Abstract
This review advances the hypothesis that the ability of integrins to engage their extracellular matrix ligands and signal can be regulated in tumor cells by vascular endothelial growth factor (VEGF), a major angiogenic factor that also has direct effects on the function of tumor cells. More specifically, we will discuss how neuropilins (NRPs), a distinct class of VEGF receptors, enable the function of specific integrins that contribute to tumor initiation and progression.
Keywords: :
Introduction
The ability of integrins to sense the extracellular matrix and alter cell behavior accordingly is a fundamental tenet of cell biology.Citation1 Given the profound effect that integrins and integrin signaling can have on cells, it is not surprising that the ability of integrins to engage the ECM is often tightly regulated by physiological and pathological stimuli. A salient mode of integrin regulation is “inside-out” signaling, a process in which intracellular signals generated by a number of physiological and pathological pathways alter the ability of integrin extracellular domains to bind their ECM ligands. This process frequently involves an increase in the affinity of a specific integrin for its ligand and the mechanisms involved are being elucidated in exquisite detail.Citation2,Citation3 Another mode of regulating integrin function is to increase the avidity of an integrin for its ligand. This mode can be accomplished by increasing the local concentration of integrins within the plasma membrane. Integrin engagement of ECM ligands, which are multi-valent, results in the clustering of integrins and a consequent increase in avidity and signaling, a process termed “outside-in” signaling.Citation4,Citation5
Another facet of integrin regulation is their ability to associate with a variety of cell surface proteins including growth factor receptorsCitation6 and tetraspanins.Citation7 Such interactions regulate integrin affinity, localization and trafficking. One aspect of particular relevance that merits renewed interest is the relationship between integrins and receptors for VEGF. Given the critical role that VEGF plays in angiogenesis and tumor biology and the fact that it is a validated target for cancer therapy,Citation8 the hypothesis that VEGF mediates its effects, in part, by modulating integrin activation and function is significant and relevant. Interestingly, some integrins such as α9β1 can bind VEGF directly,Citation9 but our focus here is on the connection between integrins and VEGF receptors and how this relationship modulates integrin function. More specifically, we will discuss a specific class of VEGF receptors termed the neuropilins (NRPs) that are distinct from the VEGF tyrosine kinase receptors (VEGFRs).Citation10 NRP1 and NRP2 were identified initially as neuronal receptors for semaphorins, which are axon guidance factors that function in the developing nervous system.Citation10 The seminal finding that neuropilins can also function as VEGF receptors and that they are expressed on endothelial and tumor cellsCitation11 launched studies aimed at understanding their contribution to angiogenesis and tumor biology. NRPs associate with plexins to facilitate semaphorin binding and signaling.Citation12-Citation14 They also interact with and modulate the function of VEGFR1 and VEGFR2,Citation15 as well as other receptors including integrins.Citation16-Citation18 The association of NRPs with specific integrins and the enhancement of integrin function by VEGF/NRP signaling have captured our attention and are the focus of this review. We are particularly interested in the emerging concept that VEGF/NRP signaling in tumor cells promotes aggressive behavior and that this effect is mediated, in part, by the ability of NRPs to modulate integrin function.
Perspective
The discovery more than 10 years ago that VEGF stimulation of endothelial and tumor cells can promote integrin activationCitation19 provided a rationale and impetus for studying the role of VEGF receptors in regulating integrin function. This particular study demonstrated that an intracellular signaling pathway involving PI3-K and initiated by autocrine/paracrine VEGF/VEGFR2 signaling increased the affinity of β1, β3 and β5 integrins for their ligands (inside-out signaling). This work also provided a paradigm for how integrin-mediated cell adhesion and migration can be regulated by VEGF signaling. These findings are buttressed by other work indicating that specific integrins such as αvβ3 can form a physical complex with VEGFR2 that is dependent on VEGF signaling and the consequent c-src-mediated phosphorylation of the β3 cytoplasmic domain.Citation20 The possibility that NRPs contribute to integrin activation was not explored in these studies but it seems likely given the fact that NRPs can associate with VEGFRs and influence their ability to bind ligand and signal.Citation15
The finding that NRPs can interact directly with specific integrins and modulate their function opened a new dimension in the study of both NRPs and integrins. For example, NRP1 was found to interact with β1 integrins in pancreatic carcinoma cells and to be important for integrin-mediated anchorage-independent growth, adhesion and invasion.Citation16 This study did not investigate the NRP1 ligand that promotes these effects but it is most likely VEGF. This assumption is based on the finding that semaphorins (Sema3A), in contrast to VEGF, can inhibit integrin function.Citation21 It is also consistent with the reports that semaphorins and VEGF compete for NRP bindingCitation22 and, consequently, that these two classes of NRP ligands can have markedly different effects on integrin function. An intriguing twist on this theme is the report that autocrine Sema3A/NRP1 signaling stimulates expression of the α2β1 integrin in breast carcinoma cells, which was shown to impede their migration and invasion.Citation23
An interesting mechanistic study found that NRP1 can associate with the α5β1 integrin in endothelial cells and enable the functional activity of this integrin.Citation17 NRP1 and the α5β1 integrin also interact in tumor cells and this interaction may contribute to VEGF regulation of cell survival.Citation24,Citation25 The interaction between NRP1 and α5β1 in endothelial cells is mediated by the PDZ-domain protein (GAIP interacting protein C terminus 1), which “bridges” these two receptors by binding to PDZ-binding domain sequences in their cytoplasmic tails. This finding is significant because it provided the first insight into the nature of the physical association between integrins and NRPs. At a functional level, the association between α5β1 and NRP1 was shown to be important for the endocytic trafficking of this integrin, resulting in increased cell adhesion to fibronectin. Another intriguing example of NRP-integrin cross-talk is the finding that the β3 integrin negatively regulates angiogenesis by blocking the formation of a VEGFR2/NRP-1 complex.Citation26 Specifically, the β3 integrin forms a complex with NRP-1 and sequesters NRP-1 from VEGFR2. These data suggest that NRP1 plays an important role in angiogenesis and can be a potential therapeutic target under pathological conditions where the expression of β3 integrins is downregulated.
