Abstract
We previously reported that expression of CD43/leukosialin induces cell rounding and microvillus formation via inhibition of cell adhesion. Here, we found that CD34, a cell surface sialomucin and marker for hematopoietic progenitor cells, also inhibited cell adhesion and induced cell rounding and microvillus formation. Forced expression of CD34-induced cell rounding, microvillus formation, and phosphorylation of ezrin/radixin/moesin (ERM) proteins in HEK293T cells, while inhibiting integrin-mediated cell re-attachment. Furthermore, CD34+ blood cells and KG-1 cells, which express endogenous CD34 on their surface, were spherical in shape, surrounded by microvilli, and non-adherent to substrata. In addition, cleavage of O-sialomucin augmented integrin-mediated cell adhesion of KG-1 cells. These results suggest the involvement of CD34 in the inhibition of integrin-mediated cell adhesion and formation of the cell surface structure. The inhibitory function of CD34 in cell adhesion may affect cell shape organization via phosphorylation of ERM proteins. Cellular structures such as the spherical shape and microvilli of CD34+ cells may also contribute to regulation of cell adhesion.
Introduction
During studies of leukocyte sialomucin family members, we previously found that exogenous expression of CD43 induces cell rounding, microvillus formation, augments phosphorylation of ezrin/radixin/moesin (ERM) proteins, and inhibits integrin-mediated re-attachment of HEK293T cells.Citation1 CD43, also called leukosialin/sialophorin, belongs to the sialomucin family and is considered to be a potent inhibitory molecule of cell adhesion.Citation2,Citation3 The highly O-glycosylated extracellular domain of CD43 is largely responsible for this phenomena. However, cleavage of the O-glycosylated extracellular domain of CD43 only partially reverses the inhibition of cell re-adhesion by CD43, suggesting there is another regulatory mechanism involving CD43.
In addition to CD43, other cell surface sialomucins are expressed in leukocytes. One of these sialomucins, CD34 shows restricted expression in a small population of bone marrow cells as well as endothelial cells.Citation4,Citation5 The CD34+ cell population in bone marrow can fully reconstitute the hematopoietic system.Citation6,Citation7 Although CD34+ bone marrow cells are considered as hematopoietic progenitor cells (HPCs) rather than hematopoietic stem cells (HSCs) in adult mice,Citation8 improved hematopoietic engraftment has been clinically achieved using sorted CD34+ human progenitor cells.Citation7,Citation9 Thus, CD34 is a widely accepted marker for HPCs and HSCs.Citation4,Citation7,Citation9
Despite its significance as a marker, the molecular functions of CD34 have not been fully revealed. Because cd34-null mice survive and have all hematopoietic lineage cells,Citation10,Citation11 CD34 does not appear to be essential for the development of hematopoietic cells. However, cd34-null embryonic stem cells and cells from the hematopoietic organs of cd34-null mice exhibit a lower colony-forming ability than that of cells from wild-type mice.Citation10 This observation suggests the involvement of CD34 in the proliferation, differentiation, maturation, and/or adhesion of HPCs and HSCs. Furthermore, previous reports have demonstrated the involvement of CD34 in regulation of cell adhesion and mobilization. Bone marrow mast cells from cd34-null mice show higher homotypic adhesion than those from wild-type mice,Citation12 indicating an anti-adhesive function of CD34. Interestingly, cd34-null cells exhibit a significant defect in the contribution to peritoneal mast cells and reconstitution of bone marrow in competitive reconstitution assays.Citation12 These results suggest that the anti-adhesive function of CD34 is involved in the migration of mobilization of HSCs and HPCs or in fixation to the niche. On the other hand, ectopically expressed human CD34 increases murine hemopoietic cell adhesion to human bone marrow stromal cells,Citation13 suggesting that human CD34 functions in cell adhesion.
Structurally, CD34 is a single-pass type-I transmembrane glycoprotein.Citation4,Citation5,Citation14 Although the human cd34 gene encodes multiple open reading frames generated by alternative splicing, each CD34 isoform consists of an N-terminal mucin domain followed by a globular domain and stalk, single-pass transmembrane region, and cytoplasmic tail. The mucin domain of CD34 is highly O-glycosylated,Citation4,Citation14 and these O-glycans with many hydroxyl and carboxyl groups confer an extreme hydrophilicity and negative charge on the CD34 molecule. Such structural characteristics indicate potential functions of CD34 in the interactions with extracellular molecules or in the inhibition of these interactions.
We were interested in CD34 because (1) CD34 is a sialomucin, (2) CD34 appears to be involved in the regulation of cell adhesion, (3) CD34 localizes at the microvillus structure of endothelial cells,Citation15 and (4 podocalyxin, a CD34-related molecule, is a potent inducer of microvillus formation.Citation5,Citation16,Citation17 In this study, we found that ectopic expression of human CD34 induces cell rounding, microvillus formation, phosphorylation of ERM proteins, and inhibition of integrin-mediated re-attachment of HEK293T cells. Because these characteristics are observed in human cells that express endogenous CD34, we believe that CD34 functions in the formation and/or maintenance of cellular structure, and in the regulation of cell adhesion.
