Abstract
Mutations in genes encoding several basal lamina components as well as their cellular receptors disrupt normal deposition and remodeling of the cortical basement membrane resulting in a disorganized cerebral and cerebellar cortex. The α6 integrin was the first α subunit associated with cortical lamination defects and formation of neural ectopias. In order to understand the precise role of α6 integrin in the central nervous system (CNS), we have generated mutant mice carrying specific deletion of α6 integrin in neuronal and glia precursors by crossing α6 conditional knockout mice with Nestin-Cre line. Cerebral cortex development occurred properly in the resulting α6fl/fl;nestin-Cre mutant animals. Interestingly, however, cerebellum displayed foliation pattern defects although granule cell (GC) proliferation and migration were not affected. Intriguingly, analysis of Bergmann glial (BG) scaffold revealed abnormalities in fibers morphology associated with reduced processes outgrowth and altered actin cytoskeleton. Overall, these data show that α6 integrin receptors are required in BG cells to provide a proper fissure formation during cerebellum morphogenesis.
Introduction
The development of mammalian cortical structures in the central nervous system (CNS) is a highly regulated process in which the radial glial cells provide an instructive scaffold for the migration and placement of neurons.Citation1 In the developing cerebral cortex, neurons are generated in the proliferative ventricular zones and migrate in distinct radial and tangential routes to the top of the embryonic cortex where they terminate their migration and start to differentiate into distinct classes of cortical neurons.Citation2 Similar migration events occur during cerebellar cortex development, which involves three principal cell types, Purkinje cells (PCs), granule cells (GCs), and Bergmann glial cells (BGs). At early embryonic stages, the granule cell precursors (GCPs) move across the surface of the developing cerebellum and form a new germinal zone, the external granular layer (EGL). In the postnatal period, the post-mitotic granule cells migrate radially along the Bergmann glial fibers through the Purkinje cell layer (PCL) to generate the internal granular layer (IGL).Citation3
Dynamic regulation of cell–extracellular matrix (ECM) and cell–cell interactions enable appropriate neuronal generation, migration and differentiation in the developing nervous system.Citation4 The integrin receptor family contributes to these interactions. Integrins are heterodimeric transmembrane glycoproteins, composed of α and β subunits, which bind different extracellular ligands.Citation5 They regulate intracellular signaling by association with adaptor molecules such as FAK (focal adhesion kinase) or ILK (integrin-linked kinase).Citation6
The α6 integrin chain can associate with the β1 or β4 subunits to form laminin receptors. In situ hybridization analyses have demonstrated that α6 subunit is present in the developing CNS mostly associated with β1, and its expression is maintained in the adult stages in the brain.Citation7
Previous studies have shown that complete deletion of α6 integrin subunit induced lamination defects in the developing cerebral cortex, associated with local disruptions of the basement membrane (BM) and consequent formation of neuronal ectopias.Citation7 The defects in neuronal migration arose close to the cortical marginal zone suggesting that the primary phenotype is due to altered BM rather than a cell-autonomous defect in neuronal migration.Citation8 Similar defects have been described with the conditional deletion of β1 integrin subunit or FAK or ILK in the neural progenitors, showing that radial glial fibers play a fundamental role in BM assembly.Citation9-Citation11 These cortical phenotypes strongly resemble those found in mice carrying mutations in the laminin α2 chain gene, in mice with a targeted deletion of the nidogen-binding site of laminin γ1 and in mice with altered glycosylation of the laminin receptor dystroglycan.Citation12-Citation14 Taken together these findings provided evidence that the signaling pathways triggered by laminin binding are essential for BM integrity and necessary for a correct cortical lamination.
Furthermore, recent studies have demonstrated that ablation of β1-class integrins or ILK in the neural progenitors induced defects in cerebellar foliation pattern associated with abnormal glial network and alteration in GCPs proliferation.Citation10,Citation15-Citation17 In addition, Watanabe et al.Citation18 have demonstrated that FAK is involved in positioning of Bergmann glial cells and dendritic innervation of Purkinje cells. Overall, these results suggest that integrin signaling is required for a proper cerebellar development.
In order to clarify the role of α6 integrin in nervous system development, we decided to inactivate α6 integrin specifically in the neural precursors using the Cre/LoxP technology. We have recently generated mice carrying a floxed α6 allele (α6fl/fl) (De Arcangelis et al., manuscript in preparation). In this study, we crossed these mice with mice expressing Cre recombinase under the control of the neuron specific enhancer of the nestin promoterCitation19 in order to delete integrin α6 in precursors of neurons and glia.Citation20
Results
To investigate the role of α6 integrin subunit in the CNS, we inactivated its expression by Cre/Lox-mediated gene deletion. For this purpose, we crossed a mouse line carrying a α6-floxed allele (α6fl) (De Arcangelis et al., manuscript in preparation) with nestin-Cre transgenic mice carrying the Cre gene under the control of nestin promoter, which has been previously shown to be active around E10.5 and efficiently induces Cre mediated gene ablation.Citation20
The α6fl/fl;nestin-Cre mice were viable and fertile. To confirm that the α6 gene was inactivated in the nervous system of the α6fl/fl;nestin-Cre mutant mice, we monitored recombination of the α6 allele at the DNA level. PCR analyses were performed with DNA obtained from neural tissue of control and mutant mice at postnatal day 0 (P0). In the wild-type animals expressing Cre recombinase, we did not detect a DNA recombination whereas the α6fl/fl;nestin-Cre animals showed an efficient DNA recombination (). Moreover, immunoblot analysis of brain or cerebellum extracts from control and mutant mice indicated a reduction of α6 protein level in the mutant samples (). The presence of low amounts of α6 protein in the extracts from dissected neural tissue from mutant animals was expected, since α6 integrin is likely to be expressed in meningeal cells and blood vessels, where Cre is not active ().
