Abstract
The binding of selectins to carbohydrate epitopes expressed on leukocytes is the first step in a multi-step cell adhesion cascade that controls the rate of leukocyte recruitment at sites of inflammation. The glycans that function as selectin-ligands are post-translationally synthesized by the serial action of Golgi resident enzymes called glycosyltransferases (glycoTs). Whereas much of our current knowledge regarding the role of glycoTs in constructing selectin-ligands comes from reconstituted biochemical investigations or murine models, tools to assess the impact of these enzymes on the human ligands are relatively underdeveloped. This is significant since the selectin-ligands, particularly those that bind E-selectin, vary between different leukocyte cell populations and they are also different in humans compared with mice. To address this shortcoming, a recent study by Buffone et al. (2013) outlines a systematic strategy to knockdown upto three glycoTs simultaneously in human leukocytes. The results suggest that the fucosyltransferases (FUTs) regulating selectin-ligand synthesis may be species-specific. In particular, they demonstrate that FUT9 plays a significant role during human, but not mouse, leukocyte-endothelial interactions. Overall, this article discusses the relative roles of the FUTs during human L-, E-, and P-selectin-ligand biosynthesis, and the potential that the knockdown strategy outlined here may assess the role of other glycoTs in human leukocytes also.
Role of Carbohydrates in the Leukocyte Adhesion Cascade
A multi-step mechanism governs leukocyte infiltration into inflamed tissue. It is initiated by the capture and subsequent rolling of circulating leukocytes on the activated vascular endothelium, followed by cell activation, firm adhesion, and trans-endothelial migration.Citation1,Citation2 Cell rolling is a crucial step in this cascade since it defines the contact time of leukocytes with chemokines bound to glycosaminoglycans expressed on the inflamed endothelium. This contact is essential for leukocyte activation so that the cells may firmly adhere on to the vessel wall prior to diapedesis. In this context, the selectins are a family of three calcium-dependent carbohydrate binding proteins or lectins (C-type) that facilitate leukocyte capture from flowing blood and subsequently mediate cell rolling interactions.Citation1,Citation2 Among the three members of the selectin family, P-selectin is expressed on activated platelets and endothelial cells, and mediates the initial leukocyte capture and rolling on the endothelium.Citation3 E-selectin is exclusively expressed on the inflamed endothelium and it supports slower rolling interactionsCitation4,Citation5 with ligation of this lectin also contributing to leukocyte activation. The leukocyte-borne L-selectin primarily supports secondary capture and this effectively enhances the number of leukocytes recruited at sites of inflammation.Citation1,Citation2
The selectins bind a variety of carbohydrate epitopes on the leukocyte cell surface, with the sialyl Lewis-X (sLeX) glycan representing the prototypic selectin-ligand.Citation4,Citation6,Citation7 This is a sialofucosylated tetrasaccharide (Neu5Acα2,3Galβ1,4[Fucα1,3]GlcNAc) containing an α(2,3)-linked sialic acid and α(1,3)-linked fucose. SLeX and related glycans are post-translationally synthesized by a family of enzymes called glycosyltransferases (glycoTs) on either cell membrane glycoprotein or glycolipid scaffolds.Citation8 Due to the high on- and off-rates of the selectin-carbohydrate molecular interaction, selectin-mediated leukocyte binding under hydrodynamic shear results in a transient cellular interaction which is characterized by the rapid formation and breakage of molecular bonds.Citation9 While the high on-rate facilitates the initial leukocyte capture/recruitment or “cell tethering” event, the force dependent off-rate regulates the “cell rolling” velocity.
Novel Tools to Study Glycosyltransferases in Human Leukocytes
The glycoTs are Golgi resident enzymes that transfer monosaccharides from sugar-nucleotide donors to protein and lipid based acceptors.Citation10 They constitute a family of ~200 enzymes or ~1% of the human genome.Citation11 Biochemical assays performed with cloned glycoTs and murine studies performed with knockouts that lack one or more of these enzymes are a major source of our current knowledge regarding the contributions of the individual glycoTs to selectin-ligand biosynthesis. The recent widespread application of RNA-interference methods provides an opportunity to determine the degree to which knowledge gained from these murine models translate to humans. Critical steps in the application of this technology to human leukocytes involves: (1) Development of quantitative methods to identify effective siRNA/shRNA that silence individual glycosyltransferases. This is important since well-characterized antibodies against most glycoTs are unavailable. (2) Stable expression of these constructs in human leukocytes that are typically hard-to-transfect.