Results
Cell rounding by CD34 in HEK293T cells
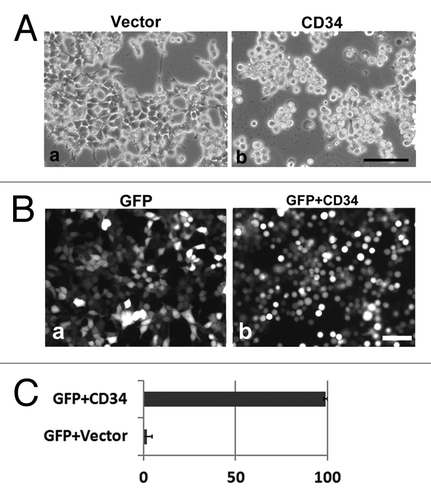
Previously, we found that ectopic expression of CD43 induces cell rounding, microvillus formation, and phosphorylation of ERM proteins, and inhibits integrin-dependent re-attachment of HEK293T cells.Citation1 Because CD43 is a cell surface sialomucin expressed in leukocytes, we performed similar analyses of another blood cell sialomucin, CD34. Endogenous CD34 is not expressed in HEK293T cells. HEK293T cells transfected with a CD34 expression vector (CD34-HEK293T) became round and detached from the substrata (, b), whereas transfectants of mock vector (Vector-HEK293T) were flat and spread (, a). Despite rounding and detachment, CD34-HEK293T cells were not dead or apoptotic, because these cells were not stained with trypan blue or contained fragmented nuclei (data not shown). To clarify the comparison, a green fluorescent protein (GFP) expression vector was co-transfected with the mock vector or CD34 expression vector into HEK293T cells. Compared with GFP expression alone, co-expression with CD34 clearly altered the shape of HEK293T cells to spherical (). The ratio of round cells was about 99% among CD34 transfectants, whereas only 2% was observed among mock transfectants ().
Figure 1. Cell rounding by CD34 in HEK293T cells. (A) Phase contrast images of HEK293T cells transfected with empty vector (HEK293T cells) or a CD34 expression vector (CD34-HEK293T cells). CD34-HEK293T cells were rounded and detached from the substrata (b), whereas HEK293T cells were flat (a) at 40 h after transfection. Scale bar: 100 µm. (B) Fluorescence images of HEK293T cells at 2 d after transfection of a GFP expression vector (a) or a GFP expression vector plus a CD34 expression vector (b). Scale bar: 100 µm. (C) Ratio of round cells among GFP+ cells shown in (B).
Microvillus formation by CD34 in HEK293T cells
We further studied localization of CD34 by expression of GFP fused to the C-terminal of CD34 (CD34-GFP). At low magnification, CD34-GFP was detected mostly at the cell surface and as a dot-like structure in the cytoplasm, presumably an intracellular transport system such as the Golgi apparatus (, a). At high magnification, CD34-GFP was detected as protrusions from cell bodies (, b). Immunohistochemical analysis with an anti-CD34 antibody and phalloidin also demonstrated long protrusions with filamentous actin from the surface of CD34-HEK293T cells (). Because ezrin, one of ERM proteins, has been detected in such protrusions, the protrusions may be microvilli.Citation18,Citation19
Figure 2. Formation of microvilli-like protrusions in CD34-HEK293T cells. (A) Subcellular localization of CD34-GFP in HEK293T cells. Images of HEK293T cells transfected with a CD34-GFP expression vector (CD34GFP-HEK293T cells) were captured at low magnification on a culture dish (a) or at high magnification on a glass coverslip (b). Scale bars: 100 µm (a) and 10 µm (b). (B) Subcellular localization of CD34 in CD34-HEK293T cells. CD34-HEK293T cells were double stained with anti-CD34 (a) or anti-Ezrin (d) antibodies and phalloidin (b and e). Staining was co-localized in microvilli-like protrusions from CD34-HEK293T cells. Merged images (c and f). Scale bars: 10 µm. (C) Electron microscopy of HEK293T transfectants. Scanning electron micrograph of Vector-HEK293T (a) and CD34-HEK293T (b) cells. Ultrathin section electron micrograph of Vector-HEK293T (c) and CD34-HEK293T (d) cells. Magnified images of microvilli-like protrusions from Vector-HEK293T (e) and CD34-HEK293T (f) cells. Arrowheads indicate lipid bilayer membranes. Arrows indicate actin filaments. Scale bars: 5 µm (a and b) and 500 nm (c–f). (D) Immunoblot analysis of ERM proteins in CD34-HEK293T cells. Alterations of ERM phosphorylation was detected by immunoblotting. (E) Immunofluorescence with an anti-p-ERM antibody. p-ERM proteins were observed at microvilli-like protrusions from CD34-HEK293T cells (b). Scale bars: 10 µm.