Figure 1. Specific deletion of α6 integrin subunit in mouse nervous system. (A) DNA was obtained from brain of control and mutant mice at P0 and analyzed by PCR for Cre-mediated recombination. The 154 bp band corresponds to the α6-floxed allele (fl) and the 120 bp band to the wild type allele (+). A 1 kb band, indicating the α6-deleted allele, was present only in the α6fl/fl;nestin-Cre brain. Primers against Cre were used to confirm the presence of nestin-Cre transgene. (B) Western blot analysis for α6 integrin in P7 brains and cerebellum. The α6 protein was detected in control mice whereas, in the α6fl/fl;nestin-Cre mice where it was expressed in very low amounts. GAPDH was used as a loading control. (C) P17 telencephalon coronal and P21 cerebellum sagittal sections of control and α6fl/fl;nestin-Cre mice stained with hematoxylin and eosin. The arrows indicate a partial defect in the formation of fissures between lobules I/II-III and VI-VII in the α6fl/fl;nestin-Cre mice. St, striatum; Se, septum; NCx, neocortex. Scale bar, 200 μm.
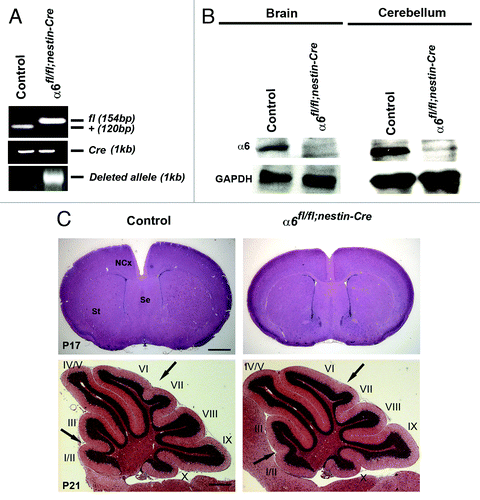
To determine the effect of α6 integrin ablation on the CNS histoarchitecture, we examined coronal histological sections of mutant brain at P17 and cerebellum at P21. The telencephalon appeared unaffected and did not display major morphological abnormalities (). To confirm the absence of lamination defects, immunostaining for layer-specific markers,Citation21,Citation22 Cdp/Cux for layer II-IV and Ctip2 for layer V were performed on P21 control and mutant brain sections. For both markers, the distribution of labeled cells was comparable in control and mutant brains as shown in Figure S1. Thus, the deletion of α6 integrin in neural progenitors, neurons and glial cells, did not induce obvious cortical lamination defects.
By contrast, we observed mild foliation pattern abnormalities in the mutant cerebellum. Indeed, the cerebellar fissures between lobules I/II and III and VI and VII were absent (). These morphological variations at P21 were present in seven mutant cerebella out of nine mutant samples.
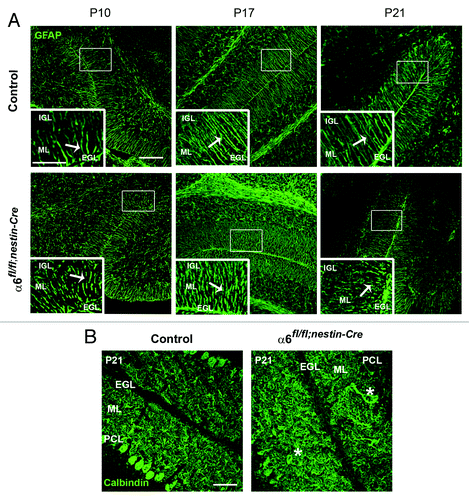
By in situ hybridization, we found that the mRNA of α6 integrin was specifically detected in the PCL, where are located the Purkinje and Bergmann glial cell bodies (). This prompted us to analyze these cell populations in the mutant cerebellum. Therefore, we performed immunostaining for GFAP and calbindin, which are markers for BGs and PCs respectively. We have focused our analyses at the following critical developmental stages: at P10 when active GC proliferation occurs, at P17, when GC proliferation and migration decline and at P21, when the histogenetic processes in the cerebellar trilaminar structure are terminated. In control mice, BG fibers spanned the molecular layer (ML) and their endfeet were anchored at the meningeal basement membrane. By contrast, the glial fibers in α6fl/fl;nestin-Cre mice appeared abnormally projected, and showed an abnormal morphology. These glial fiber defects were particularly observed in portions of mutant lobules IV/V, VI, IX, and X at P10 (n = 3), P17 (n = 4) and P21 (n = 5) with various degrees of severity (). All together these findings suggested that α6 integrin is required for a proper BG scaffold organization while being dispensable for the basement membrane integrity maintenance during cerebellum development. We further characterized the PCL organization. In both control and mutant mice, PCs formed a continuous layer and extended well-arborized dendrites into the ML. We occasionally observed a misplacement of PC bodies in some restricted areas of α6fl/fl;nestin-Cre cerebellum at P21 (n = 7) (), suggesting a role for α6 integrin in the proper localization of the PCs.