VEGF/NRP2 Regulation of the α6β1 Integrin
We focus now on the regulation of the α6β1 integrin by VEGF/NRP2 signaling because it provides new insight into how integrins are regulated by NRPs and it has important implications for tumor biology. The α6β1 integrin (CD49f) functions primarily as a receptor for basement membrane laminins.Citation27 Notably, this integrin has been implicated in the aggressive behavior of several cancers. For example, an early study demonstrated that the expression of α6 correlates with poor outcome in breast cancer patients.Citation28 More recent studies have revealed that high α6β1 expression is characteristic of tumor initiating or stem cells and, importantly, that it has a critical role in the function of these cells.Citation29-Citation31 The assumption can be made based on these data that the function of α6β1 is regulated in cancer to control its potent effects on tumor cells. In fact, previous work in leukocytes demonstrated that the ability of α6β1 to engage laminin and mediate cell adhesion and migration is regulated by stimuli that induce leukocyte activation,Citation32,Citation33 providing a mechanism for the interaction of leukocytes with basement membrane proteins that occurs during diapedesis. Surprisingly, however, the issue of whether the function of α6β1 is regulated in tumor cells and the mechanisms involved had not been addressed directly.
Our discovery that the α6β1 integrin can associate with NRP2 in breast and prostate carcinoma cells provided the first clue that the function of this integrin may be linked to the NRPs in cancer.Citation18,Citation34 Interestingly, an association between α6β1 and NRP1 was not detected in these cells. These findings are relevant because NRP2 expression is low in normal epithelia and it is induced by oncogenic transformation and it correlates with more aggressive disease.Citation34,Citation35 Based on these data, we formulated the hypothesis that the induction of NRP2 expression in some malignant cells enables the formation of a NRP2/α6β1 complex that regulates the function of this integrin in response to VEGF stimulation. This hypothesis was validated by demonstrating that inhibition of NRP2 expression or function diminished the ability of breast carcinoma cells to adhere to laminin but not to other ECM proteins.Citation18 Unexpectedly, we discovered that NRP2 is localized with α6β1 in focal adhesions formed by tumor cells adherent to lamininCitation18 (). This localization of NRP2 in focal adhesions provided the first evidence linking these receptors to the dynamics of adhesive structures. Moreover, NRP2 is actually necessary for the localization of α6β1 in focal adhesionsCitation18 (). The ability of NRP2 to regulate α6β1 localization and function is dependent on VEGF. The validity of these findings was strengthened by our analysis of tumor cells analyzed directly after resection of human breast tumors. These cells were fractionated into NRP2high and NRP2low populations, and the NRP2high population exhibited high α6β1 expression and was able to form focal adhesions on laminin compared with the NRP2low populationCitation18 ().