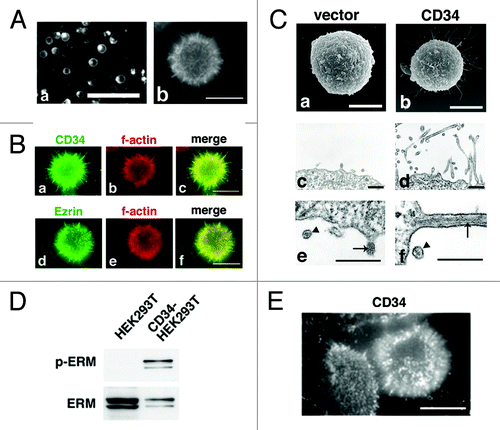
To ascertain augmentation of these protrusions by CD34, we examined Vector-HEK293T and CD34-HEK293T cells by electron microscopy. Many long protrusions from CD34-HEK293T cell bodies were observed by scanning electron microscopy (SEM) (, b) and ultrathin section electron microscopy (, d), whereas a few short protrusions were observed in Vector-HEK293T cells (, a and c). Parallel fibers of filamentous actin were observed within the protrusions by ultrathin section electron microscopy (, e and f, arrows), confirming these protrusions as microvilli. We also observed that the glycocalyx on the microvillar surface was thick in CD34-HEK293T cells, whereas it was thin in Vector transfectants (, f and e, arrowheads).
To confirm augmentation of microvilli by CD34, we digitalized the number and length of cell surface protrusions from HEK293T transfectants by a method described previously.Citation1 In brief, mCherry with the myristoylation site of c-Src (Myr-mCherry) or CD34-mCherry was expressed in HEK293T cells. Cell surface protrusions were then detected by mCherry fluorescence. Fluorescence images were captured at middle phases, but not at attachment phases, of transfectants to measure the number and length of microvilli. As summarized in , the number of microvillus protrusions per cell was 22 ± 11 (mean ± SD) for CD34-mCherry-HEK293T cells and 9 ± 8 for Myr-mCherry-HEK293T cells. The length of protrusions was 2.5 ± 1.3 μm for CD34-mCherry-HEK293T cells and 1.0 ± 0.8 μm for Myr-mCherry-HEK293T cells. These data indicated that the microvillus protrusions of HEK293T cells were augmented by CD34.
Table 1. Table of the length and number of microvilli
As a potential biochemical mechanism that leads to both cell rounding and microvillus formation, we previously found phosphorylation of ERM proteins in CD43-HEK293T cells.Citation1 Because phosphorylation of the C-terminal Thr residue is the activating mechanism for ERM proteins,Citation18,Citation20 we investigated phosphorylation of ERM proteins in CD34-HEK293T cells. Immunoblotting with an anti-phosphorylated-ERM (p-ERM) antibody showed an increase of p-ERM by CD34 expression (). Moreover, significant immunostaining of p-ERM was detected mostly at the microvillus protrusions of CD34-HEK293T cells (). p-ERM was hardly detected in non-transfectant HEK293T cells (data not shown). Thus, phosphorylation of ERM proteins is augmented by CD34, and may be involved in the cell shape alterations observed in CD34-HEK293T cells.
Inhibition of integrin-mediated cell re-attachment by CD34
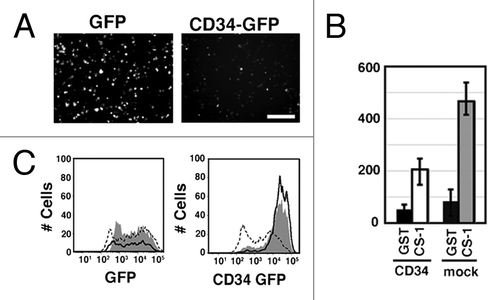
When CD34 was expressed, HEK293T cells became spherical in shape, formed microvilli, and showed augmented phosphorylation of ERM proteins. We previously reported similar phenomena by expression of CD43/leukosialin, an inhibitory molecule of cell adhesion, and also showed that detachment of HEK293T cells by trypsin augments phosphorylation of ERM proteins.Citation1 These findings suggest that CD34 is an anti-adhesion molecule. To test the effect of CD34 on cell adhesion, we studied the effect of CD34 on integrin-mediated re-attachment of HEK293T cells. GFP or CD34-GFP were transiently expressed in α4-HEK293T cells that stably express α4 integrin.Citation1 In addition, tissue culture plates were coated with GST or a fusion protein of GST and fibronectin CS-1 peptide (GST-CS-1), a ligand of α4β1 integrin. The cells were detached by pipetting, incubated on the CS-1-coated substrata, and then evaluated for re-attachment. After incubation followed by washing, GFP-α4-HEK293T cells were mostly bound and spread (, a), whereas CD34-GFP-α4-HEK293T cells were hardly attached to the CS-1-coated substrata (, b). The numbers of bound fluorescent cells are shown in . The number of CD34-GFP transfectants bound to the CS-1-coated substrata was much lower than that of GFP transfectants. Flow cytometry showed that the expression level of CD34-GFP in unbound cells (, continuous line) was higher than that in bound cells (dashed line), further suggesting that high expression of CD34-GFP inhibited integrin-mediated cell adhesion. Moreover, the GFP levels in unbound and bound GFP-HEK293T cells were indistinguishable. Thus, CD34 significantly inhibits α4β1 integrin-mediated cell re-attachment.