Figure 2. Expression of the integrin α6 subunit mRNA in the P10 mouse cerebellum. (A) In situ hybridization analysis of the α6 integrin mRNA on sagittal sections of P10 mouse cerebellum. The α6 mRNA was present in the PCL. Scale bar, 100 µm. (B) High magnification view of the boxed area in (A) showed a strong expression in the cell bodies of Purkinje cells (arrows). Scale bar, 40 µm. EGL, external granular layer; ML, molecular layer; PCL, Purkinje cellular layer; IGL, internal granular layer.
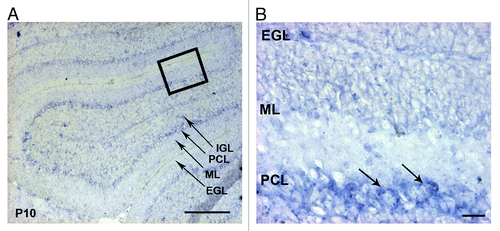
Figure 3. Defects in Bergmann glial fibers morphology and Purkinje cell position. (A) Staining with anti-GFAP antibody on sagittal sections from P10, P17, and P21 control and α6fl/fl;nestin-Cre cerebellum to reveal Bergmann glial fibers. In the control samples the glial processes normally extended through the ML and branched to the basement membrane. In contrast, the glial fibers lacking α6 integrin appeared irregular with abnormal extensions. Glial fiber morphology is shown in each panel in high magnification views of the corresponding boxed area. The white arrows indicate the glial fibers. Scale bars, 100 μm; 20 μm. (B) P21 sagittal cerebellar sections stained with an anti-calbindin antibody to visualize Purkinje cells. In the control mice, Purkinje cells were aligned along the PCL, whereas in the α6fl/fl;nestin-Cre mice, some Purkinje cell bodies were ectopically localized in the ML. The asterisks indicate the mispositioned Purkinje cell in the mutant cerebellum. Scale bar, 50 μm. EGL, external granular layer; ML, molecular layer; PCL, Purkinje cellular layer; IGL, internal granular layer.
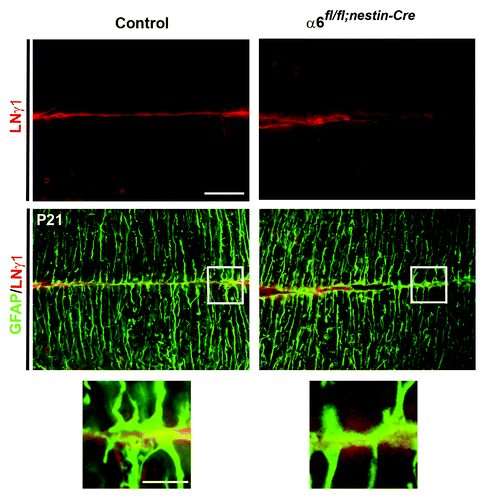
Several studies suggested that integrin signaling regulates the interactions between radial glial fibers and meningeal cells during the formation and maintenance of basement membrane.Citation7,Citation9-Citation11,Citation13 To investigate whether the loss of α6 integrin could affect the attachment of glial fibers to the basement membrane with a consequent defect in basement membrane assembly, we performed a double immunostaining for GFAP and laminin γ1 chain on coronal sections of P21 control and mutant cerebellum. However, in both genotypes, we observed a general correct formation of glial endfeets and absence of obvious basement membrane defects (). These findings suggested that the α6 integrin-deficient Bergmann glial fibers are still able to functionally interact with components of the basement membrane during cerebellum development.
Figure 4. Analyses of glial endfeet formation and basement membrane structure. P21 sagittal sections from control and mutant cerebellum were double stained for GFAP (green) and laminin γ1 (red). Higher magnification view of the areas, indicated by the white square, did not show gross defects in the attachment of glial fibers to the basement membrane in the mutant mice compared with the control sample.