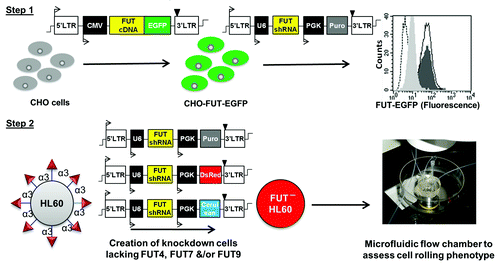
In the manuscript by Buffone et al.,Citation12 these challenges were overcome by developing a streamlined two-step methodology (). In the first step, glycoT fusion proteins containing a C-terminal enhanced green fluorescent protein (EGFP) were expressed in the trans-Golgi network (TGN) of either Chinese hamster ovary (CHO) or human embryonic kidney (HEK293T) cell lines. Lentiviral vectors carrying shRNA were transduced into these cells in order to identify shRNA that reduced EGFP fluorescence in the fusion proteins by >85%. In the second step, these shRNA were transduced into hard to transfect human promyelocytic leukemia HL-60 cells using either fluorescence reporters or drug resistance cassettes to monitor stable gene expression. In this manner, cell lines lacking one to three different fucosyltransferases were developed. Cell adhesion studies were performed with these knock-down cells in order to assess the functional effect of gene silencing.
Figure 1. Workflow for the study of fucosyltransferases in human leukocytes. In step 1, CHO cells expressing FUT4-, FUT7-, or FUT9-EGFP fusion proteins were constructed. Various FUT shRNA with puromycin selection cassette were introduced into these cells and their ability to reduce cellular EGFP fluorescence was assessed using flow cytometry. Green fluorescence signal was reduced from the dark shaded histogram without shRNA to light shaded histogram upon addition of efficient shRNA. Empty and dashed histograms represent cells transduced with non-silencing lentivirus and cells lacking EGFP respectively. In step 2, efficient shRNA containing either a puromycin cassette or fluorescence reporter (DsRed or Cerulean) were transduced into HL-60 cells to create stable knockdowns. Cell function was measured under fluid shear using flow chamber substrates bearing either CHO cells expressing P-selectin, recombinant L-selectin, E-selectin expressing L-cells, or IL-1β stimulated HUVECs.
FUTs Regulating P- and L-Selectin Binding Are Similar in Humans and Mice
In the case of P- and L-selectin mediated cell adhesion, an O-linked glycan located at the N-terminus of the glycoprotein PSGL-1 (P-selectin glycoprotein ligand-1) is the major selectin-ligand expressed on both murine and human leukocytes.Citation13 In humans, this glycan resides at Threonine 57 (T57), 15 amino acids from the N-terminus of mature PSGL-1. Further, in order to enhance its accessibility to the selectins, PSGL-1 is localized at the tips of the leukocyte microvilli.Citation14 While there is a heterogeneous distribution of O-glycans at T57, the functional selectin-ligand at this site is a non-extended sialyl Lewis-X glycan contained within a core-2 motifCitation7 ().
Figure 2. Selectin ligand biosynthetic pathway on PSGL-1: A core-2 O-glycan carrying the sLeX tetrasaccharide is expressed at the N-terminus of PSGL-1. This is synthesized in the Golgi by the action of various glycosyltransferases (GlycoTs).
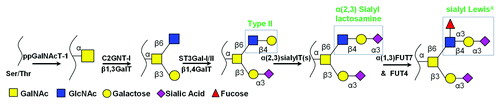
Transgenic mouse studies where putative glycoTs responsible for the construction of this carbohydrate epitope were knocked out suggest a role for polypeptide α-GalNAcT ppGalNAcT-1,Citation15 core-1 β1,3GalactosylT T-synthase,Citation10 core-2 β1,6GlcNAcT C2GnT-I,Citation16 β1,4GalactosylT β4GalT-I,Citation17 α(2,3)sialylT ST3GalT-IV and VI,Citation18,Citation19 and α(1,3) fucosyltansferases (FUTs), FUT7Citation20 and FUT4,Citation21 during the synthesis of such structures (). Sulfation of the peptide backbone by tyrosine sulfotransferases is also important for functional selectin ligand biosynthesis on PSGL-1.
