 - 8; PMID: 22495062
- Mani SA, Guo W, Liao M-J, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133:704 - 15; http://dx.doi.org/10.1016/j.cell.2008.03.027; PMID: 18485877
- Wu K-J, Yang M-H. Epithelial-mesenchymal transition and cancer stemness: the Twist1-Bmi1 connection. Biosci Rep 2011; 31:449 - 55; PMID: 21919891
- Strathdee G. Epigenetic versus genetic alterations in the inactivation of E-cadherin. Semin Cancer Biol 2002; 12:373 - 9; http://dx.doi.org/10.1016/S1044-579X(02)00057-3; PMID: 12191636
- van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci 2008; 65:3756 - 88; http://dx.doi.org/10.1007/s00018-008-8281-1; PMID: 18726070
- Rahnama F, Shafiei F, Gluckman PD, Mitchell MD, Lobie PE. Epigenetic regulation of human trophoblastic cell migration and invasion. Endocrinology 2006; 147:5275 - 83; http://dx.doi.org/10.1210/en.2006-0288; PMID: 16887905
- Rahnama F, Thompson B, Steiner M, Shafiei F, Lobie PE, Mitchell MD. Epigenetic regulation of E-cadherin controls endometrial receptivity. Endocrinology 2009; 150:1466 - 72; http://dx.doi.org/10.1210/en.2008-1142; PMID: 18974268
- Rountree MR, Bachman KE, Baylin SB. DNMT1 binds HDAC2 and a new co-repressor, DMAP1, to form a complex at replication foci. Nat Genet 2000; 25:269 - 77; http://dx.doi.org/10.1038/77023; PMID: 10888872
- Fuks F, Burgers WA, Brehm A, Hughes-Davies L, Kouzarides T. DNA methyltransferase Dnmt1 associates with histone deacetylase activity. Nat Genet 2000; 24:88 - 91; http://dx.doi.org/10.1038/71750; PMID: 10615135
- Suzuki M, Sunaga N, Shames DS, Toyooka S, Gazdar AF, Minna JD. RNA interference-mediated knockdown of DNA methyltransferase 1 leads to promoter demethylation and gene re-expression in human lung and breast cancer cells. Cancer Res 2004; 64:3137 - 43; http://dx.doi.org/10.1158/0008-5472.CAN-03-3046; PMID: 15126351
- Shieh Y-S, Shiah S-G, Jeng H-H, Lee H-S, Wu C-W, Chang L-C. DNA methyltransferase 1 expression and promoter methylation of E-cadherin in mucoepidermoid carcinoma. Cancer 2005; 104:1013 - 21; http://dx.doi.org/10.1002/cncr.21278; PMID: 15999364
- Ehrlich M, Woods CB, Yu MC, Dubeau L, Yang F, Campan M, et al. Quantitative analysis of associations between DNA hypermethylation, hypomethylation, and DNMT RNA levels in ovarian tumors. Oncogene 2006; 25:2636 - 45; http://dx.doi.org/10.1038/sj.onc.1209145; PMID: 16532039
- Arora P, Kim E-O, Jung JK, Jang KL. Hepatitis C virus core protein downregulates E-cadherin expression via activation of DNA methyltransferase 1 and 3b. Cancer Lett 2008; 261:244 - 52; http://dx.doi.org/10.1016/j.canlet.2007.11.033; PMID: 18164808
- Kautiainen TL, Jones PA. DNA methyltransferase levels in tumorigenic and nontumorigenic cells in culture. J Biol Chem 1986; 261:1594 - 8; PMID: 2418016
- De Marzo AM, Marchi VL, Yang ES, Veeraswamy R, Lin X, Nelson WG. Abnormal regulation of DNA methyltransferase expression during colorectal carcinogenesis. Cancer Res 1999; 59:3855 - 60; PMID: 10463569
- Eads CA, Danenberg KD, Kawakami K, Saltz LB, Danenberg PV, Laird PW. CpG island hypermethylation in human colorectal tumors is not associated with DNA methyltransferase overexpression. Cancer Res 1999; 59:2302 - 6; PMID: 10344733
- Robertson KD, Uzvolgyi E, Liang G, Talmadge C, Sumegi J, Gonzales FA, et al. The human DNA methyltransferases (DNMTs) 1, 3a and 3b: coordinate mRNA expression in normal tissues and overexpression in tumors. Nucleic Acids Res 1999; 27:2291 - 8; http://dx.doi.org/10.1093/nar/27.11.2291; PMID: 10325416