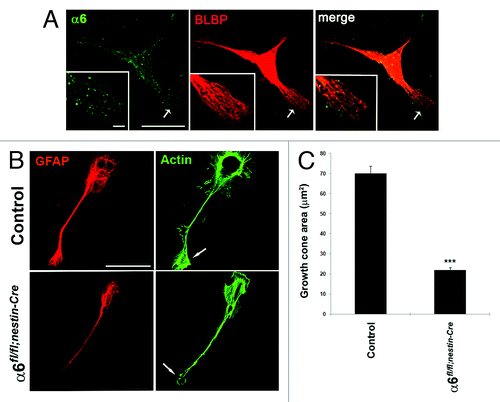
To better characterize the role of α6 integrin in BG fibers morphology, we analyzed the behavior of BG cells on primary cultures. In wild-type glial cells, positive for the glial marker BLBP (brain lipid binding protein), α6 integrin subunit is distributed throughout the plasma membrane both in the cell bodies and the glial processes (). As reported by Belvindrah et al.,Citation15 primary cerebellar glial cells rapidly attached to laminin (LN) substrates and initiated glial process outgrowth with concentration of actin in cell membrane protrusions. Glial processes contained a network of GFAP positive filaments that extended into glial growth cones. In our experiments, cerebellar glial cells obtained from P7 control or mutant cerebellum were cultured on an EHS-LN (Engelbreth-Holm-Swarm) substrate, a major ligand of α6 receptors. Interestingly, in contrast to control conditions where glial cells grew extensive processes on EHS-LN, with actin positive filaments that accumulated into glial growth cones, α6-deficient glial cells extended smaller processes displaying altered growth cones with reduced actin accumulation (). A quantitative analysis confirmed that the absence of α6 integrin leads to a dramatic reduction in size of the glial growth cone (). These data indicate that α6 integrin is involved in BG fibers extension acting at level of actin remodeling.
Figure 5. Absence of α6 integrin subunit impairs process outgrowth in Bergmann glial growth cones. (A and B) Primary cerebellar glial cells were plated onto LN/Poly-D-Lysine substrates and analyzed by immunostaining. (A) Detection of α6 integrin subunit (green) and the glial marker BLBP (red).The α6 integrin subunit was expressed throughout glial fibers and located at the tip of glial processes. Insets represent enlargements of the glial process (arrow). Scale bars, 20 μm, 2.4 μm. (B) Immunostaining of GFAP (red) and actin (visualized by staining with phalloidin) (green). Control cells showed actin accumulation at level of the membrane ruffles and the tips of growth cone. By contrast, in α6-deficient glial cells the cell periphery and the leading edge of glial growth cone contained reduced actin amounts. The arrows indicate sites of actin accumulation in the glial cells. Scale bar, 20 μm. (C) Quantification of growth cone area measurements in control and α6fl/fl;nestin-Cre glial cells (n = 10, Student t test ***P < 0.001).
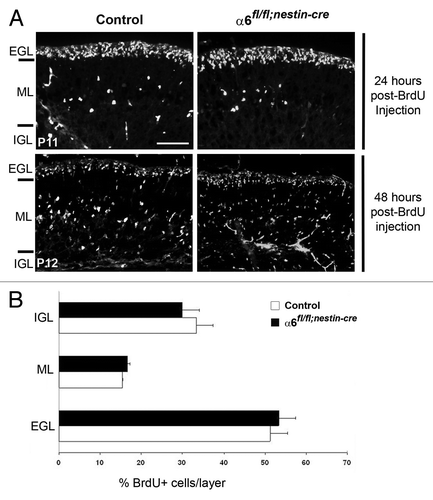
At postnatal stages, during formation of the cerebellar cortex, the post-mitotic granule cells migrate radially along the Bergmann glial processes from the EGL, through the ML, to reach the IGL. Therefore, a proper interaction between the granule cells and the glial fibers is essential for a correct formation of the cerebellar layers. Since we observed morphological abnormalities of the Bergmann glial processes in the absence of α6 integrin, we examined whether these defects could alter radial granule cell migration. For this purpose, we labeled a cohort of granule cells born at P10 with a pulse of BrdU and followed migration of these cells over the next 2 d. Twenty-four hours post-labeling, the majority of BrdU-positive granule cells had not yet begun to migrate and remained in the EGL of both control and α6fl/fl;nestin-Cre mice (). By contrast, 48 h post-labeling, granule cells had extensively migrated from the EGL to the IGL. We quantified the distribution of BrdU-positive cells in the three different layers, at the level of lobules IV/V, VI, IX, and X, where the morphological defects of Bergmann glial processes were observed (). The quantification analysis revealed that the percentage of migrating granular cells in the three layers was not significantly different between control (n = 2; EGL: 51.2 ± 4.2%; ML: 15.3 ± 0.12%; IGL: 33.5 ± 4.1%) and α6fl/fl;nestin-Cre mice (n = 3; EGL: 53.4 ± 4%; ML: 16.6 ± 0.5%; IGL: 30 ± 4.2%) (). Thus, radial granule cell migration is not significantly affected in α6 integrin mutant mice.