In human systems, a number of studies that characterize the O-glycans of PSGL-1 have been performed using the HL-60 cells,Citation22,Citation23 since these cells exhibit similar rolling phenotype as primary human neutrophils.Citation24,Citation25 Leveraging the knowledge from this heavily characterized cell system and applying lentivirus to silence the α(1,3) fucosyltansferases FUT4 and FUT7, either individually or in tandem, Buffone et al. demonstrate that primarily FUT7 and secondarily FUT4 contribute to leukocyte rolling on substrates composed of either L- or P-selectinCitation12 (). These experiments performed using the gene knockdown strategy, are in agreement with prior studies that either utilize mouse models or that overexpress the FUTs in heterologous cells.Citation21,Citation26
Table 1. Effect of FUT silencing on leukocyte rolling density (% reduction)*
FUTs Regulating E-Selectin Ligand Biosynthesis are Distinct in Human vs. Mouse Leukocytes
While many potential leukocyte E-selectin ligands have been proposed in literature, the quantitation of the relative importance of these candidate glycoconjugates remains an active area of investigation. This is in part due to missing information on the precise O- or N-glycan structure(s) on glycoproteins and also human gangliosides that bind E-selectin under physiologically relevant hydrodynamic shear conditions. In addition, except for PSGL-1, which is a relatively minor E-selectin ligand, there are no monoclonal antibodies that can individually block the contributions of other potential E-selectin ligands. For example, while some studies suggest a prominent role for the N-glycans of ESL-1 (E-selectin ligand-1) and CD44 in binding mouse E-selectin,Citation27 others suggest that E-selectin-mediated cell adhesion is largely driven by O-linked glycans.Citation16,Citation28 In the latter investigations, granulocytes from mice lacking the core-2 GlcNAc transferase C2GnT-ICitation16 and also mice lacking T-synthase activityCitation28 exhibit dramatic loss of E-selectin binding function. Adding to this complication, the E-selectin ligands in different leukocyte populations may be distinct. For example, while CD43 is considered to be an E-selectin ligand on T-cells,Citation29,Citation30 ESL-1 is a more potent ligand on mouse granulocytes.Citation27 In this regard, the expression pattern of scaffold proteins bearing the carbohydrate-ligands and also the level of cellular glycosyltransferase activity are likely to be important parameters that define the E-selectin ligand in specific cell types.
In addition to differences in the nature of the E-selectin ligands between different leukocyte sub-populations, even in a single cell type like granulocytes, the E-selectin ligands in humans are likely to be different from that in mice. In support of this, the treatment of mouse neutrophils with pronase results in a complete loss of leukocyte adhesion to all three selectins.Citation31,Citation32 In contrast, the addition of pronase to both primary human neutrophils and HL-60 cells abrogates cell binding to L- and P-selectin, but this treatment has little effect on cell adhesion to E-selectin (refs. Citation31 and Citation32 and our unpublished data). The possibility that the pronase-insensitive feature of human E-selectin ligands may be attributed to cell-surface glycosphingolipids has been tested by several investigators.Citation33,Citation34 These studies show that sialylated glycosphingolipids or gangliosides extracted from primary human neutrophils and HL-60 cells contain repeating N-acetyl-lactosamine units with internal fucosylation that support E-selectin binding under static and fluid flow conditions. Inhibition of ganglioside biosynthesis with a small molecule inhibitor of glucosylceramide synthase reduces E-selectin mediated cell rolling by ~50%.Citation33
In further support of this species-specific difference: (1) L-selectin in human but not mouse leukocytes is reported to act as an E-selectin ligand.Citation35 (2) ESL-1 has been shown to be a functional E-selectin ligand on murine but not human myeloid cells.Citation36 (3) An N-linked sialofucosylated glycoform of CD44 called “Hematopoietic cell E and L-selectin ligand (HCELL)” is thought to represent a functional E-/L-selectin ligand on human but not murine hematopoietic stem and progenitor cells (hHSPCs).Citation37 (4) CD44 expressed on mature neutrophils and also some lymphocytes is thought to act as an E-selectin ligand in murine but not human cells.Citation27
