Abstract
After central nervous system (CNS) insults, such as spinal cord injury or traumatic brain injury, neurons encounter a complex microenvironment where mechanisms that promote regeneration compete with inhibitory processes. Sprouting and axonal re-growth are key components of functional recovery, but are often counteracted by inhibitory molecules. Several strategies are being pursued whereby these inhibitory molecules are either being neutralized with blocking antibodies, with enzymatic degradation or downstream signaling events are being interfered with. Two recent studiesCitation1,Citation2 show that activating integrin signaling in dorsal root ganglion (DRG) neurons renders them able to overcome inhibitory signals, and could possibly lead to new strategies to improve neuronal regeneration.
Keywords: :
Background
The successful outcome of peripheral nerve regeneration is attributed both to the growth permissive milieu through which the neuronal growth cone advances toward its target and to the intrinsic ability of the neuron to initiate appropriate cellular responses such as changes in gene expression and cytoskeletal rearrangements. While injuries in the peripheral nervous system heal relatively well, after a CNS injury, various supporting cells start to produce growth-inhibiting molecules, including myelin-associated glycoprotein (MAG) oligodendrocyte-myelin glycoprotein (OMgp), Nogo and chondroitin sulfate proteoglycans (CSPGs), thus limiting regeneration.
Manipulating Integrin Function to Improve Neuronal Regeneration
Axonal growth cones interact with the extracellular matrix (ECM), by cell-surface receptors such as members of the integrin family. These molecules are essential to neuronal regeneration in the peripheral nervous system, reviewed in reference Citation3. Integrins are cell-surface receptors consisting of one α and one β chain and elicit various intracellular signaling cascades upon activation that follows ligand binding (“outside-in” signaling). In addition, integrins have binding sites for divalent cations and the addition of manganese ions can activate integrins and has been shown to increase growth from retinal ganglions cells (RGC) in culture,Citation4 showing that the developmental decrease in neurite growth capacity observed in RGCs could be reversed by integrin activation. This observation and the fact that the neurite growth inhibitor Nogo exerts at least part of its effect via inactivation of integrins,Citation5 lead Tan and coworkers to investigate the role of integrin activation in relation to the growth inhibition effects by CSPGs.Citation2 Addition of CSPGs to dorsal root ganglion (DRG) (the pseudounipolar neurons responsible for conveying sensory information from the periphery to the CNS) cultures resulted in a reduction of activated integrins [as determined by a decrease in phosphorylated focal adhesion kinase (FAK)] and growth inhibition. By adding manganese to the cultures, both FAK phosphorylation and growth response was restored, indicating that integrin activation is sufficient to override the inhibitory effects of CSPGs.
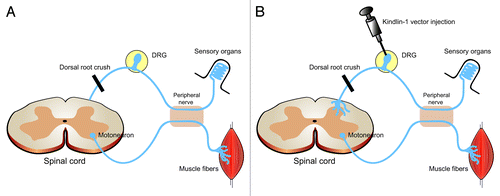
In addition to the above-mentioned “outside-in” signaling, the affinity with which the integrin binds its ligand can be regulated from within the cell (“inside-out” signaling). This process is important to make sure that an appropriate level of adhesiveness is achieved. “Inside-out” signaling is regulated by the activity of proteins that interact with the intracellular domain of the integrin and one such group of proteins is the kindlin family (kindlin-1, -2 and -3). Inspired by the discovery that kindlin-1 can rescue the phenotype of keratinocytes from patients with Kindler syndrome (a hereditary disease caused by mutation in the kindlin-1 gene, leading to skin blistering and photosensitivity), by enhancing integrin activation, Tan and coworkers overexpressed this molecule in DRG neurons.Citation1 The outcome was an increase in integrin activation (as determined by staining with an antibody specific for activated integrins) and an increase in phosphorylation of FAK in axons. Interestingly, knockdown of kindlin-2 (which, in contrast to kindlin-1, is expresses in the nervous system) in DRG neurons did not affect axonal growth and neither did overexpression of this molecule. Finally, to examine if kindlin-1 overexpression also increases regeneration in vivo, the authors utilized the dorsal root crush model. In this injury paradigm, re-growing axons from the DRG neurons are unable to enter the spinal cord because of inhibitory molecules at the dorsal root entry zone (). Injection of virus vectors carrying kindlin-1 constructs into the DRG lead to ingrowth of axons into the dorsal horn and dorsal column of the spinal cord (). Importantly, this treatment also led to functional recovery of thermal but not pressure sensation in the forepaw. The reason for this is difference is currently unclear. Possible explanations could be that the virus vector affects different subpopulations of neurons within the DRG differently, or that pressure-sensing DRGs regenerate at a slower rate or that integrin expression is different in subpopulations of DRG neurons. On a similar note, it should be noted that the number of regenerating nerve fibers was quite low and whether this was due to low efficiency of the viral construct (no quantification of the percentage of successfully transfected neurons was presented) or if kindlin-1 overexpression by itself is not enough to induce more robust regeneration in vivo is currently not known.