Figure 6. Granule cell migration occurred normally in the α6fl/fl;nestin-Cre mice. (A) Dividing granule cells in P10 control and α6fl/fl;nestin-Cre littermates were labeled by systemic injection of BrdU. Cerebellar tissue was then collected and processed for BrdU immunohistochemistry at the indicated post-injection times. Twenty-four hours post-BrdU labeling, the granule migration had not yet begun. Forty-eight hours post-BrdU injection the BrdU-positive cells were migrating from the EGL, through the ML, to reach the IGL. Scale bar, 40 µm. (B) Laminar distribution of migrating granular cells in cerebellum from P12 control and α6fl/fl;nestin-Cre mice, 48 h after BrdU injection. Four sections were counted per animal (2 controls and 3 mutants). The percentage of BrdU-labeled cells in each layer was not significantly different in control compared with mutant mice. Error bars indicate SD. Scale bar, 50 μm. EGL, external granular layer; ML, molecular layer; IGL, internal granular layer.
Previous studies using in vivo approaches have shown that the integrin pathway regulates granule cell precursors (GCPs) proliferation in the developing mouse cerebellum.Citation16,Citation17 To verify whether the loss of α6 integrin could also affect the postnatal GCPs proliferation, we measured the number of BrdU-positive cells in the EGL of control (n = 3) and α6fl/fl;nestin-Cre (n = 3) cerebella at P10. BrdU pulse labeling did not reveal significant differences in GCP proliferation between control and mutant mice in the three analyzed regions (Fig. S2). These data indicate that the absence of α6 integrin had not altered GCP proliferation.
Discussion
Total ablation of α6 integrin altered cortical lamination, causing gaps in the basement membrane where neurons invade the cortical marginal zone. Importantly, these migratory defects are most pronounced close to the marginal zone suggesting that they are in large part a secondary consequence of perturbed basement membrane. Thus, we assume that α6 integrin could be involved in basement membrane assembly, regulating interaction between glial fibers and meningeal cells. In agreement with this hypothesis, specific deletion of β1-class integrins in cortical migrating neurons did not perturb radial migration, providing evidence that α6β1 laminin receptor may be not essential in migrating neurons for glia-guided migration.Citation23 In the current study, we have selectively inactived the integrin α6 gene in the precursors of neurons and glia by the Cre-LoxP techonology. The mutant mice did not display major abnormalities in the laminar organization of the cerebral cortex. One possible explanation could be that the expression of other laminin receptors, such as α3β1 integrin, could compensate the absence of α6 integrin in the glial cells, restoring the interactions between glial processes and pial surface. This compensation effect may not be present in the total knockout for α6 integrin where α6 integrin is deleted in both glial and meningeal cells. In this regard, the complete absence of β1-class integrins in the neural progenitors is sufficient to induce neuronal ectopias suggesting that integrin receptors in the glial cells are necessary for the interactions with the basement membrane.Citation10
Furthermore, no defects in meningeal basement membranes in the forebrain were observed also in mice where Fak or Ilk genes were inactivated after crosses with nestin-Cre mice.Citation15,Citation19 However, basement membrane defects in the forebrain have been reported for mice where Fak or Ilk genes were inactived with Emx1-Cre.Citation9,Citation11 It is at present unclear why different Cre lines give different results. The Emx-1 promoter is active in neuroepithelial precursors of developing neocortex and hippocampus at E9 before initiation of cortical neurogenesis.Citation24 By contrast, as mentioned in the results, the nestin promoter begins to be active around E10.5.Citation19 Alternatively, the two different lines may express Cre recombinase in different cell types. Indeed, in nestin-Cre mice, the Cre is active in all pyramidal neurons and inhibitory interneurons, while in Emx1-Cre mice, Cre is only active in cortical pyramidal neurons and not in interneurons.Citation25 Therefore, it is possible that the two different Cre lines, depending of timing and cell-specificity of Cre-mediated recombination, allow or not compensatory mechanisms. Further analysis using Emx1-Cre or other Cre lines to induce selective deletion of α6 integrin in the neural progenitors or specific inactivation of α6 integrin in the meningeal cells could address the role of α6β1 laminin receptors during cerebral cortex development.