Buffone et al.Citation12 addressed the hypothesis that differences in the relative roles of glycoTs in human vs. mouse leukocytes may, at least partially, drive the synthesis of distinct E-selectin ligands in the two species. More specifically, the authors compared the relative roles of all three myeloid α(1,3)FUTs, FUT4, FUT7, and FUT9, in the two species. Here, consistent with previous studies,Citation21 predominantly FUT7 and secondarily FUT4 synthesized all the E-selectin ligands on mouse granulocytes. In contrast, FUT9 played an important role during human leukocyte binding to E-selectin with the relative roles of the three α(1,3)FUTs during HL-60 E-selectin ligand biosynthesis varying as FUT9 > FUT7 > FUT4 (). In these assays, the number of HL-60 interacting with E-selectin under flow decreased by 30% in dual knockouts lacking FUT4 and FUT 7 (FUT4−7− HL-60) while this was reduced by 70% in the FUT7−9− HL-60s. Since the differentiation of myeloid precursor cells to mature human neutrophils is accompanied by a substantial increase in FUT9 expression,Citation12,Citation38 the contribution of FUT9 to primary human neutrophils may also be significant and this can impact human inflammatory ailments.
Conclusions and Future Directions
The framework established by Buffone et al.Citation12 provides a streamlined strategy to examine the impact of the glycoTs in regulating human leukocyte cell adhesion function. While the current studies focus on the α(1,3)FUTs, extensions of this approach can also be applied to study the impact of other members of the glycoT family. Further advancement of this approach to primary cells can also enable testing of these hypotheses in human leukocyte populations derived from peripheral blood and other sources. These efforts can help identify the molecular players and corresponding drug targets for diverse human inflammatory ailments.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL63014 and HL103411).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Neelamegham S. Transport features, reaction kinetics and receptor biomechanics controlling selectin and integrin mediated cell adhesion. Cell Commun Adhes 2004; 11:35 - 50; http://dx.doi.org/10.1080/15419060490471793; PMID: 15500296
- Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood 2011; 118:6743 - 51; http://dx.doi.org/10.1182/blood-2011-07-343566; PMID: 22021370
- Geng JG, Bevilacqua MP, Moore KL, McIntyre TM, Prescott SM, Kim JM, et al. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature 1990; 343:757 - 60; http://dx.doi.org/10.1038/343757a0; PMID: 1689464
- Phillips ML, Nudelman E, Gaeta FC, Perez M, Singhal AK, Hakomori S, et al. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science 1990; 250:1130 - 2; http://dx.doi.org/10.1126/science.1701274; PMID: 1701274
- Walz G, Aruffo A, Kolanus W, Bevilacqua M, Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science 1990; 250:1132 - 5; http://dx.doi.org/10.1126/science.1701275; PMID: 1701275
- Foxall C, Watson SR, Dowbenko D, Fennie C, Lasky LA, Kiso M, et al. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewis(x) oligosaccharide. J Cell Biol 1992; 117:895 - 902; http://dx.doi.org/10.1083/jcb.117.4.895; PMID: 1374413
- Lo CY, Antonopoulos A, Gupta R, Qu J, Dell A, Haslam SM, et al. Competition between core-2 GlcNAc transferase and ST6GalNAc transferase regulates the synthesis of the leukocyte selectin-ligand on human P-selectin glycoprotein ligand-1. J Biol Chem 2013; In press http://dx.doi.org/10.1074/jbc.M113.463653; PMID: 23548905
- Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat Rev Immunol 2008; 8:874 - 87; http://dx.doi.org/10.1038/nri2417; PMID: 18846099
- Beauharnois ME, Lindquist KC, Marathe D, Vanderslice P, Xia J, Matta KL, et al. Affinity and kinetics of sialyl Lewis-X and core-2 based oligosaccharides binding to L- and P-selectin. Biochemistry 2005; 44:9507 - 19; http://dx.doi.org/10.1021/bi0507130; PMID: 15996105
- Taniguchi N. Handbook of Glycosyltransferases and Related Genes. Springer, 2002.