Figure 1. Neuropilin-2 regulates the localization of the α6β1 integrin in focal adhesions. (A) Breast carcinoma cells (MDA-MB-435) in which NRP2 expression was depleted using shRNAs (shGFP, shNRP2–1 and shNRP2–2) were plated on laminin or collagen and stained with a p-FAK (Y397) Ab. The percentage of cells with focal adhesions (indicated by arrows) was quantified as shown in the bar graph. (B) MDA-MB-435 transfectants (shGFP, shNRP2–1 or shNRP2–2) were used to localize α6β1 by immunofluorescence microscopy using an α6-specific Ab. The number of cells with discrete localization of α6β1 integrin in focal adhesions was counted and plotted as a percentage of total cells. Note that loss of NRP2 expression reduces the number of focal adhesions formed on laminin significantly and the localization of α6β1 in focal adhesions. These effects were not seen in cells adherent to collagen. For details see Goel et al.Citation18
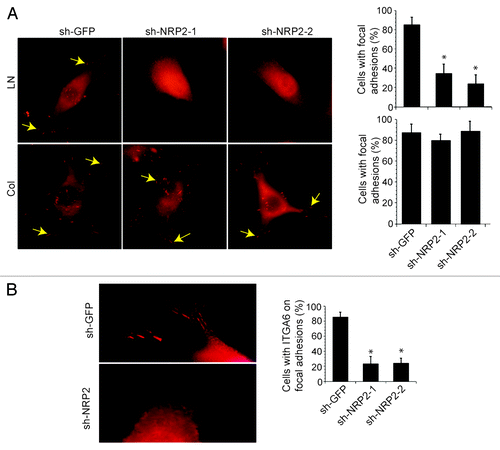
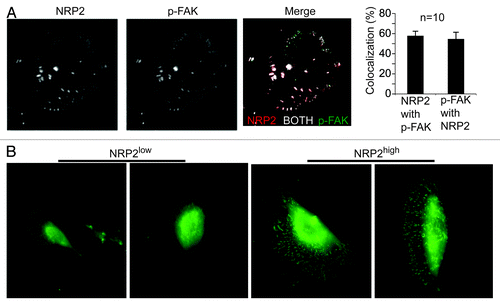
Figure 2. Neuropilin-2 localizes in focal adhesions and contributes to focal adhesion formation on laminin. (A) MDA-MB-435 cells were plated on laminin and immunofluorescence staining was performed using Abs to p-FAK (Y397) and NRP2. Samples were imaged using TIRF microscopy. Averaging all experiments, the colocalization of NRP-2 with FAK is 57.8% ± 4.6 and FAK with NRP-2 is 54.6% ± 6.8 (mean ± SEM, n = 10). These data demonstrate that NRP2 can localize in focal adhesions. (B) NRP2high and NRP2low populations isolated from breast tumors were plated on laminin and stained with a p-FAK (Y397) Ab. These data indicate that the NRP2high population in human breast tumors, which comprises only ~8% of total tumor cells and expresses high levels of α6β1 (CD49f), has the capacity to form focal adhesions on laminin in contrast to the NRP2low population. For details see Goel et al.Citation18
The foregoing discussion raises several important issues that bear on the general question of how VEGF/NRP2 signaling regulates integrin function. These issues include the nature of the association between NRPs and integrins, the signaling pathways controlled by VEGF/NRP that impact integrin function and the fundamental issue of how VEGF/NRP regulation of integrin function contributes to tumor biology.
How Do NRPs and Integrins Associate?
There are now several reports that NRPs and integrin can associate physically as evidenced primarily by co-immunoprecipitation experiments.Citation16-Citation18 As mentioned above, a mechanism has been proposed for how NRP1 and α5β1 interact that involves the binding of the cytoplasmic domains of these receptors to a PDZ-containing protein, which promotes their association by bridging.Citation17 This is an attractive model but other modes of association may also exist. Our finding that NRP2 and α6β1 co-localize in focal adhesions is worth noting in this regardCitation18 because it raises the concept that NRPs and integrins associate in microdomains on the cell surface and that these microdomains may consist of multiple proteins. It is well established, for example, that some integrins including α6β1 bind avidly to tetraspanins and that tetraspanins influence integrin function.Citation7 In this context, tetraspanin-enriched microdomains (TEMs) have been postulated that contain integrins and growth factor receptors,Citation7 and the possibility that NRPs are components of such TEMs merits investigation. Although the direct interaction of tetraspanins with integrin α subunits has been demonstrated, it may be that the association between integrins and NRPs is not direct in such complexes. This putative association of tetraspanins with an α6β1/NRP2 complex implies that tetraspanins can localize in focal adhesions, an issue that has not been resolved. An alternative hypothesis, albeit speculative at this point, is that α6β1 can interact with either tetraspanins or NRP2 and that such interactions have distinct functional consequences. Regardless, it seems probable that integrins and NRPs associate in larger macromolecular complexes on the cell surface. Whether the formation of these complexes is dependent upon VEGF stimulation and attachment to specific ECM ligands are key issues that need to be resolved. Also, an overriding question is whether tyrosine kinase VEGF receptors are present in these integrin-NRP complexes and, if so, how they impact VEGF signaling (see below).
There appears to be specificity in the association of NRP1 and NRP2 with specific integrins. NRP1 has been shown to associate with several β1 integrins including α5β1 but the possible association with NRP2 was not assessed in these studies.Citation17 In contrast, we observed that α6β1 associates with NRP2 but not with NRP1.Citation18 Given that NRP1 and NRP2 have similar domain structures and are 45–50% homologous at the amino acid level,Citation36 an intriguing question is what determines their specificity of integrin association? Indeed, there is precedence that NRP1 and NRP2 differ in their pattern of expression and function.Citation37,Citation38 Going forward, it will be important to refine the structural determinants of integrin association with NRPs. The association of NRP1 and NRP2 with different integrins may also reflect their differential localization in microdomains as mentioned above.
How Do VEGF/NRP-Mediated Signaling Pathways Regulate Integrin Function?