- Etoh T, Kanai Y, Ushijima S, Nakagawa T, Nakanishi Y, Sasako M, et al. Increased DNA methyltransferase 1 (DNMT1) protein expression correlates significantly with poorer tumor differentiation and frequent DNA hypermethylation of multiple CpG islands in gastric cancers. Am J Pathol 2004; 164:689 - 99; http://dx.doi.org/10.1016/S0002-9440(10)63156-2; PMID: 14742272
- Oh B-K, Kim H, Park H-J, Shim Y-H, Choi J, Park C, et al. DNA methyltransferase expression and DNA methylation in human hepatocellular carcinoma and their clinicopathological correlation. Int J Mol Med 2007; 20:65 - 73; PMID: 17549390
- Kwon O, Jeong SJ, Kim SO, He L, Lee HG, Jang KL, et al. Modulation of E-cadherin expression by K-Ras; involvement of DNA methyltransferase-3b. Carcinogenesis 2010; 31:1194 - 201; http://dx.doi.org/10.1093/carcin/bgq071; PMID: 20375073
- Papageorgis P, Lambert AW, Ozturk S, Gao F, Pan H, Manne U, et al. Smad signaling is required to maintain epigenetic silencing during breast cancer progression. Cancer Res 2010; 70:968 - 78; http://dx.doi.org/10.1158/0008-5472.CAN-09-1872; PMID: 20086175
- Zhang Q, Chen L, Helfand BT, Jang TL, Sharma V, Kozlowski J, et al. TGF-β regulates DNA methyltransferase expression in prostate cancer, correlates with aggressive capabilities, and predicts disease recurrence. PLoS One 2011; 6:e25168; http://dx.doi.org/10.1371/journal.pone.0025168; PMID: 21980391
- Kokura K, Sun L, Bedford MT, Fang J. Methyl-H3K9-binding protein MPP8 mediates E-cadherin gene silencing and promotes tumour cell motility and invasion. EMBO J 2010; 29:3673 - 87; http://dx.doi.org/10.1038/emboj.2010.239; PMID: 20871592
- Peng L, Yuan Z, Ling H, Fukasawa K, Robertson K, Olashaw N, et al. SIRT1 deacetylates the DNA methyltransferase 1 (DNMT1) protein and alters its activities. Mol Cell Biol 2011; 31:4720 - 34; http://dx.doi.org/10.1128/MCB.06147-11; PMID: 21947282
- Tachibana M, Matsumura Y, Fukuda M, Kimura H, Shinkai Y. G9a/GLP complexes independently mediate H3K9 and DNA methylation to silence transcription. EMBO J 2008; 27:2681 - 90; http://dx.doi.org/10.1038/emboj.2008.192; PMID: 18818694
- Espada J, Peinado H, Lopez-Serra L, Setién F, Lopez-Serra P, Portela A, et al. Regulation of SNAIL1 and E-cadherin function by DNMT1 in a DNA methylation-independent context. Nucleic Acids Res 2011; 39:9194 - 205; http://dx.doi.org/10.1093/nar/gkr658; PMID: 21846773
- Dong C, Wu Y, Yao J, Wang Y, Yu Y, Rychahou PG, et al. G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. J Clin Invest 2012; 122:1469 - 86; http://dx.doi.org/10.1172/JCI57349; PMID: 22406531
- Dong C, Wu Y, Wang Y, Wang C, Kang T, Rychahou PG, et al. Interaction with Suv39H1 is critical for Snail-mediated E-cadherin repression in breast cancer. Oncogene 2012; In press http://dx.doi.org/10.1038/onc.2012.169; PMID: 22562246
- Viré E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature 2006; 439:871 - 4; http://dx.doi.org/10.1038/nature04431; PMID: 16357870
- Hock H. A complex Polycomb issue: the two faces of EZH2 in cancer. Genes Dev 2012; 26:751 - 5; http://dx.doi.org/10.1101/gad.191163.112; PMID: 22508723
- Richly H, Aloia L, Di Croce L. Roles of the Polycomb group proteins in stem cells and cancer. Cell Death Dis 2011; 2:e204; http://dx.doi.org/10.1038/cddis.2011.84; PMID: 21881606
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 2002; 298:1039 - 43; http://dx.doi.org/10.1126/science.1076997; PMID: 12351676