Figure 1. Schematic figure of the in vivo experiment by Tan and coworkers. (A) After a dorsal root crush, regrowing axons are halted at the interface between the dorsal root and spinal cord. (B) Injection of kindlin-1 viral vectors into the DRG allows growing axons to enter the spinal cord and sprout into the dorsal horn and -column of the spinal cord and leads to recovery of function.
Too Much of a Good Thing, Are There Causes for Concern?
The authors did not see any signs of pain-related side-effects (a condition that can be caused by aberrant neuronal sprouting and can be ameliorated by integrin antagonistsCitation6) and no other side effects were reported. However, it is currently not known if a more robust growth of axons would induce side effects. Given the relatively low number of regenerating axons and that the authors did not examine if the observed growth of neurites came from any particular subtype (one would probably expect more pain-related side effects if nociceptive DRGs were stimulated to grow vigorously), it seems premature at current to conclude that the strategy presented by Tan and coworkers is free from side-effects.
Moreover, a number of previous studies have shown that integrin activation can have deleterious effects on recovery after CNS injury. Fibrinogen that leaks into the CNS from the bloodstream after trauma binds to neuronal integrin αVβ3, which in turn activates the epidermal growth factor receptor (EGFR) and leads to growth cone collapse.Citation7 Further, integrin β1 can bind the growth-inhibitory molecule MAG,Citation8 and using an in vitro growth-cone turning assay, results from the same study showed that β1 integrin is essential for the repulsive signal from MAG.Citation8 However, if integrin β1 is also responsible for MAG-induced repulsion and/or growth cone collapse in vivo is currently not known. Finally, when neurons are presented with a favorable substrate (laminin), combined with increased integrin activation (manganese administration) and elevated intracellular cAMP (which has been shown to increase the regenerative capacity of several neuronal types), the outcome is not an additive effect on growth, but rather the opposite: the combination of these manipulations instead activates Rho and leads to growth cone collapse.Citation9 This raises concern that “overloading” the neurons with positive enforcement might actually trigger regeneration failure.
Conclusion and Future Directions
Although previous studies have shown that overexpression or activation of integrins can increase neurite growth on permissive substrates, such as laminin,Citation4 fibronectinCitation10,Citation11 and tenascin-C,Citation12 the studies by Tan and coworkersCitation1,Citation2 provide important new proofs-of-concept that enhancing integrin function, both “outside-in” (manganese administration) and “inside-out” (kindlin-1 overexpression) can overcome CSPG-mediated inhibition. It has been argued that growth-inhibition by CSPGs is not the most likely mechanism underlying the regenerative failure after dorsal root crush model used by Tan and coworkers: Di Maio and colleagues claim that axons instead stop growing and form non-functional pre-synaptic terminals in the spinal cordCitation13 and Zhang and coworkers did not detect substantial expression of CSPGs in the DREZ.Citation14 However, other studies have claimed that CSPGs at the DREZ are contributing to the failed regeneration after dorsal root injury.Citation15
Future studies will have to be performed to elucidate if the strategies developed by Tan and coworkers also function in other injury paradigms and on other neuronal types such as corticospinal neurons, responsible for much of our voluntary movement and thus crucial for recovery of locomotion after spinal cord injury. Much work is still ahead, but given the heterogeneous and wide-spread expression of integrin subunits in the CNS,Citation16 the studies by Tan and coworkers has presented us with new promising possibilities for neuronal regeneration.
| Abbreviations: | ||
| CNS | = | central nervous system |
| CSPG | = | chondroitin sulphate proteoglycans |
| DRG | = | dorsal root ganglion |
| ECM | = | extracellular matrix |
| FAK | = | focal adhesion kinase |
| MAG | = | myelin-associated glycoprotein |
| OMgp | = | oligodendrocyte-myelin glycoprotein |
| RGC | = | retinal ganglion cell |
Acknowledgments
The author wishes to thank Fredrik Almgren for help with the illustration. S.P. is supported by a scholarship from the Swedish Brain Foundation (Hjärnfonden).