The data on NRP1 and α5β1 trafficking indicated that NRP1 binds to this integrin and regulates its function independently of ligand (VEGF165 or Sema3A).Citation17 Another scenario, however, is that signaling pathways initiated by autocrine or paracrine VEGF impinge upon integrins to modulate their affinity or avidity for their ECM ligands. The pioneering example of this model mentioned above is that VEGF stimulation mediated by VEGFR2 can increase the affinity of several integrins by activating the PI3-K/AKT pathway.Citation19 This study did not define the mechanism by which this signaling pathway altered integrin affinity nor did it investigate the contribution of NRPs. Our investigation of the mechanism by which VEGF/NRP2 signaling enables the laminin-binding function of the α6β1 integrin led to the finding that this signaling activates protein kinase C (PKC) and PKC is necessary for adhesion to laminin and focal adhesion formation.Citation18 In this context, we note that VEGF/NRP2 can activate TORC2Citation39 and that TORC2 can phosphorylate and stabilize conventional PKCs.Citation40 Interestingly, there is also evidence that tetraspanins interact with activated PKCs.Citation41 The data on PKC are of interest because previous work by our group demonstrated that PKC-mediated phosphorylation of the α6 cytoplasmic domain contributes to the activation of the α6β1 integrin in macrophages and promotes it association with the actin cytoskeleton.Citation42 Collectively, these observations form the novel hypothesis that VEGF/NRP2 signaling activates a TORC2/PKC pathway that activates the α6β1 integrin and promotes its association with F-actin, possibly by phosphorylation of the α6 subunit, enabling this integrin to nucleate focal adhesion formation and, consequently, focal adhesion signaling. Another significant question is whether VEGF/NRP2 signaling in this context involves VEGFRs and, if so, how these two distinct types of receptors function in concert to modulate integrin activation. We do know that NRP2 is essential for autocrine VEGF signaling in the tumor cells studied because inhibition of its function or expression impedes activation of α6β1.Citation18
Another potential aspect of VEGF signaling that needs to be considered with regard to integrin activation is the role of intracellular VEGF receptors and VEGF signaling. It has become apparent that VEGF receptors, including VEGFRs and NRPs, undergo endocytic recycling in response to ligand binding and that they are capable of signaling intracellularly.Citation17,Citation43,Citation44 Some integrins are also recycled and this process has been implicated in cell migration among other processes.Citation45 We have already discussed how NRPs can influence integrin trafficking and function but much more work needs to be done to understand the possible impact of intracellular VEGF signaling on integrin function.
How Does VEGF/NRP Regulation of Integrin Function Impact Tumor Biology?
The overarching issue in this review is how VEGF/NRP regulation of integrin function impacts the behavior of tumor cells. This issue has significance because VEGF,Citation8 NRPsCitation36,Citation46,Citation47 and integrinsCitation48,Citation49 are all validated drug targets and a better understanding of the mechanisms by which they function has the real possibility of improving cancer therapy. To put this issue in perspective, paracrine and autocrine VEGF signaling in tumor cells has been implicated in the initiation and progression of several cancersCitation43,Citation50,Citation51 and some of these seminal studies have invoked a key role for NRPs.Citation43,Citation51 Any discussion of VEGF/NRP signaling in tumor cells must consider the tumor microenvironment and the differentiation state of the tumor. Tumor hypoxia can induce VEGF expression, providing ligand for VEGF receptor signaling. For example, elevated VEGF expression is characteristic of the tumor microenvironment in glioblastoma,Citation52 and VEGF/NRP1 signaling has been implicated in the genesis of this cancer.Citation43 Along the same line, loss of estrogen receptor β expression in prostate cancer mimics hypoxia by stabilizing HIF-1α and inducing VEGF expression.Citation53 A relevant finding here is that autocrine VEGF/NRP signaling promotes a more de-differentiated, mesenchymal phenotype associated with highly aggressive tumor behavior.Citation53,Citation54 This finding is consistent with our observation that the expression of both VEGF and NRP2 in prostate tumor cells correlates inversely with Gleason grade,Citation34,Citation53 a measure of tumor de-differentiation and aggressiveness.Citation55 What is less known is the contribution of integrin regulation by this signaling pathway to these processes. In other terms, does VEGF/NRP signaling enhance integrin-mediated signaling pathways that contribute to tumor initiation and progression? Related to this discussion is our finding that metabolic stress including hypoxia induces the lysosomal degradation of NRP1 but not NRP2.Citation56 Given that the tumor microenvironment can have a profound effect of the metabolic state of tumor cells and consequent modulation of signaling responses, these results reinforce the role of the microenvironment in VEGF/NRP signaling and integrin activation. NRPs may also alter the tumor microenvironment as evidenced by the ability of NRP1 to stimulate the α5β1 integrin-dependent formation of fibronectin fibrils.Citation57
Recently, we made an interesting observation that bears directly on this question of how VEGF/NRP2 impacts tumor behavior by regulating integrin function. VEGF/NRP2 signaling in breast carcinoma cells activates focal adhesion kinase (FAK).Citation18,Citation58 FAK is not activated directly by VEGF/NRP2, however, but it is the engagement of laminin by the α6β1 integrin that results in FAK activation.Citation18 The role of VEGF/NRP2 signaling is to activate the laminin binding function of α6β1 and promote its localization in focal adhesions. An obvious issue that needs to be addressed is why this effect is specific to α6β1/laminin given that other integrins can activate FAK. The fact that NRP2 does not interact with α3β1, another laminin-binding integrin,Citation18 provides some indication that the selectivity of NRP2 association contributes to the specificity of integrin-mediated signaling.