- Song L-B, Li J, Liao W-T, Feng Y, Yu C-P, Hu L-J, et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J Clin Invest 2009; 119:3626 - 36; http://dx.doi.org/10.1172/JCI39374; PMID: 19884659
- Guo B-H, Feng Y, Zhang R, Xu L-H, Li M-Z, Kung H-F, et al. Bmi-1 promotes invasion and metastasis, and its elevated expression is correlated with an advanced stage of breast cancer. Mol Cancer 2011; 10:10; http://dx.doi.org/10.1186/1476-4598-10-10; PMID: 21276221
- Herranz N, Pasini D, Díaz VM, Francí C, Gutierrez A, Dave N, et al. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol Cell Biol 2008; 28:4772 - 81; http://dx.doi.org/10.1128/MCB.00323-08; PMID: 18519590
- Tong Z-T, Cai M-Y, Wang X-G, Kong L-L, Mai S-J, Liu Y-H, et al. EZH2 supports nasopharyngeal carcinoma cell aggressiveness by forming a co-repressor complex with HDAC1/HDAC2 and Snail to inhibit E-cadherin. Oncogene 2012; 31:583 - 94; PMID: 21685935
- Rao Z-Y, Cai M-Y, Yang G-F, He L-R, Mai S-J, Hua W-F, et al. EZH2 supports ovarian carcinoma cell invasion and/or metastasis via regulation of TGF-beta1 and is a predictor of outcome in ovarian carcinoma patients. Carcinogenesis 2010; 31:1576 - 83; http://dx.doi.org/10.1093/carcin/bgq150; PMID: 20668008
- Yang M-H, Hsu DS-S, Wang H-W, Wang H-J, Lan H-Y, Yang W-H, et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol 2010; 12:982 - 92; http://dx.doi.org/10.1038/ncb2099; PMID: 20818389
- Hong S, Troyanovsky RB, Troyanovsky SM. Spontaneous assembly and active disassembly balance adherens junction homeostasis. Proc Natl Acad Sci U S A 2010; 107:3528 - 33; http://dx.doi.org/10.1073/pnas.0911027107; PMID: 20133579
- Nishimura T, Takeichi M. Remodeling of the adherens junctions during morphogenesis. Curr Top Dev Biol 2009; 89:33 - 54; http://dx.doi.org/10.1016/S0070-2153(09)89002-9; PMID: 19737641
- Sigismund S, Confalonieri S, Ciliberto A, Polo S, Scita G, Di Fiore PP. Endocytosis and signaling: cell logistics shape the eukaryotic cell plan. Physiol Rev 2012; 92:273 - 366; http://dx.doi.org/10.1152/physrev.00005.2011; PMID: 22298658
- Mustonen H, Lepistö A, Lehtonen S, Lehtonen E, Puolakkainen P, Kivilaakso E. CD2AP contributes to cell migration and adhesion in cultured gastric epithelium. Biochem Biophys Res Commun 2005; 332:426 - 32; http://dx.doi.org/10.1016/j.bbrc.2005.04.140; PMID: 15910750
- van Duijn TJ, Anthony EC, Hensbergen PJ, Deelder AM, Hordijk PL. Rac1 recruits the adapter protein CMS/CD2AP to cell-cell contacts. J Biol Chem 2010; 285:20137 - 46; http://dx.doi.org/10.1074/jbc.M109.099481; PMID: 20404345
- de Beco S, Amblard F, Coscoy S. New insights into the regulation of E-cadherin distribution by endocytosis. Int Rev Cell Mol Biol 2012; 295:63 - 108; http://dx.doi.org/10.1016/B978-0-12-394306-4.00008-3; PMID: 22449487
- Insall RH, Machesky LM. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell 2009; 17:310 - 22; http://dx.doi.org/10.1016/j.devcel.2009.08.012; PMID: 19758556
- Platta HW, Stenmark H. Endocytosis and signaling. Curr Opin Cell Biol 2011; 23:393 - 403; http://dx.doi.org/10.1016/j.ceb.2011.03.008; PMID: 21474295
- Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, et al. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol 1993; 120:757 - 66; http://dx.doi.org/10.1083/jcb.120.3.757; PMID: 8425900
- Ishiyama N, Lee S-H, Liu S, Li G-Y, Smith MJ, Reichardt LF, et al. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion. Cell 2010; 141:117 - 28; http://dx.doi.org/10.1016/j.cell.2010.01.017; PMID: 20371349
- Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HEM, Behrens J, et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol 2002; 4:222 - 31; http://dx.doi.org/10.1038/ncb758; PMID: 11836526
- Yang J-Y, Zong CS, Xia W, Wei Y, Ali-Seyed M, Li Z, et al. MDM2 promotes cell motility and invasiveness by regulating E-cadherin degradation. Mol Cell Biol 2006; 26:7269 - 82; http://dx.doi.org/10.1128/MCB.00172-06; PMID: 16980628
- Mukherjee M, Chow SY, Yusoff P, Seetharaman J, Ng C, Sinniah S, et al. Structure of a novel phosphotyrosine-binding domain in Hakai that targets E-cadherin. EMBO J 2012; 31:1308 - 19; http://dx.doi.org/10.1038/emboj.2011.496; PMID: 22252131
- Wadhawan A, Smith C, Nicholson RI, Barrett-Lee P, Hiscox S. Src-mediated regulation of homotypic cell adhesion: implications for cancer progression and opportunities for therapeutic intervention. Cancer Treat Rev 2011; 37:234 - 41; http://dx.doi.org/10.1016/j.ctrv.2010.08.003; PMID: 20888696
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001; 2:127 - 37; http://dx.doi.org/10.1038/35052073; PMID: 11252954
- Hiscox S, Jiang WG. Association of the HGF/SF receptor, c-met, with the cell-surface adhesion molecule, E-cadherin, and catenins in human tumor cells. Biochem Biophys Res Commun 1999; 261:406 - 11; http://dx.doi.org/10.1006/bbrc.1999.1002; PMID: 10425198
- Hoschuetzky H, Aberle H, Kemler R. Beta-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol 1994; 127:1375 - 80; http://dx.doi.org/10.1083/jcb.127.5.1375; PMID: 7962096
- Pece S, Gutkind JS. Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J Biol Chem 2000; 275:41227 - 33; http://dx.doi.org/10.1074/jbc.M006578200; PMID: 10969083
- Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J 2004; 23:1739 - 48; http://dx.doi.org/10.1038/sj.emboj.7600136; PMID: 15057284
- Sallee JL, Wittchen ES, Burridge K. Regulation of cell adhesion by protein-tyrosine phosphatases: II. Cell-cell adhesion. J Biol Chem 2006; 281:16189 - 92; http://dx.doi.org/10.1074/jbc.R600003200; PMID: 16497667
- Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, et al. Protein tyrosine phosphatases in the human genome. Cell 2004; 117:699 - 711; http://dx.doi.org/10.1016/j.cell.2004.05.018; PMID: 15186772
- Lin G, Aranda V, Muthuswamy SK, Tonks NK. Identification of PTPN23 as a novel regulator of cell invasion in mammary epithelial cells from a loss-of-function screen of the ‘PTP-ome’. Genes Dev 2011; 25:1412 - 25; http://dx.doi.org/10.1101/gad.2018911; PMID: 21724833
- Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell 2008; 15:209 - 19; http://dx.doi.org/10.1016/j.devcel.2008.06.012; PMID: 18694561
- Kazazic M, Roepstorff K, Johannessen LE, Pedersen NM, van Deurs B, Stang E, et al. EGF-induced activation of the EGF receptor does not trigger mobilization of caveolae. Traffic 2006; 7:1518 - 27; http://dx.doi.org/10.1111/j.1600-0854.2006.00487.x; PMID: 16984407
- Lu Z, Ghosh S, Wang Z, Hunter T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell 2003; 4:499 - 515; http://dx.doi.org/10.1016/S1535-6108(03)00304-0; PMID: 14706341
- Orlichenko L, Weller SG, Cao H, Krueger EW, Awoniyi M, Beznoussenko G, et al. Caveolae mediate growth factor-induced disassembly of adherens junctions to support tumor cell dissociation. Mol Biol Cell 2009; 20:4140 - 52; http://dx.doi.org/10.1091/mbc.E08-10-1043; PMID: 19641024