Figure 3. Inhibition of integrin-mediated cell adhesion by CD34. (A) Effect of CD34 on integrin-mediated re-attachment of α4-HEK293T cells. α4-HEK293T cells were transfected with a GFP expression vector (GFP-α4-HEK293T cells) or a CD34-GFP expression vector (CD34-GFP-α4-HEK293T cells). Cells were harvested, re-plated, and incubated in GST-CS1-coated wells, and then washed with medium. Adherent cells were evaluated by fluorescence microscopy. Scale bar: 200 µm. (B) Comparison of the number of bound cells. The average numbers of adherent cells with even weak fluorescence was calculated from three experiments. The histogram indicates the average numbers of adherent fluorescent cells on the coated surface. GFP-α4-HEK293T cells, CD34-GFP-α4-HEK293T cells, GST, and GST-CS1 are indicated as G, 34G, GST, and CS1, respectively. (C) Flow cytometric analysis of GFP in bound and unbound cells. The fluorescent intensity of GFP and CD34-GFP in cells bound (dashed line) or unbound (continuous line) to the GST-CS-1-coated substrata is shown. Cells prior to the binding assay are indicated by the shaded areas. The expression level of CD34-GFP in unbound CD34-GFP-α4-HEK293T cells in GST-CS1-coated wells was higher than that in bound cells.
Identification of microvilli in CD34+ blood cells and the acute myelogenous leukemic cell line, KG-1
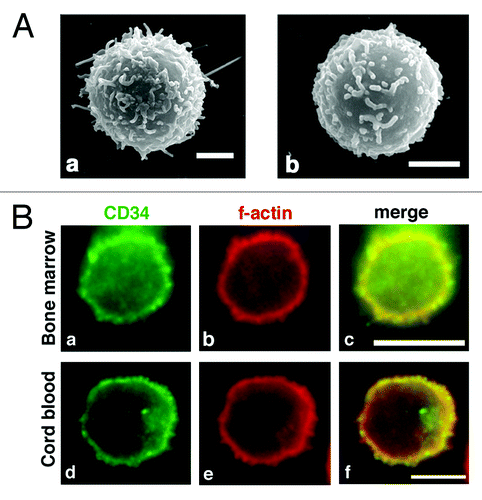
Because ectopic expression of CD34 induced various cellular phenomena in HEK293T cells, we examined cells expressing endogenous CD34. CD34 is a marker for HPCs and HSCs. Therefore, we investigated CD34+ blood cells from bone marrow and cord blood. Using SEM, we observed microvillus protrusions at the surface of both types of CD34+ blood cells (). Immunofluorescence further revealed protrusions with filamentous actin outgrown from the cell bodies (, b and e) and co-localization of CD34 at these protrusions and the cell surface (, a, c, d, and f). Thus, CD34+ primary blood cells possess microvilli on their surface, and CD34 is localized at these microvilli and the surface of spherical cells.
Figure 4. Microvilli in human CD34+ HPCs. (A) SEM of a CD34+ bone marrow cell (a) and CD34+ cord blood cell (b). Scale bars: 2 µm. (B) Immunofluorescence of a CD34+ bone marrow cell (a–c) and a CD34+ cord blood cell (d–f). CD34+ cells were double stained with an anti-CD34 antibody (a and d) and phalloidin (b and e). Merged images (c and f). Scale bars: 5 µm.
For a more precise analysis of the surface morphology of CD34+ cells, we investigated the surface structure of KG-1 cells by electron microscopy. KG-1 is an acute myelogenous leukemia (AML) cell line that abundantly expresses endogenous CD34.Citation21 As demonstrated by SEM (, a), a KG-1 cell was spherical in shape and surrounded by microvilli-like protrusions. In ultrathin section electron microscopy, parallel fibers of filamentous actin were observed in these protrusions (, b, arrow), indicating that the protrusions were indeed microvilli.
Figure 5. CD34 is localized at microvilli in KG-1 cells. (A) Electron microscopy of KG-1 cells. Scanning electron micrograph (a) and ultrathin section electron micrograph (b) of KG-1 cells. KG-1 cells were covered with numerous microvilli. Arrows indicate parallel filamentous actin. Scale bars: 2 µm (a), 500 nm (b), and 125 nm (inset). (B) Subcellular localization of CD34 in KG-1 cells. KG-1 cells were double stained with anti-CD34 (a), anti-ezrin (d), or anti-β1-integrin antibody (g) and phalloidin (b, e, and h). Merged images (c, f, and i). Scale bars: 10 µm.