The observation that VEGF/NRP2 signaling enables the α6β1 integrin to activate FAK is intriguing, because all of these molecules are emerging as central players in the initiation of breast cancer, as well as other cancers, and the biology of aggressive disease. As mentioned, α6β1 is a marker of breast tumor stem or initiating cells and it has been implicated in the function of these cells and other tumor stem cells.Citation29-Citation31 The expression of NRP2 in breast and prostate cancer correlates with aggressive disease.Citation34,Citation35 Along the same lines, FAK has been shown to be important for the function of breast tumor stem cells.Citation59 Together, these observations form the novel hypothesis that VEGF/NRP2 signaling contributes to the function of tumor initiating cells by promoting the α6β1-mediated activation of FAK. This hypothesis is supported, in part, by the finding that a VEGF/NRP1 autocrine loop regulates the initiation and stemness of skin tumors.Citation51 It remains to be determined whether VEGF/NRP2 signaling is necessary for the initiation of breast or other tumors and the extent to which this signaling involves α6β1-mediated FAK activation. In this direction, we discovered recently that NRP2/α6β1-mediated FAK activation in prostate cancer induces the expression of Bmi-1,Citation34 a Polycomb transcriptional repressor that has been implicated in tumor initiation.Citation60 These findings also bear on the biological basis of highly aggressive cancers because such cancers are known to be de-differentiatedCitation61 and contain a high frequency of tumor initiating cells.Citation62 The possibility that VEGF/NRP signaling contributes to the differentiation state and behavior of these cancers by regulating integrin signaling is likely.
Concluding Comments
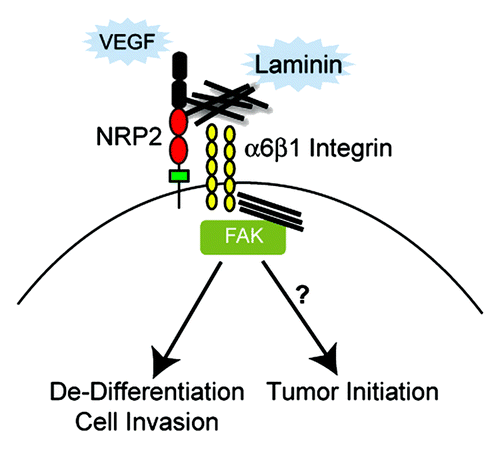
The intent of this review was to illustrate how two seemingly disparate signaling pathways (VEGF/NRP and ECM/integrin) interface to impact the behavior of cells, especially tumor cells (). Clearly, the hypothesis that VEGF/NRP signaling enables the activation and signaling potential of specific integrins is valid and highly relevant to cancer. Although VEGF signaling in tumor cells affects much more than integrin function and many integrins can function independently of VEGF signaling, the intersection of VEGF signaling with integrin regulation can have profound effects on tumors. This hypothesis is illustrated best by our analysis of VEGF/NRP2 regulation of α6β1 integrin function and signaling, and the potential role of this pathway in tumor initiating cells and aggressive carcinomas ().
Figure 3. The function of the α6β1 integrin is regulated by VEGF/NRP2 signaling in tumor cells. An integrated model is presented based on the data and discussion highlighted in this review. Oncogenic stimuli such as loss of PTEN function induce NRP2 expression.Citation34 VEGF produced by tumor cells binds NRP2 (autocrine signaling) and NRP2 associates with α6β1 enabling this integrin to interact with laminin, engage the cytoskeleton and form focal adhesions. A critical component of this mechanism is that VEGF/NRP2 signaling facilitates FAK activation by α6β1. The possibility that this concerted activation of FAK by VEGF/NRP1 and α6β1 signaling contributes to tumor de-differentiation and initiation by regulating the expression of key stem cell factors should be investigated.
Another goal of this review was to pinpoint key areas for future study. Indeed, much more needs to be learned about the biochemical basis of how NRPs and integrins interact, and whether they associate in specific membrane microdomains. Related issues are the role of NRP ligands including the different VEGF isoforms and the semaphorins in modulating the association of NRPs with specific integrins. Although some work has been done on the role of tyrosine kinase VEGF receptors with respect to integrin activation, the contribution of these VEGFRs to VEGF/NRP regulation of integrin function needs to be evaluated more rigorously. From a functional perspective, the time is ripe to test some of the key aspects of integrin regulation by VEGF/NRP signaling in mouse models. The culmination of this work with respect to cancer will be to evaluate the efficacy of targeting NRPs, VEGF and integrins either alone or in combination to enhance tumor regression and impede progression. The fact that function-blocking antibodies are available should facilitate this work and provide a foundation for clinical trials.