References
- Tan CL, Andrews MR, Kwok JC, Heintz TG, Gumy LF, Fässler R, et al. Kindlin-1 enhances axon growth on inhibitory chondroitin sulfate proteoglycans and promotes sensory axon regeneration. J Neurosci 2012; 32:7325 - 35; http://dx.doi.org/10.1523/JNEUROSCI.5472-11.2012; PMID: 22623678
- Tan CL, Kwok JC, Patani R, Ffrench-Constant C, Chandran S, Fawcett JW. Integrin activation promotes axon growth on inhibitory chondroitin sulfate proteoglycans by enhancing integrin signaling. J Neurosci 2011; 31:6289 - 95; http://dx.doi.org/10.1523/JNEUROSCI.0008-11.2011; PMID: 21525268
- Lemons ML, Condic ML. Integrin signaling is integral to regeneration. Exp Neurol 2008; 209:343 - 52; http://dx.doi.org/10.1016/j.expneurol.2007.05.027; PMID: 17727844
- Ivins JK, Yurchenco PD, Lander AD. Regulation of neurite outgrowth by integrin activation. J Neurosci 2000; 20:6551 - 60; PMID: 10964960
- Hu F, Strittmatter SM. The N-terminal domain of Nogo-A inhibits cell adhesion and axonal outgrowth by an integrin-specific mechanism. J Neurosci 2008; 28:1262 - 9; http://dx.doi.org/10.1523/JNEUROSCI.1068-07.2008; PMID: 18234903
- Fu WM, Chang TK, Sun WZ, Ling QD, Peng HC, Liou HC, et al. Inhibition of neuropathic pain by a potent disintegrin--triflavin. Neurosci Lett 2004; 368:263 - 8; http://dx.doi.org/10.1016/j.neulet.2004.06.035; PMID: 15364408
- Schachtrup C, Lu P, Jones LL, Lee JK, Lu J, Sachs BD, et al. Fibrinogen inhibits neurite outgrowth via beta 3 integrin-mediated phosphorylation of the EGF receptor. Proc Natl Acad Sci U S A 2007; 104:11814 - 9; http://dx.doi.org/10.1073/pnas.0704045104; PMID: 17606926
- Goh EL, Young JK, Kuwako K, Tessier-Lavigne M, He Z, Griffin JW, et al. beta1-integrin mediates myelin-associated glycoprotein signaling in neuronal growth cones. Mol Brain 2008; 1:10; http://dx.doi.org/10.1186/1756-6606-1-10; PMID: 18922173
- Lemons ML, Condic ML. Combined integrin activation and intracellular cAMP cause Rho GTPase dependent growth cone collapse on laminin-1. Exp Neurol 2006; 202:324 - 35; http://dx.doi.org/10.1016/j.expneurol.2006.06.008; PMID: 16899244
- Condic ML. Adult neuronal regeneration induced by transgenic integrin expression. J Neurosci 2001; 21:4782 - 8; PMID: 11425905
- Vogelezang MG, Liu Z, Relvas JB, Raivich G, Scherer SS, ffrench-Constant C. Alpha4 integrin is expressed during peripheral nerve regeneration and enhances neurite outgrowth. J Neurosci 2001; 21:6732 - 44; PMID: 11517262
- Andrews MR, Czvitkovich S, Dassie E, Vogelaar CF, Faissner A, Blits B, et al. Alpha9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. J Neurosci 2009; 29:5546 - 57; http://dx.doi.org/10.1523/JNEUROSCI.0759-09.2009; PMID: 19403822
- Di Maio A, Skuba A, Himes BT, Bhagat SL, Hyun JK, Tessler A, et al. In vivo imaging of dorsal root regeneration: rapid immobilization and presynaptic differentiation at the CNS/PNS border. J Neurosci 2011; 31:4569 - 82; http://dx.doi.org/10.1523/JNEUROSCI.4638-10.2011; PMID: 21430157
- Zhang Y, Tohyama K, Winterbottom JK, Haque NS, Schachner M, Lieberman AR, et al. Correlation between putative inhibitory molecules at the dorsal root entry zone and failure of dorsal root axonal regeneration. Mol Cell Neurosci 2001; 17:444 - 59; http://dx.doi.org/10.1006/mcne.2000.0952; PMID: 11273641
- Steinmetz MP, Horn KP, Tom VJ, Miller JH, Busch SA, Nair D, et al. Chronic enhancement of the intrinsic growth capacity of sensory neurons combined with the degradation of inhibitory proteoglycans allows functional regeneration of sensory axons through the dorsal root entry zone in the mammalian spinal cord. J Neurosci 2005; 25:8066 - 76; http://dx.doi.org/10.1523/JNEUROSCI.2111-05.2005; PMID: 16135764
- Pinkstaff JK, Detterich J, Lynch G, Gall C. Integrin subunit gene expression is regionally differentiated in adult brain. J Neurosci 1999; 19:1541 - 56; PMID: 10024342