- Neelamegham S, Liu G. Systems glycobiology: biochemical reaction networks regulating glycan structure and function. Glycobiology 2011; 21:1541 - 53; http://dx.doi.org/10.1093/glycob/cwr036; PMID: 21436236
- Buffone A Jr., Mondal N, Gupta R, McHugh KP, Lau JTY, Neelamegham S. Silencing α1,3-fucosyltransferases in human leukocytes reveals a role for FUT9 enzyme during E-selectin-mediated cell adhesion. J Biol Chem 2013; 288:1620 - 33; http://dx.doi.org/10.1074/jbc.M112.400929; PMID: 23192350
- McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest 1997; 100:Suppl S97 - 103; http://dx.doi.org/10.1172/JCI119556; PMID: 9413410
- Moore KL, Patel KD, Bruehl RE, Li F, Johnson DA, Lichenstein HS, et al. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol 1995; 128:661 - 71; http://dx.doi.org/10.1083/jcb.128.4.661; PMID: 7532174
- Tenno M, Ohtsubo K, Hagen FK, Ditto D, Zarbock A, Schaerli P, et al. Initiation of protein O glycosylation by the polypeptide GalNAcT-1 in vascular biology and humoral immunity. Mol Cell Biol 2007; 27:8783 - 96; http://dx.doi.org/10.1128/MCB.01204-07; PMID: 17923703
- Ellies LG, Tsuboi S, Petryniak B, Lowe JB, Fukuda M, Marth JD. Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity 1998; 9:881 - 90; http://dx.doi.org/10.1016/S1074-7613(00)80653-6; PMID: 9881978
- Asano M, Nakae S, Kotani N, Shirafuji N, Nambu A, Hashimoto N, et al. Impaired selectin-ligand biosynthesis and reduced inflammatory responses in β-1,4-galactosyltransferase-I-deficient mice. Blood 2003; 102:1678 - 85; http://dx.doi.org/10.1182/blood-2003-03-0836; PMID: 12714507
- Ellies LG, Sperandio M, Underhill GH, Yousif J, Smith M, Priatel JJ, et al. Sialyltransferase specificity in selectin ligand formation. Blood 2002; 100:3618 - 25; http://dx.doi.org/10.1182/blood-2002-04-1007; PMID: 12393657
- Yang WH, Nussbaum C, Grewal PK, Marth JD, Sperandio M. Coordinated roles of ST3Gal-VI and ST3Gal-IV sialyltransferases in the synthesis of selectin ligands. Blood 2012; 120:1015 - 26; http://dx.doi.org/10.1182/blood-2012-04-424366; PMID: 22700726
- Malý P, Thall AD, Petryniak B, Rogers CE, Smith PL, Marks RM, et al. The α(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 1996; 86:643 - 53; http://dx.doi.org/10.1016/S0092-8674(00)80137-3; PMID: 8752218
- Weninger W, Ulfman LH, Cheng G, Souchkova N, Quackenbush EJ, Lowe JB, et al. Specialized contributions by alpha(1,3)-fucosyltransferase-IV and FucT-VII during leukocyte rolling in dermal microvessels. Immunity 2000; 12:665 - 76; http://dx.doi.org/10.1016/S1074-7613(00)80217-4; PMID: 10894166
- Sako D, Chang XJ, Barone KM, Vachino G, White HM, Shaw G, et al. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell 1993; 75:1179 - 86; http://dx.doi.org/10.1016/0092-8674(93)90327-M; PMID: 7505206
- Wilkins PP, McEver RP, Cummings RD. Structures of the O-glycans on P-selectin glycoprotein ligand-1 from HL-60 cells. J Biol Chem 1996; 271:18732 - 42; http://dx.doi.org/10.1074/jbc.271.31.18732; PMID: 8702529
- Marathe DD, Buffone A Jr., Chandrasekaran EV, Xue J, Locke RD, Nasirikenari M, et al. Fluorinated per-acetylated GalNAc metabolically alters glycan structures on leukocyte PSGL-1 and reduces cell binding to selectins. Blood 2010; 115:1303 - 12; http://dx.doi.org/10.1182/blood-2009-07-231480; PMID: 19996411