Acknowledgments
Work from the authors’ lab cited in this review was funded by NIH Grants CA168464 and DOD Prostate Cancer Grant PC111410.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002; 110:673 - 87; http://dx.doi.org/10.1016/S0092-8674(02)00971-6; PMID: 12297042
- Kim C, Ye F, Ginsberg MH. Regulation of integrin activation. Annu Rev Cell Dev Biol 2011; 27:321 - 45; http://dx.doi.org/10.1146/annurev-cellbio-100109-104104; PMID: 21663444
- Springer TA, Dustin ML. Integrin inside-out signaling and the immunological synapse. Curr Opin Cell Biol 2012; 24:107 - 15; http://dx.doi.org/10.1016/j.ceb.2011.10.004; PMID: 22129583
- Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized?. Curr Opin Cell Biol 2003; 15:547 - 56; http://dx.doi.org/10.1016/j.ceb.2003.08.003; PMID: 14519389
- Margadant C, Monsuur HN, Norman JC, Sonnenberg A. Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol 2011; 23:607 - 14; http://dx.doi.org/10.1016/j.ceb.2011.08.005; PMID: 21924601
- Ivaska J, Heino J. Cooperation between integrins and growth factor receptors in signaling and endocytosis. Annu Rev Cell Dev Biol 2011; 27:291 - 320; http://dx.doi.org/10.1146/annurev-cellbio-092910-154017; PMID: 21663443
- Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol 2005; 6:801 - 11; http://dx.doi.org/10.1038/nrm1736; PMID: 16314869
- Ferrara N. VEGF as a therapeutic target in cancer. Oncology 2005; 69:Suppl 3 11 - 6; http://dx.doi.org/10.1159/000088479; PMID: 16301831
- Vlahakis NE, Young BA, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J Biol Chem 2005; 280:4544 - 52; http://dx.doi.org/10.1074/jbc.M412816200; PMID: 15590642
- Uniewicz KA, Fernig DG. Neuropilins: a versatile partner of extracellular molecules that regulate development and disease. Front Biosci 2008; 13:4339 - 60; http://dx.doi.org/10.2741/3008; PMID: 18508514
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998; 92:735 - 45; http://dx.doi.org/10.1016/S0092-8674(00)81402-6; PMID: 9529250
- Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, et al. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 1999; 99:59 - 69; http://dx.doi.org/10.1016/S0092-8674(00)80062-8; PMID: 10520994
- Nasarre C, Koncina E, Labourdette G, Cremel G, Roussel G, Aunis D, et al. Neuropilin-2 acts as a modulator of Sema3A-dependent glioma cell migration. Cell Adh Migr 2009; 3:383 - 9; http://dx.doi.org/10.4161/cam.3.4.9934; PMID: 19855168
- Gaur P, Bielenberg DR, Samuel S, Bose D, Zhou Y, Gray MJ, et al. Role of class 3 semaphorins and their receptors in tumor growth and angiogenesis. Clin Cancer Res 2009; 15:6763 - 70; http://dx.doi.org/10.1158/1078-0432.CCR-09-1810; PMID: 19887479
- Neufeld G, Kessler O, Herzog Y. The interaction of Neuropilin-1 and Neuropilin-2 with tyrosine-kinase receptors for VEGF. Adv Exp Med Biol 2002; 515:81 - 90; http://dx.doi.org/10.1007/978-1-4615-0119-0_7; PMID: 12613545
- Fukasawa M, Matsushita A, Korc M. Neuropilin-1 interacts with integrin beta1 and modulates pancreatic cancer cell growth, survival and invasion. Cancer Biol Ther 2007; 6:1173 - 80; PMID: 17726369
- Valdembri D, Caswell PT, Anderson KI, Schwarz JP, König I, Astanina E, et al. Neuropilin-1/GIPC1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS Biol 2009; 7:e25; http://dx.doi.org/10.1371/journal.pbio.1000025; PMID: 19175293
- Goel HL, Pursell B, Standley C, Fogarty K, Mercurio AM. Neuropilin-2 regulates α6β1 integrin in the formation of focal adhesions and signaling. J Cell Sci 2012; 125:497 - 506; http://dx.doi.org/10.1242/jcs.094433; PMID: 22302985
- Byzova TV, Goldman CK, Pampori N, Thomas KA, Bett A, Shattil SJ, et al. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell 2000; 6:851 - 60; PMID: 11090623
- Mahabeleshwar GH, Feng W, Reddy K, Plow EF, Byzova TV. Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ Res 2007; 101:570 - 80; http://dx.doi.org/10.1161/CIRCRESAHA.107.155655; PMID: 17641225
- Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 2003; 424:391 - 7; http://dx.doi.org/10.1038/nature01784; PMID: 12879061
- Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, et al. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res 2003; 63:5230 - 3; PMID: 14500350