Interestingly, we observed mild defects in the cerebellar foliation of α6fl/fl;nestin-Cre mice, with reduction of the fissure separating lobules I/II and III and VI and VII. In this context, Sudarov et al.Citation26 proposed a mechanism to explain the generation of fissure during embryonic cerebellar foliation. Their studies indicated that the initiation of cerebellar fissures is characterized by the formation of multi-cellular anchoring centers, containing granule cells, Purkinje cells, and Bergmann glial cells. Indeed, folding of the Purkinje cell layer, proliferation of granule cell precursors, and orientation of BG fibers are the three fundamental processes required for anchoring centers organization. Therefore, the absence of α6 integrin may affect coordinated actions of all these three cellular components during embryonic stages leading to foliation pattern defects in the adult cerebellum. In agreement with this hypothesis, we detected abnormalities in BG fibers morphology and displacement of Purkinje cell bodies in α6fl/fl;nestin-Cre adult cerebellum. Alterations in BG processes were confirmed by an in vitro approach, showing that absence of α6 integrin in the glial cells impaired concentration of actin filaments at level of the leading edge of glial growth cones. However, α6 integrin is dispensable for GC proliferation and migration supporting previous findings, which demonstrated that β1 integrins are required in BG but not in GC for proper cerebellar development.Citation27
Several in vitro and in vivo studies demonstrated that signaling triggered by laminin binding is required during cerebellar development.Citation28,Citation29 It is known that at least five integrin receptors are able to bind laminin, α1β1, α2β1, α3β1, α6β1, and α7β1.Citation30 In particular, in the cerebellum the laminin receptors α6β1 and α3β1 are expressed in the PCL whereas the receptor α7β1 is mostly expressed in the EGL.Citation31 This redundancy could explain the mild phenotype observed in the α6fl/fl;nestin-Cre cerebellum. Interestingly, double mutant mice carrying null mutation for α7 integrin and specific deletion of α6 integrin in the nervous system (α7−/−; α6fl/fl;nestin-Cre) did not show increase of cerebellar defects (data not shown). This observation suggests that α3β1 receptor might be the good candidate for playing a compensatory role in the α6fl/fl;nestin-Cre cerebellum. It will be therefore interesting in the future to generate mouse model carrying double mutations for α6-α3 integrins specifically in the cerebellum to reduce the compensatory mechanisms and clarify the laminin receptors functions during cerebellum development.
Materials and Methods
Mice
The α6fl/fl mouse line will be described elsewhere (De Arcangelis et al., manuscript in preparation). The nestin-Cre mice were provided by Dr Ryoichiro Kageyama (Institute for Virus Research, Kyoto University).Citation20 The animals used as control were α6fl/fl or α6fl/+;nestin-Cre. All animal experimentations were approved by regional ethical committee for animal experimentation (CREMEAS) and in accordance with French regulations.
Antibodies
Primary antibodies used in this study were against integrin α6 (rat,1:50, PharMingen), bromodeoxyuridine (BrdU) (mouse, 1:100, Boehringer), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (mouse, 1:300, Chemicon), CCAAT displacement protein (CDP) (rabbit, 1:50, Santa Cruz Biotechnology), chicken ovalbumin upstream promoter transcription factor-interacting proteins 2 (Ctip2) (rat, 1:500, Abcam), glial fibrillary acidic protein (GFAP) (rabbit, 1:200, DAKO), brain lipid-binding protein (BLBP) (rabbit,1:500, Chemicon), Calbindin D-28k (rabbit, 1:200, Swant), laminin γ1 chain (rat, 1:100, Chemicon). Secondary antibodies used for immunochemistry or in western blotting analyses were Alexa fluor 488 goat anti-rabbit IgG, Alexa 594 fluor goat anti-mouse IgG, Alexa fluor 488 phalloidin (Molecular Probes), HRP goat anti-mouse, and anti-rat (Jackson ImmunoResearch Laboratories).
Western blot analysis
The cerebellum from P7 mice were lysed in RIPA buffer (150 mM NaCl, 10 mM TRIS-HCl pH 7.4, 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate, 5mM EDTA and a protease inhibitor cocktail [Roche]). The lysates were cleared by centrifugation at 13 000 rpm for 15 min at 4 °C. The proteins were separated on 8% SDS-PAGE and transferred to nitrocellulose membranes (Schleicher and Schuell) for western blotting.
Histology and immunohistochemistry
Post-natal mice were perfused using 4% paraformaldehyde. Brains and cerebella were either cryoprotected by 20% sucrose /phosphate buffer saline (PBS), frozen over dry ice and sectioned on a cryostat (10 µm), or embedded in paraffin and sectioned on a microtome (7 µm). Sections were stained with hematoxylin-eosin, or processed for immunostaining using standard procedures. Nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI) (Molecular Probes). All images were captured using Macroconfocal LSI (Leica-microsystems), a laser scanning confocal microscope (Leica) with a 40×/1.25 or 63×/1.40 oil-immersion objective or a Leica DMRBP microscope.
Cerebellar cell cultures
Primary cultures of cerebellar glial cells were performed as described in Belvindrah et al.Citation15 Experiments were performed two times in duplicate. Image J software was used to measure the growth cone areas by framing the actin-positive extension using the freehand modus. Values for each condition were pooled and a Student t test performed.
In vivo BrdU labeling and quantitative analysis
Mice were injected intraperitoneally with 5-Bromodeoxyuridine (BrdU, Boehringer, 50 mg/kg body weight) and were sacrificed at the indicated times. In migration experiments the number of labeled cells in each cortical layer was counted in four non-adjacent sagittal sections on selected regions in the lobules IV/V, VI, IX, and X of control (n = 3) and mutant mice (n = 3). In proliferation assay, BrdU-labeled cells and DAPI-positive cells were assessed in four non-adjacent sagittal sections on selected regions of the EGL of control (n = 2) and mutant mice (n = 3). The rate of proliferation was expressed as the percentage of BrdU-labeled granule cells per total number of DAPI-positive cells in the EGL. Statistical analysis was performed using the Student t test, with a P value of < 0.05 being considered statistically significant.