- Masuelli L, Budillon A, Marzocchella L, Mrozek M-A, Vitolo D, Di Gennaro E, et al. Caveolin-1 overexpression is associated with simultaneous abnormal expression of the E-cadherin/α-β catenins complex and multiple ErbB receptors and with lymph nodes metastasis in head and neck squamous cell carcinomas. J Cell Physiol 2012; 227:3344 - 53; http://dx.doi.org/10.1002/jcp.24034; PMID: 22213373
- Katanaev VL, Solis GP, Hausmann G, Buestorf S, Katanayeva N, Schrock Y, et al. Reggie-1/flotillin-2 promotes secretion of the long-range signalling forms of Wingless and Hedgehog in Drosophila. EMBO J 2008; 27:509 - 21; http://dx.doi.org/10.1038/sj.emboj.7601981; PMID: 18219274
- Otto GP, Nichols BJ. The roles of flotillin microdomains--endocytosis and beyond. J Cell Sci 2011; 124:3933 - 40; http://dx.doi.org/10.1242/jcs.092015; PMID: 22194304
- Stuermer CAO. How reggies regulate regeneration and axon growth. Cell Tissue Res 2012; 349:71 - 7; PMID: 22350847
- Málaga-Trillo E, Solis GP, Schrock Y, Geiss C, Luncz L, Thomanetz V, et al. Regulation of embryonic cell adhesion by the prion protein. PLoS Biol 2009; 7:e55; http://dx.doi.org/10.1371/journal.pbio.1000055; PMID: 19278297
- Solis GP, Schrock Y, Hülsbusch N, Wiechers M, Plattner H, Stuermer CAO. Reggies/flotillins regulate E-cadherin-mediated cell contact formation by affecting EGFR trafficking. Mol Biol Cell 2012; 23:1812 - 25; http://dx.doi.org/10.1091/mbc.E11-12-1006; PMID: 22438585
- Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell 2004; 15:176 - 88; http://dx.doi.org/10.1091/mbc.E03-05-0319; PMID: 14528017
- Gulino A, Di Marcotullio L, Screpanti I. The multiple functions of Numb. Exp Cell Res 2010; 316:900 - 6; http://dx.doi.org/10.1016/j.yexcr.2009.11.017; PMID: 19944684
- Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol 1997; 137:1421 - 31; http://dx.doi.org/10.1083/jcb.137.6.1421; PMID: 9182672
- Kitt KN, Nelson WJ. Rapid suppression of activated Rac1 by cadherins and nectins during de novo cell-cell adhesion. PLoS One 2011; 6:e17841; http://dx.doi.org/10.1371/journal.pone.0017841; PMID: 21412440
- Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol 2007; 178:517 - 27; http://dx.doi.org/10.1083/jcb.200701058; PMID: 17646397
- Lau KM, McGlade CJ. Numb is a negative regulator of HGF dependent cell scattering and Rac1 activation. Exp Cell Res 2011; 317:539 - 51; http://dx.doi.org/10.1016/j.yexcr.2010.12.005; PMID: 21147098
- Wang Z, Sandiford S, Wu C, Li SS-C. Numb regulates cell-cell adhesion and polarity in response to tyrosine kinase signalling. EMBO J 2009; 28:2360 - 73; http://dx.doi.org/10.1038/emboj.2009.190; PMID: 19609305
- Pece S, Confalonieri S, R Romano P, Di Fiore PP. NUMB-ing down cancer by more than just a NOTCH. Biochim Biophys Acta 2011; 1815:26 - 43; PMID: 20940030
- Ozdamar B, Bose R, Barrios-Rodiles M, Wang H-R, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science 2005; 307:1603 - 9; http://dx.doi.org/10.1126/science.1105718; PMID: 15761148
- Viloria-Petit AM, David L, Jia JY, Erdemir T, Bane AL, Pinnaduwage D, et al. A role for the TGFbeta-Par6 polarity pathway in breast cancer progression. Proc Natl Acad Sci U S A 2009; 106:14028 - 33; http://dx.doi.org/10.1073/pnas.0906796106; PMID: 19667198
- Sato K, Watanabe T, Wang S, Kakeno M, Matsuzawa K, Matsui T, et al. Numb controls E-cadherin endocytosis through p120 catenin with aPKC. Mol Biol Cell 2011; 22:3103 - 19; http://dx.doi.org/10.1091/mbc.E11-03-0274; PMID: 21775625