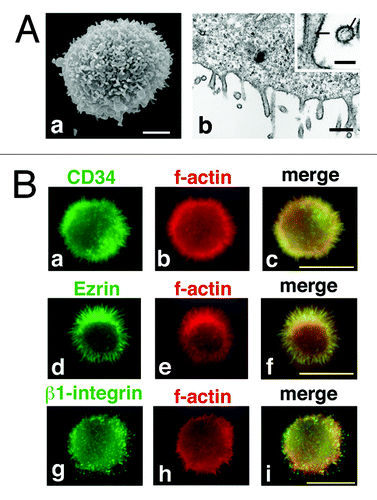
Immunofluorescence with an anti-CD34 antibody showed localization of CD34 on the protrusions from the cell surface (, a), and co-localization of CD34 with filamentous actin (, b and c). Co-localization of ezrin with filamentous actin at the protrusions further demonstrated the protrusions from KG-1 cells as microvilli (, d–f). These data were consistent with the data obtained in CD34-HEK293T cells. We also observed β1 integrin (, g–i) and α4 integrin (data not shown) at the protrusions. However, unlike immunostaining with anti-CD34 and anti-ERM antibodies or phalloidin, which showed homogenous staining of protrusions, immunostaining with anti-integrin antibodies showed a patchy staining pattern on the cell surface protrusions.
Augmented integrin-mediated KG-1 cell adhesion by O-sialoglycosylpeptide endopeptidase treatment
KG-1 and CD34+ blood cells are non-adherent in culture with a spherical shape and microvilli. These characteristics suggest the involvement of CD34 in the inhibition of cell adhesion of these cells. Therefore, we tried to elucidate regulatory function of CD34 in cell adhesion of KG-1 cells. Because α4β1 integrin was observed on the surface of KG-1 cells, we investigated α4β1 integrin-mediated adhesion of KG-1 cells. As shown in , KG-1 cell adhesion to a GST-CS1-coated plate was marginally augmented compared with that to a GST-coated plate. However, pre-treatment of KG-1 cells with O-sialoglycoprotein endopeptidase (OSGEPase) largely augmented GST-CS1-dependent KG-1 cell adhesion. OSGEPase treatment significantly reduced the OSGEPase-sensitive class II epitope of CD34 (QBEnd10), while not affecting the OSGEPase-resistant class III epitope (581) (). Thus, OSGEPase cleaves the O-sialoglycosylated N-terminal portion of the CD34 extracellular region.Citation22 Since other sialomucins can be also cleaved by OSGEPase, target molecule of OSGEPase in KG-1 cells is not limited to CD34. However, CD34 is cleaved by OSGEPase and abundantly expressed in KG-1 cells. Thus, the N-terminal mucin domain of CD34 can be involved in the inhibition of integrin-mediated adhesion of KG-1 cells.
Figure 6. Effect of OSGEPase treatment on integrin-mediated adhesion of KG-1 cells. (A) Numbers of unattached KG-1 cells in GST-CS1-coated wells. KG-1 cells were treated with or without OSGEPase for 30 min. Cells were incubated in tissue culture plates coated with GST or GST-CS1, washed, and then the numbers of unattached cells were counted. (B) Flow cytometric analysis of OSGEPase-treated and untreated KG-1 cells. Cells were double immunostained with fluorophore-labeled anti-CD34 antibodies, 581 (PE) and QBEnd10 (FITC). OSGEPase treatment reduced QBEnd10 on KG-1 cells. (C) Phalloidin staining of KG-1 cells. OSGEPase-treated KG-1 cells were attached to GST-CS-1-coated glass chamber, fixed, permeabilized, and then stained with phalloidin. Images of attachment, middle, and top phases are shown. Scale bar: 1.25 μm.
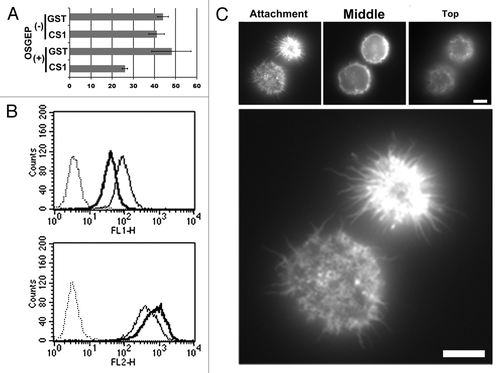
At the same time, however, only some of the OSGEPase-treated KG-1 cells remained attached to the GST-CS-1-coated substrata after washing. This result suggests another mechanism for the regulation of cell adhesion other than direct inhibition by sialomucin domains. To better understand the integrin-mediated adhesion of OSGEPase-treated KG-1 cells, adherent cells in GST-CS-1-coated glass chambers were investigated by fluorescence microscopy. As shown in , microvillus protrusions were observed at attachment sites to the substrata. Moreover, the area covered by each attached cell at the attachment phase was the same or less than the area at the middle phase, indicating that these attached KG-1 cells were not spread on the coated substrata. These data indicate that OSGEPase treatment is not sufficient for the collapse of microvilli, alteration of the spherical cell shape, and spreading of KG-1 cells. Moreover, interactions between GST-CS-1 and α4β1 integrin did not cause either the collapse of micorvilli or cell spreading. Given the rather weak attachment of OSGEPase-treated KG-1 cells to GST-CS-1-coated substrata, the collapse of microvilli and cell spreading may be important to establish strong cell adhesion. Therefore, the spherical cell shape and cell surface microvilli themselves function as regulators of cell adhesion.