In situ hybridization
Frozen sections were processed for in situ hybridization with digoxigenin-labeled antisense probes as described in Dupé et al.Citation32 A 500 bp fragment of α6 integrin cDNA was used as probe.Citation7
| Abbreviations: | ||
| BG | = | Bergmann glia |
| BLBP | = | brain lipid binding protein |
| BM | = | basement membrane |
| BrdU | = | bromodeoxyuridine |
| Cdp/Cux | = | CCAAT displacement protein |
| CNS | = | central nervous system |
| Ctip2 | = | chicken ovalbumin upstream promoter transcription factor-interacting proteins 2 |
| DAPI | = | 4',6-diamidino-2-phenylindole |
| ECM | = | extracellular matrix |
| EGL | = | external granular layer |
| EHS-LN | = | Engelbreth-Holm-Swarm-Laminin |
| FAK | = | focal adhesion kinase |
| GAPDH | = | glyceraldehyde 3-phosphate dehydrogenase |
| GC | = | granular cells |
| GCP | = | granular cell progenitors |
| GFAP | = | glial fibrillary acidic protein |
| IGL | = | internal granular layer |
| ILK | = | integrin linked kinase |
| ML | = | molecular layer |
| LN | = | laminin |
| PC | = | Purkinje cells |
| PCL | = | Purkinje cell layer |
Additional material
Download Zip (2.7 MB)Acknowledgments
We thank the IGBMC animal facility staff for their help and M. Koch for his help in confocal imaging. We are grateful to Dr U. Mayer and Dr R. Kageyama who made available to us the α7−/− and Nestin-Cre mice respectively. We are grateful to M. Labouesse for reading of the manuscript. This work was supported by funds from the CNRS, Inserm, and Université de Strasbourg, as well as Association pour la recherche sur le cancer (ARC), Ligue régionale contre le cancer (LRC), and Association française contre les myopathies (AFM). G.M. was supported by doctoral fellowships from ARC and LRC.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Rakic P. Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex 2003; 13:541 - 9; http://dx.doi.org/10.1093/cercor/13.6.541; PMID: 12764027
- Nadarajah B, Alifragis P, Wong RO, Parnavelas JG. Neuronal migration in the developing cerebral cortex: observations based on real-time imaging. Cereb Cortex 2003; 13:607 - 11; http://dx.doi.org/10.1093/cercor/13.6.607; PMID: 12764035
- Hatten ME. Central nervous system neuronal migration. Annu Rev Neurosci 1999; 22:511 - 39; http://dx.doi.org/10.1146/annurev.neuro.22.1.511; PMID: 10202547
- De Arcangelis A, Georges-Labouesse E. Integrin and ECM functions: roles in vertebrate development. Trends Genet 2000; 16:389 - 95; http://dx.doi.org/10.1016/S0168-9525(00)02074-6; PMID: 10973067
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002; 110:673 - 87; http://dx.doi.org/10.1016/S0092-8674(02)00971-6; PMID: 12297042
- Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci 2009; 122:159 - 63; http://dx.doi.org/10.1242/jcs.018093; PMID: 19118207
- Georges-Labouesse E, Mark M, Messaddeq N, Gansmüller A. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr Biol 1998; 8:983 - 6; http://dx.doi.org/10.1016/S0960-9822(98)70402-6; PMID: 9742403
- Haubst N, Georges-Labouesse E, De Arcangelis A, Mayer U, Götz M. Basement membrane attachment is dispensable for radial glial cell fate and for proliferation, but affects positioning of neuronal subtypes. Development 2006; 133:3245 - 54; http://dx.doi.org/10.1242/dev.02486; PMID: 16873583
- Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, et al. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron 2003; 40:501 - 14; http://dx.doi.org/10.1016/S0896-6273(03)00666-4; PMID: 14642275
- Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, et al. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron 2001; 31:367 - 79; http://dx.doi.org/10.1016/S0896-6273(01)00374-9; PMID: 11516395
- Niewmierzycka A, Mills J, St-Arnaud R, Dedhar S, Reichardt LF. Integrin-linked kinase deletion from mouse cortex results in cortical lamination defects resembling cobblestone lissencephaly. J Neurosci 2005; 25:7022 - 31; http://dx.doi.org/10.1523/JNEUROSCI.1695-05.2005; PMID: 16049178
- Costell M, Gustafsson E, Aszódi A, Mörgelin M, Bloch W, Hunziker E, et al. Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol 1999; 147:1109 - 22; http://dx.doi.org/10.1083/jcb.147.5.1109; PMID: 10579729
- Halfter W, Dong S, Yip YP, Willem M, Mayer U. A critical function of the pial basement membrane in cortical histogenesis. J Neurosci 2002; 22:6029 - 40; PMID: 12122064