- Pan H, Wanami LS, Dissanayake TR, Bachelder RE. Autocrine semaphorin3A stimulates alpha2 beta1 integrin expression/function in breast tumor cells. Breast Cancer Res Treat 2009; 118:197 - 205; http://dx.doi.org/10.1007/s10549-008-0179-y; PMID: 18787945
- Hervé MA, Buteau-Lozano H, Vassy R, Bieche I, Velasco G, Pla M, et al. Overexpression of vascular endothelial growth factor 189 in breast cancer cells leads to delayed tumor uptake with dilated intratumoral vessels. Am J Pathol 2008; 172:167 - 78; http://dx.doi.org/10.2353/ajpath.2008.070181; PMID: 18079435
- Vintonenko N, Pelaez-Garavito I, Buteau-Lozano H, Toullec A, Lidereau R, Perret GY, et al. Overexpression of VEGF189 in breast cancer cells induces apoptosis via NRP1 under stress conditions. Cell Adh Migr 2011; 5:332 - 43; http://dx.doi.org/10.4161/cam.5.4.17287; PMID: 21897119
- Robinson SD, Reynolds LE, Kostourou V, Reynolds AR, da Silva RG, Tavora B, et al. Alphav beta3 integrin limits the contribution of neuropilin-1 to vascular endothelial growth factor-induced angiogenesis. J Biol Chem 2009; 284:33966 - 81; http://dx.doi.org/10.1074/jbc.M109.030700; PMID: 19837659
- Mercurio AM. Laminin: multiple forms, multiple receptors. Curr Opin Cell Biol 1990; 2:845 - 9; http://dx.doi.org/10.1016/0955-0674(90)90082-P; PMID: 2150589
- Friedrichs K, Ruiz P, Franke F, Gille I, Terpe HJ, Imhof BA. High expression level of alpha 6 integrin in human breast carcinoma is correlated with reduced survival. Cancer Res 1995; 55:901 - 6; PMID: 7850807
- Cariati M, Naderi A, Brown JP, Smalley MJ, Pinder SE, Caldas C, et al. Alpha-6 integrin is necessary for the tumourigenicity of a stem cell-like subpopulation within the MCF7 breast cancer cell line. Int J Cancer 2008; 122:298 - 304; http://dx.doi.org/10.1002/ijc.23103; PMID: 17935134
- Yu KR, Yang SR, Jung JW, Kim H, Ko K, Han DW, et al. CD49f enhances multipotency and maintains stemness through the direct regulation of OCT4 and SOX2. Stem Cells 2012; 30:876 - 87; http://dx.doi.org/10.1002/stem.1052; PMID: 22311737
- Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 2010; 6:421 - 32; http://dx.doi.org/10.1016/j.stem.2010.02.018; PMID: 20452317
- Mercurio AM, Shaw LM. Macrophage interactions with laminin: PMA selectively induces the adherence and spreading of mouse macrophages on a laminin substratum. J Cell Biol 1988; 107:1873 - 80; http://dx.doi.org/10.1083/jcb.107.5.1873; PMID: 2972733
- Shaw LM, Mercurio AM. Interferon gamma and lipopolysaccharide promote macrophage adherence to basement membrane glycoproteins. J Exp Med 1989; 169:303 - 8; http://dx.doi.org/10.1084/jem.169.1.303; PMID: 2491881
- Goel HL, Chang C, Pursell B, Leav I, Lyle S, Xi HS, et al. VEGF/Neuropilin-2 Regulation of Bmi-1 and Consequent Repression of IGF-IR Define a Novel Mechanism of Aggressive Prostate Cancer. Cancer Discov 2012; 2:906 - 21; http://dx.doi.org/10.1158/2159-8290.CD-12-0085; PMID: 22777769
- Yasuoka H, Kodama R, Tsujimoto M, Yoshidome K, Akamatsu H, Nakahara M, et al. Neuropilin-2 expression in breast cancer: correlation with lymph node metastasis, poor prognosis, and regulation of CXCR4 expression. BMC Cancer 2009; 9:220; http://dx.doi.org/10.1186/1471-2407-9-220; PMID: 19580679
- Bagri A, Tessier-Lavigne M, Watts RJ. Neuropilins in tumor biology. Clin Cancer Res 2009; 15:1860 - 4; http://dx.doi.org/10.1158/1078-0432.CCR-08-0563; PMID: 19240167
- Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, et al. A requirement for neuropilin-1 in embryonic vessel formation. Development 1999; 126:4895 - 902; PMID: 10518505
- Chen H, Bagri A, Zupicich JA, Zou Y, Stoeckli E, Pleasure SJ, et al. Neuropilin-2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron 2000; 25:43 - 56; http://dx.doi.org/10.1016/S0896-6273(00)80870-3; PMID: 10707971
- Muders MH, Zhang H, Wang E, Tindall DJ, Datta K. Vascular endothelial growth factor-C protects prostate cancer cells from oxidative stress by the activation of mammalian target of rapamycin complex-2 and AKT-1. Cancer Res 2009; 69:6042 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-09-0552; PMID: 19638584
- Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J 2008; 27:1932 - 43; http://dx.doi.org/10.1038/emboj.2008.120; PMID: 18566586