Discussion
In this study, we have demonstrated that CD34, a leukocyte surface sialomucin and marker for HPCs and HSCs, has similar functions as those of CD43 when expressed in HEK293T cells. Expression of CD34 induced augmentation of microvilli, a switch from a polarized to spherical shape, and phosphorylation of ERM proteins. Integrin-mediated cell re-attachment was also inhibited in CD34-HEK293T cells. The cell shape and lack of cell adhesion of CD34+ blood cells and KG-1 cells, which express endogenous CD34, were consistent with the phenotypes of CD34-HEK293T cells. Treatment with OSGEPase resulted in augmented integrin-mediated KG-1 cell attachment to the substrata. Although other sialomucins can be also cleaved by OSGEPase, CD34 is abundantly expressed in KG-1 cells, and its mucin domain is cleaved off by OSGEPase. Thus, CD34 can be involved in the formation and/or maintenance of spherical cell morphology with microvilli and regulates integrin-mediated adhesion CD34+ cells. Then, how are these phenomena related each other?
We would like to start with a potential cause of cell shape alterations by CD34. Similar to CD43, CD34 augmented phosphorylation of ERM proteins. Recent studies have demonstrated that phosphorylated ERM proteins are essential for rounding of cells during mitosis.Citation23,Citation24 Actin cortex is observed just under the plasma membrane in spherical cells, which is essential for the formation and maintenance of round cell shape. In addition, phosphorylated and activated ERM proteins function as a linker between the membrane component and actin cytoskeleton.Citation18,Citation25 Thus, ERM proteins and their phosphorylation are critical elements for the formation and/or maintenance of a spherical cell shape during mitosis. In addition to mitotic cells, circulating lymphocytes and other leukocytes are spherical and relatively rigid. The spherical shape of leukocytes is also maintained by phosphorylated ERM proteins and an actin cortex.Citation26 Furthermore, phosphorylated ERM proteins and actin filaments are localized at microvilli.Citation18,Citation19 Antisense oligonucleotides against ERM expression perturb microvilli,Citation27 indicating that ERM proteins are essential for microvillus formation and/or maintenance. Thus, phosphorylated ERM proteins are essential for cell rounding and microvillus formation. Our findings of phosphorylated ERM proteins in microvilli and at the surface of spherical CD34-HEK293T and CD34+ KG-1 cells are consistent with these previous reports.
Then, how does CD34 induce ERM phosphorylation? We have previously reported induction of ERM phosphorylation as a result of cell detachment either by expression of CD43 or by keeping cells swirling in BSA-coated dish after trypsinization.Citation1 Like CD43, CD34 was reported as an inhibitor of cell adhesion,Citation12 and expression of CD34 inhibited integrin-mediated HEK293T cell re-attachment (). Therefore, it is conceivable that CD34 induces ERM phosphorylation via inhibition of cell adhesion. Then, how does CD34 inhibit cell adhesion? CD34 has a highly O-glycosylated region in the N-terminal of its extracellular domain. In fact, a much thicker glycocalyx was observed in CD34-HEK293T cells than that in Vector transfectants (), indicating augmented glycosylation on the surface of CD34-HEK293T cells. Cleavage of sialomucin by OSGEPase also augmented integrin-mediated adhesion of KG-1 cells () and in CD34-α4-HEK293T cells (data not shown). Taken together, these findings strongly suggest that large numbers of hydroxyl and carboxyl groups on the O-glycosylated region of CD34 affect other membrane proteins and/or modify the characteristics of the cell membrane to inhibit integrin-mediated cell adhesion.
Meanwhile, the function of CD34 as an anchor for ERM proteins remains unclear. Phosphorylated ERM proteins bind directly to the cytoplasmic domain of CD43,Citation28 and bind indirectly to that of podocalyxin, a CD34 family protein, via an interaction with another cytoplasmic protein, NHERF2.Citation5,Citation16,Citation17 However, CD34 does not bind to ERM proteins or NHERF family members.Citation5,Citation17 Thus, no mechanism as a membrane anchor for phosphorylated ERM proteins has been established for CD34. However, because CD34 is localized at microvilli and the surface of spherical cells, it is reasonable to assume that CD34 functions as a membrane anchor for ERM proteins. Expression of human CD34 transcription variant 2, which has a different cytoplasmic tail from that of variant 1, caused similar phenomena in HEK293T cells (data not shown). Therefore, we believe the linkage to ERM proteins is within the common juxtamembrane sequence in the cytoplasmic domain or within extracellular-transmembrane domains.