- Takeda S, Kondo M, Sasaki J, Kurahashi H, Kano H, Arai K, et al. Fukutin is required for maintenance of muscle integrity, cortical histiogenesis and normal eye development. Hum Mol Genet 2003; 12:1449 - 59; http://dx.doi.org/10.1093/hmg/ddg153; PMID: 12783852
- Belvindrah R, Nalbant P, Ding S, Wu C, Bokoch GM, Müller U. Integrin-linked kinase regulates Bergmann glial differentiation during cerebellar development. Mol Cell Neurosci 2006; 33:109 - 25; http://dx.doi.org/10.1016/j.mcn.2006.06.013; PMID: 16914328
- Blaess S, Graus-Porta D, Belvindrah R, Radakovits R, Pons S, Littlewood-Evans A, et al. Beta1-integrins are critical for cerebellar granule cell precursor proliferation. J Neurosci 2004; 24:3402 - 12; http://dx.doi.org/10.1523/JNEUROSCI.5241-03.2004; PMID: 15056720
- Mills J, Niewmierzycka A, Oloumi A, Rico B, St-Arnaud R, Mackenzie IR, et al. Critical role of integrin-linked kinase in granule cell precursor proliferation and cerebellar development. J Neurosci 2006; 26:830 - 40; http://dx.doi.org/10.1523/JNEUROSCI.1852-05.2006; PMID: 16421303
- Watanabe F, Miyazaki T, Takeuchi T, Fukaya M, Nomura T, Noguchi S, et al. Effects of FAK ablation on cerebellar foliation, Bergmann glia positioning and climbing fiber territory on Purkinje cells. Eur J Neurosci 2008; 27:836 - 54; http://dx.doi.org/10.1111/j.1460-9568.2008.06069.x; PMID: 18279360
- Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, et al. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron 1994; 12:11 - 24; http://dx.doi.org/10.1016/0896-6273(94)90148-1; PMID: 8292356
- Isaka F, Ishibashi M, Taki W, Hashimoto N, Nakanishi S, Kageyama R. Ectopic expression of the bHLH gene Math1 disturbs neural development. Eur J Neurosci 1999; 11:2582 - 8; http://dx.doi.org/10.1046/j.1460-9568.1999.00699.x; PMID: 10383648
- Leid M, Ishmael JE, Avram D, Shepherd D, Fraulob V, Dollé P. CTIP1 and CTIP2 are differentially expressed during mouse embryogenesis. Gene Expr Patterns 2004; 4:733 - 9; http://dx.doi.org/10.1016/j.modgep.2004.03.009; PMID: 15465497
- Nieto M, Monuki ES, Tang H, Imitola J, Haubst N, Khoury SJ, et al. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J Comp Neurol 2004; 479:168 - 80; http://dx.doi.org/10.1002/cne.20322; PMID: 15452856
- Belvindrah R, Graus-Porta D, Goebbels S, Nave KA, Müller U. Beta1 integrins in radial glia but not in migrating neurons are essential for the formation of cell layers in the cerebral cortex. J Neurosci 2007; 27:13854 - 65; http://dx.doi.org/10.1523/JNEUROSCI.4494-07.2007; PMID: 18077697
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci 2002; 22:6309 - 14; PMID: 12151506
- Gu X, Li C, Wei W, Lo V, Gong S, Li SH, et al. Pathological cell-cell interactions elicited by a neuropathogenic form of mutant Huntingtin contribute to cortical pathogenesis in HD mice. Neuron 2005; 46:433 - 44; http://dx.doi.org/10.1016/j.neuron.2005.03.025; PMID: 15882643
- Sudarov A, Joyner AL. Cerebellum morphogenesis: the foliation pattern is orchestrated by multi-cellular anchoring centers. Neural Dev 2007; 2:26; http://dx.doi.org/10.1186/1749-8104-2-26; PMID: 18053187
- Frick A, Grammel D, Schmidt F, Pöschl J, Priller M, Pagella P, et al. Proper cerebellar development requires expression of β1-integrin in Bergmann glia, but not in granule neurons. Glia 2012; 60:820 - 32; http://dx.doi.org/10.1002/glia.22314; PMID: 22374686
- Heng C, Lefebvre O, Klein A, Edwards MM, Simon-Assmann P, Orend G, et al. Functional role of laminin α1 chain during cerebellum development. Cell Adh Migr 2011; 5:480 - 9; http://dx.doi.org/10.4161/cam.5.6.19191; PMID: 22274713
- Pons S, Trejo JL, Martínez-Morales JR, Martí E. Vitronectin regulates Sonic hedgehog activity during cerebellum development through CREB phosphorylation. Development 2001; 128:1481 - 92; PMID: 11290288
- Venstrom KA, Reichardt LF. Extracellular matrix. 2: Role of extracellular matrix molecules and their receptors in the nervous system. FASEB J 1993; 7:996 - 1003; PMID: 8370483
- Pinkstaff JK, Detterich J, Lynch G, Gall C. Integrin subunit gene expression is regionally differentiated in adult brain. J Neurosci 1999; 19:1541 - 56; PMID: 10024342
- Dupé V, Matt N, Garnier JM, Chambon P, Mark M, Ghyselinck NB. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc Natl Acad Sci U S A 2003; 100:14036 - 41; http://dx.doi.org/10.1073/pnas.2336223100; PMID: 14623956