- Xu XX, Yang W, Jackowski S, Rock CO. Cloning of a novel phosphoprotein regulated by colony-stimulating factor 1 shares a domain with the Drosophila disabled gene product. J Biol Chem 1995; 270:14184 - 91; http://dx.doi.org/10.1074/jbc.270.23.14184; PMID: 7775479
- Mok SC, Chan WY, Wong KK, Cheung KK, Lau CC, Ng SW, et al. DOC-2, a candidate tumor suppressor gene in human epithelial ovarian cancer. Oncogene 1998; 16:2381 - 7; http://dx.doi.org/10.1038/sj.onc.1201769; PMID: 9620555
- Kleeff J, Huang Y, Mok SC, Zimmermann A, Friess H, Büchler MW. Down-regulation of DOC-2 in colorectal cancer points to its role as a tumor suppressor in this malignancy. Dis Colon Rectum 2002; 45:1242 - 8; http://dx.doi.org/10.1007/s10350-004-6399-2; PMID: 12352243
- Karam JA, Shariat SF, Huang H-Y, Pong R-C, Ashfaq R, Shapiro E, et al. Decreased DOC-2/DAB2 expression in urothelial carcinoma of the bladder. Clin Cancer Res 2007; 13:4400 - 6; http://dx.doi.org/10.1158/1078-0432.CCR-07-0287; PMID: 17671122
- Fazili Z, Sun W, Mittelstaedt S, Cohen C, Xu XX. Disabled-2 inactivation is an early step in ovarian tumorigenicity. Oncogene 1999; 18:3104 - 13; http://dx.doi.org/10.1038/sj.onc.1202649; PMID: 10340382
- Anupam K, Tusharkant C, Gupta SD, Ranju R. Loss of disabled-2 expression is an early event in esophageal squamous tumorigenesis. World J Gastroenterol 2006; 12:6041 - 5; PMID: 17009406
- Hocevar BA, Prunier C, Howe PH. Disabled-2 (Dab2) mediates transforming growth factor beta (TGFbeta)-stimulated fibronectin synthesis through TGFbeta-activated kinase 1 and activation of the JNK pathway. J Biol Chem 2005; 280:25920 - 7; http://dx.doi.org/10.1074/jbc.M501150200; PMID: 15894542
- Prunier C, Howe PH. Disabled-2 (Dab2) is required for transforming growth factor beta-induced epithelial to mesenchymal transition (EMT). J Biol Chem 2005; 280:17540 - 8; http://dx.doi.org/10.1074/jbc.M500974200; PMID: 15734730
- Chaudhury A, Hussey GS, Ray PS, Jin G, Fox PL, Howe PH. TGF-beta-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nat Cell Biol 2010; 12:286 - 93; PMID: 20154680
- Hussey GS, Chaudhury A, Dawson AE, Lindner DJ, Knudsen CR, Wilce MCJ, et al. Identification of an mRNP complex regulating tumorigenesis at the translational elongation step. Mol Cell 2011; 41:419 - 31; http://dx.doi.org/10.1016/j.molcel.2011.02.003; PMID: 21329880
- Hannigan A, Smith P, Kalna G, Lo Nigro C, Orange C, O’Brien DI, et al. Epigenetic downregulation of human disabled homolog 2 switches TGF-beta from a tumor suppressor to a tumor promoter. J Clin Invest 2010; 120:2842 - 57; http://dx.doi.org/10.1172/JCI36125; PMID: 20592473
- Martin JC, Herbert B-S, Hocevar BA. Disabled-2 downregulation promotes epithelial-to-mesenchymal transition. Br J Cancer 2010; 103:1716 - 23; http://dx.doi.org/10.1038/sj.bjc.6605975; PMID: 21063401
- Chao A, Lin C-Y, Lee Y-S, Tsai C-L, Wei P-C, Hsueh S, et al. Regulation of ovarian cancer progression by microRNA-187 through targeting Disabled homolog-2. Oncogene 2012; 31:764 - 75; http://dx.doi.org/10.1038/onc.2011.269; PMID: 21725366
- Yang D-H, Cai KQ, Roland IH, Smith ER, Xu X-X. Disabled-2 is an epithelial surface positioning gene. J Biol Chem 2007; 282:13114 - 22; http://dx.doi.org/10.1074/jbc.M611356200; PMID: 17339320
- Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol 2010; 188:11 - 9; http://dx.doi.org/10.1083/jcb.200909003; PMID: 19951899
- Rodriguez FJ, Lewis-Tuffin LJ, Anastasiadis PZ. E-cadherin’s dark side: Possible role in tumor progression. Biochim Biophys Acta 2012; 1826:23 - 31; PMID: 22440943
- Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol 2009; 11:1287 - 96; http://dx.doi.org/10.1038/ncb1973; PMID: 19838175
- Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol 2000; 148:779 - 90; http://dx.doi.org/10.1083/jcb.148.4.779; PMID: 10684258
- Maeda M, Johnson KR, Wheelock MJ. Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci 2005; 118:873 - 87; http://dx.doi.org/10.1242/jcs.01634; PMID: 15713751
- Alexander NR, Tran NL, Rekapally H, Summers CE, Glackin C, Heimark RL. N-cadherin gene expression in prostate carcinoma is modulated by integrin-dependent nuclear translocation of Twist1. Cancer Res 2006; 66:3365 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-05-3401; PMID: 16585154
- Hao L, Ha JR, Kuzel P, Garcia E, Persad S. Cadherin switch from E- to N-cadherin in melanoma progression is regulated by the PI3K/PTEN pathway through Twist and Snail. Br J Dermatol 2012; 166:1184 - 97; http://dx.doi.org/10.1111/j.1365-2133.2012.10824.x; PMID: 22332917
- Ganz A, Lambert M, Saez A, Silberzan P, Buguin A, Mège RM, et al. Traction forces exerted through N-cadherin contacts. Biol Cell 2006; 98:721 - 30; http://dx.doi.org/10.1042/BC20060039; PMID: 16895521
- Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, et al. Collective chemotaxis requires contact-dependent cell polarity. Dev Cell 2010; 19:39 - 53; http://dx.doi.org/10.1016/j.devcel.2010.06.012; PMID: 20643349
- Yano H, Mazaki Y, Kurokawa K, Hanks SK, Matsuda M, Sabe H. Roles played by a subset of integrin signaling molecules in cadherin-based cell-cell adhesion. J Cell Biol 2004; 166:283 - 95; http://dx.doi.org/10.1083/jcb.200312013; PMID: 15263022
- Shih W, Yamada S. N-cadherin-mediated cell-cell adhesion promotes cell migration in a three-dimensional matrix. J Cell Sci 2012; In press http://dx.doi.org/10.1242/jcs.103861; PMID: 22467866
- Kubow KE, Horwitz AR. Reducing background fluorescence reveals adhesions in 3D matrices. Nat Cell Biol 2011; 13:3 - 5, author reply 5-7; http://dx.doi.org/10.1038/ncb0111-3; PMID: 21173800
- Fraley SI, Feng Y, Krishnamurthy R, Kim D-H, Celedon A, Longmore GD, et al. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat Cell Biol 2010; 12:598 - 604; http://dx.doi.org/10.1038/ncb2062; PMID: 20473295
- Kim J-H, Asthagiri AR. Matrix stiffening sensitizes epithelial cells to EGF and enables the loss of contact inhibition of proliferation. J Cell Sci 2011; 124:1280 - 7; http://dx.doi.org/10.1242/jcs.078394; PMID: 21429934
- Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS. Matrix rigidity regulates a switch between TGF-β1-induced apoptosis and epithelial-mesenchymal transition. Mol Biol Cell 2012; 23:781 - 91; http://dx.doi.org/10.1091/mbc.E11-06-0537; PMID: 22238361
- Ladoux B, Anon E, Lambert M, Rabodzey A, Hersen P, Buguin A, et al. Strength dependence of cadherin-mediated adhesions. Biophys J 2010; 98:534 - 42; http://dx.doi.org/10.1016/j.bpj.2009.10.044; PMID: 20159149
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, et al. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 2004; 303:848 - 51; http://dx.doi.org/10.1126/science.1090922; PMID: 14764882
- Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol 2007; 9:1392 - 400; http://dx.doi.org/10.1038/ncb1658; PMID: 18037882
- Peinado H, Moreno-Bueno G, Hardisson D, Pérez-Gómez E, Santos V, Mendiola M, et al. Lysyl oxidase-like 2 as a new poor prognosis marker of squamous cell carcinomas. Cancer Res 2008; 68:4541 - 50; http://dx.doi.org/10.1158/0008-5472.CAN-07-6345; PMID: 18559498