Lastly, what is the potential function of microvilli and a spherical cell shape in regulation of cell adhesion? OSGEPase treatment augmented the attachment of many KG-1 cells to GST-CS1-coated substrata. However, the cell attachment was weak because sequential washing gradually washed out the cells. In addition, OSGEPase-treated KG-1 cells attached to GST-CS-1-coated or poly-l-lysine (PLL)-coated substrata maintained a spherical shape and cell surface microvilli, and did not spread on the substrata ( and data not shown). These findings indicate that (1) cleavage of sialomucins including the N-terminal sialomucin domain of CD34 does not induce microvillus collapse or alteration of the spherical shape of KG-1 cells at least over a few hours. (2) α4β1 integrin on the surface of KG-1 cells is active and can interact with its ligand, GST-CS1. (3) This integrin-ligand interaction does not induce a cell shape alteration and is not sufficient to support stable cell adhesion. Although it requires further study, our hypothesis for this limited cell adhesion is as follows. The spherical shape and surface microvilli of KG-1 cells restrict the attachment sites to a small area. Only limited numbers of adhesion molecules such as α4β1 integrin are exposed to the substrata because of the restriction of the attachment area. Because the number of adhesion molecules attached to the substrata is limited, cell adhesion to the substrata is vulnerable. If this is true, the spherical cell shape and microvilli themselves are the elements that regulate cell adhesion. For release from this regulation and formation of stable adhesion, alteration of the spherical cell shape and cell surface microvilli may be essential. Brown et al. reported microvillus collapse by stromal-derived factor-1 (SDF-1) in peripheral T lymphocytes,Citation29 suggesting that chemokines regulate cell shape. Taken together, these findings indicate a two-step mechanism for leukocyte adhesion, restructuring of the cell shape by chemokines, and then cell adhesion with spreading via adhesion molecule–ligand interactions.
Then, what can we infer as the functions of CD34 in HPCs and HSCs based on our findings? Cell adhesion, homing, and trafficking are all critical for HPCs and HSCs. Expression of CD34 alone inhibits integrin-mediated cell adhesion directly or indirectly via formation and/or maintenance of a spherical cell shape and cell surface microvilli. Considering the circulation of leukocytes, CD34 directly or indirectly inhibits cell adhesion of HPCs and HSCs with other blood cells and endothelial cells, while the spherical cell shape may promote HPC/HSC survival in the hemodynamic rigors of circulation. Furthermore, restructuring of the cytoskeleton and cell shape may be essential for stable cell adhesion such as that in the niche or during extravasation. It has been reported that the SDF-1-CXCR4 interaction is the major regulatory element for the trafficking, retention, and adhesion of HSCs and HPCs.Citation30,Citation31 Taken together with the effect of SDF-1 on microvillus collapse of lymphocytes,Citation26,Citation29 alterations of HPC/HSC morphology can be caused by the SDF-1-CXCR4 interaction. Thus, cell adhesion of HPCs and HSCs may be regulated by cell shape, and CD34 might play significant roles in the formation and/or maintenance of cell shape and adhesion.
Materials and Methods
Cells and reagents
KG-1, HEK293T, and bone marrow CD34+ cells were purchased from RIKEN BioResource Center or Lonza. Cord blood CD34+ cells were purified with a CD34+ MicroBead kit (Miltenyi Biotec) from cord blood samples that were obtained from full-term deliveries according to the institutional guidelines approved by the Tokai University Committee on Clinical Investigation. HEK293T and KG-1 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) and RPMI 1640, respectively, supplemented with 10% fetal calf serum (FCS). PLL, puromycin, paraformaldehyde, bovine serum albumin (BSA), and TRITC-labeled phalloidin were obtained from Sigma. Mowiol 4-88 Reagent was obtained from CALBIOCHEM. OSGEPase was purchased from CEDARLANE Laboratories. Anti-CD34 (clone 581), anti-β1 integrin (MAR4), and anti-Ezrin (clone 18) antibodies were obtained from BD Biosciences. Anti-p-ERM and anti-ERM antibodies were purchased from Cell Signaling Technologies. An anti-CD34 (QBEnd10) antibody was obtained from Santa Cruz Biotechnology. Alexa Fluor 488-labeled goat anti-mouse IgG was purchased from Invitrogen.