- Marathe DD, Chandrasekaran EV, Lau JTY, Matta KL, Neelamegham S. Systems-level studies of glycosyltransferase gene expression and enzyme activity that are associated with the selectin binding function of human leukocytes. FASEB J 2008; 22:4154 - 67; http://dx.doi.org/10.1096/fj.07-104257; PMID: 18716032
- Wagers AJ, Stoolman LM, Kannagi R, Craig R, Kansas GS. Expression of leukocyte fucosyltransferases regulates binding to E-selectin: relationship to previously implicated carbohydrate epitopes. J Immunol 1997; 159:1917 - 29; PMID: 9257857
- Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity 2007; 26:477 - 89; http://dx.doi.org/10.1016/j.immuni.2007.03.011; PMID: 17442598
- Yago T, Fu J, McDaniel JM, Miner JJ, McEver RP, Xia L. Core 1-derived O-glycans are essential E-selectin ligands on neutrophils. Proc Natl Acad Sci U S A 2010; 107:9204 - 9; http://dx.doi.org/10.1073/pnas.1003110107; PMID: 20439727
- Matsumoto M, Atarashi K, Umemoto E, Furukawa Y, Shigeta A, Miyasaka M, et al. CD43 functions as a ligand for E-Selectin on activated T cells. J Immunol 2005; 175:8042 - 50; PMID: 16339541
- Stockton BM, Cheng G, Manjunath N, Ardman B, von Andrian UH. Negative regulation of T cell homing by CD43. Immunity 1998; 8:373 - 81; http://dx.doi.org/10.1016/S1074-7613(00)80542-7; PMID: 9529154
- Larsen GR, Sako D, Ahern TJ, Shaffer M, Erban J, Sajer SA, et al. P-selectin and E-selectin. Distinct but overlapping leukocyte ligand specificities. J Biol Chem 1992; 267:11104 - 10; PMID: 1375936
- Kobzdej MM, Leppänen A, Ramachandran V, Cummings RD, McEver RP. Discordant expression of selectin ligands and sialyl Lewis x-related epitopes on murine myeloid cells. Blood 2002; 100:4485 - 94; http://dx.doi.org/10.1182/blood-2002-06-1799; PMID: 12393554
- Nimrichter L, Burdick MM, Aoki K, Laroy W, Fierro MA, Hudson SA, et al. E-selectin receptors on human leukocytes. Blood 2008; 112:3744 - 52; http://dx.doi.org/10.1182/blood-2008-04-149641; PMID: 18579791
- Handa K, Stroud MR, Hakomori S-i. Sialosyl-fucosyl Poly-LacNAc without the sialosyl-Lex epitope as the physiological myeloid cell ligand in E-selectin-dependent adhesion: studies under static and dynamic flow conditions. Biochemistry 1997; 36:12412 - 20; http://dx.doi.org/10.1021/bi971181t; PMID: 9376344
- Picker LJ, Warnock RA, Burns AR, Doerschuk CM, Berg EL, Butcher EC. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell 1991; 66:921 - 33; http://dx.doi.org/10.1016/0092-8674(91)90438-5; PMID: 1716182
- Levinovitz A, Mühlhoff J, Isenmann S, Vestweber D. Identification of a glycoprotein ligand for E-selectin on mouse myeloid cells. J Cell Biol 1993; 121:449 - 59; http://dx.doi.org/10.1083/jcb.121.2.449; PMID: 7682218
- Merzaban JS, Burdick MM, Gadhoum SZ, Dagia NM, Chu JT, Fuhlbrigge RC, et al. Analysis of glycoprotein E-selectin ligands on human and mouse marrow cells enriched for hematopoietic stem/progenitor cells. Blood 2011; 118:1774 - 83; http://dx.doi.org/10.1182/blood-2010-11-320705; PMID: 21659548
- Nakayama F, Nishihara S, Iwasaki H, Kudo T, Okubo R, Kaneko M, et al. CD15 expression in mature granulocytes is determined by α 1,3-fucosyltransferase IX, but in promyelocytes and monocytes by α 1,3-fucosyltransferase IV. J Biol Chem 2001; 276:16100 - 6; http://dx.doi.org/10.1074/jbc.M007272200; PMID: 11278338