- Zhang XA, Bontrager AL, Hemler ME. Transmembrane-4 superfamily proteins associate with activated protein kinase C (PKC) and link PKC to specific beta(1) integrins. J Biol Chem 2001; 276:25005 - 13; http://dx.doi.org/10.1074/jbc.M102156200; PMID: 11325968
- Shaw LM, Messier JM, Mercurio AM. The activation dependent adhesion of macrophages to laminin involves cytoskeletal anchoring and phosphorylation of the alpha 6 beta 1 integrin. J Cell Biol 1990; 110:2167 - 74; http://dx.doi.org/10.1083/jcb.110.6.2167; PMID: 2141029
- Hamerlik P, Lathia JD, Rasmussen R, Wu Q, Bartkova J, Lee M, et al. Autocrine VEGF-VEGFR2-Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J Exp Med 2012; 209:507 - 20; http://dx.doi.org/10.1084/jem.20111424; PMID: 22393126
- Berger P, Ballmer-Hofer K. The reception and the party after: how vascular endothelial growth factor receptor 2 explores cytoplasmic space. Swiss Med Wkly 2011; 141:w13318; PMID: 22180219
- Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol 2009; 10:843 - 53; http://dx.doi.org/10.1038/nrm2799; PMID: 19904298
- Caunt M, Mak J, Liang WC, Stawicki S, Pan Q, Tong RK, et al. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell 2008; 13:331 - 42; http://dx.doi.org/10.1016/j.ccr.2008.01.029; PMID: 18394556
- Gray MJ, Van Buren G, Dallas NA, Xia L, Wang X, Yang AD, et al. Therapeutic targeting of neuropilin-2 on colorectal carcinoma cells implanted in the murine liver. J Natl Cancer Inst 2008; 100:109 - 20; http://dx.doi.org/10.1093/jnci/djm279; PMID: 18182619
- Hehlgans S, Haase M, Cordes N. Signalling via integrins: implications for cell survival and anticancer strategies. Biochim Biophys Acta 2007; 1775:163 - 80; PMID: 17084981
- Millard M, Odde S, Neamati N. Integrin targeted therapeutics. Theranostics 2011; 1:154 - 88; http://dx.doi.org/10.7150/thno/v01p0154; PMID: 21547158
- Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell 2010; 140:268 - 79; http://dx.doi.org/10.1016/j.cell.2009.12.046; PMID: 20141840
- Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature 2011; 478:399 - 403; http://dx.doi.org/10.1038/nature10525; PMID: 22012397
- Takano S, Yoshii Y, Kondo S, Suzuki H, Maruno T, Shirai S, et al. Concentration of vascular endothelial growth factor in the serum and tumor tissue of brain tumor patients. Cancer Res 1996; 56:2185 - 90; PMID: 8616870
- Mak P, Leav I, Pursell B, Bae D, Yang X, Taglienti CA, et al. ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell 2010; 17:319 - 32; http://dx.doi.org/10.1016/j.ccr.2010.02.030; PMID: 20385358
- Cao Y, Wang L, Nandy D, Zhang Y, Basu A, Radisky D, et al. Neuropilin-1 upholds dedifferentiation and propagation phenotypes of renal cell carcinoma cells by activating Akt and sonic hedgehog axes. Cancer Res 2008; 68:8667 - 72; http://dx.doi.org/10.1158/0008-5472.CAN-08-2614; PMID: 18974107
- Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 1974; 111:58 - 64; PMID: 4813554
- Bae D, Lu S, Taglienti CA, Mercurio AM. Metabolic stress induces the lysosomal degradation of neuropilin-1 but not neuropilin-2. J Biol Chem 2008; 283:28074 - 80; http://dx.doi.org/10.1074/jbc.M804203200; PMID: 18708346
- Yaqoob U, Cao S, Shergill U, Jagavelu K, Geng Z, Yin M, et al. Neuropilin-1 stimulates tumor growth by increasing fibronectin fibril assembly in the tumor microenvironment. Cancer Res 2012; 72:4047 - 59; http://dx.doi.org/10.1158/0008-5472.CAN-11-3907; PMID: 22738912
- Goel HL, Bae D, Pursell B, Gouvin LM, Lu S, Mercurio AM. Neuropilin-2 promotes branching morphogenesis in the mouse mammary gland. Development 2011; 138:2969 - 76; http://dx.doi.org/10.1242/dev.051318; PMID: 21693513
- Guan JL. Integrin signaling through FAK in the regulation of mammary stem cells and breast cancer. IUBMB Life 2010; 62:268 - 76; PMID: 20101634
- Lukacs RU, Memarzadeh S, Wu H, Witte ON. Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell 2010; 7:682 - 93; http://dx.doi.org/10.1016/j.stem.2010.11.013; PMID: 21112563
- Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 2008; 40:499 - 507; http://dx.doi.org/10.1038/ng.127; PMID: 18443585
- Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell 2010; 140:62 - 73; http://dx.doi.org/10.1016/j.cell.2009.12.007; PMID: 20074520