Plasmids and transfection
The retroviral expression vector pCpuroCMVS and the expression vector with the myristoylation site of c-Src, pJ3SrcMS, have been described previously.Citation1 Human cd34 transcription variant 1 cDNA was cloned by RT-PCR, fused to DNA fragments of EGFP or mCherry (Clontech) at the CD34 C terminus, and subcloned into pCpuroCMVS. Establishment of α4-HEK293T cells has been described previously.Citation1 Expression vectors were transfected with Lipofectamine 2000 (Invitrogen). pGEX-CS1 was a kind gift from Dr Kenjiro Kamiguchi.Citation32
Electron and immunofluorescence microscopy
Scanning and ultrathin section electron microscopy were preformed as described previously.Citation1
For immunofluorescence microscopy, cells grown on glass coverslips were fixed with 4% paraformaldehyde in PBS for 10 min at room temperature, washed three times with PBS, permeabilized with 0.2% Triton X-100 in PBS for 5 min, and then washed three times with PBS. After blocking with 1% BSA in PBS for 10 min, samples were incubated with primary antibodies for 1 h, washed three times with PBS, incubated with the secondary antibody for 30 min, and then washed three times with PBS. After mounting with Mowiol 4-88, specimens were observed under a fluorescence microscope (IX70 or IX71; OLYMPUS).
Cell adhesion assays
For the adhesion assay of HEK293T transfectants, tissue culture plates were coated with either 10 μg/ml GST or GST-CS1 in PBS at 37 °C for 3 h, washed three times with PBS, blocked with PBS containing 1% BSA, and then washed three times with PBS. HEK293T transfectants were harvested, washed, re-suspended in DMEM, and plated onto the coated plates. After incubation at 37 °C for 30 min in a CO2 incubator, the cells were washed three times with DMEM and images were captured of the bound cells.
For OSGEPase treatment, 1 × 106 KG-1 cells were incubated with 36 μg OSGEPase in 0.5 ml RPMI 1640 at 37 °C in a CO2 incubator for 30 min. Then, the cells were incubated in coated tissue culture plates in RPMI 1640 supplemented with FCS at 37 °C for 30 min, and then unbound cells were collected and counted. For immunohistochemistry, OSGEPase-treated KG-1 cells were incubated in GST-CS1-coated glass chambers (AGC Techno Glass Co., Ltd.).
Immunoblotting
HEK293T cells and transfectants were washed with PBS, lysed in 1% Nonidet-P40 lysis buffer, and then subjected to immunoblot analysis as described previously.Citation1 After the first immunoblotting with an anti-phospho-ERM antibody, the membranes were stripped with WB Stripping Solution (Nacalai Tesque, Inc.), re-blocked, and then re-analyzed with an anti-ERM antibody.
Flow cytometry
HEK293T transfectants were washed with RPMI 1640 and fixed with 0.5% paraformaldehyde in PBS. Cells were analyzed by a FACSCanto II (BD Biosciences).
| Abbreviations: | ||
| HSCs | = | hematopoietic stem cells |
| HPCs | = | hematopoietic progenitor cells |
| AML | = | acute myelogenous leukemia |
| ERM | = | ezrin/radixin/moesin |
| p-ERM | = | phosphorylated-ERM |
| PLL | = | poly-L-lysine |
| BSA | = | bovine serum albumin |
| OSGEPase | = | O-sialoglycoprotein endopeptidase |
| SDF-1 | = | stromal-derived factor-1 |
| SEM | = | scanning electron microscopy |
Acknowledgments
We thank Dr Kenjiro Kamiguchi for providing the pGST-CS-1 vector and Mr Hideki Saito for technical assistance. This work was supported in part by Grants-in-Aid for Scientific Research (KAKENHI 10011601) and a grant from the New Energy and Industrial Technology Development Organization (NEDO) of Japan.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Yamane J, Ohnishi H, Sasaki H, Narimatsu H, Ohgushi H, Tachibana K. Formation of microvilli and phosphorylation of ERM family proteins by CD43, a potent inhibitor for cell adhesion: cell detachment is a potential cue for ERM phosphorylation and organization of cell morphology. Cell Adh Migr 2011; 5:119 - 32; http://dx.doi.org/10.4161/cam.5.2.13908; PMID: 21045567
- Carlsson SR, Fukuda M. Isolation and characterization of leukosialin, a major sialoglycoprotein on human leukocytes. J Biol Chem 1986; 261:12779 - 86; PMID: 2943740
- Rosenstein Y, Santana A, Pedraza-Alva G. CD43, a molecule with multiple functions. Immunol Res 1999; 20:89 - 99; http://dx.doi.org/10.1007/BF02786465; PMID: 10580634
- Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood 1996; 87:1 - 13; PMID: 8547630
- Furness SGB, McNagny K. Beyond mere markers: functions for CD34 family of sialomucins in hematopoiesis. Immunol Res 2006; 34:13 - 32; http://dx.doi.org/10.1385/IR:34:1:13; PMID: 16720896
- Berenson RJ, Andrews RG, Bensinger WI, Kalamasz D, Knitter G, Buckner CD, Bernstein ID. Antigen CD34+ marrow cells engraft lethally irradiated baboons. J Clin Invest 1988; 81:951 - 5; http://dx.doi.org/10.1172/JCI113409; PMID: 2893812
- Berenson RJ, Bensinger WI, Hill RS, Andrews RG, Garcia-Lopez J, Kalamasz DF, Still BJ, Spitzer G, Buckner CD, Bernstein ID, et al. Engraftment after infusion of CD34+ marrow cells in patients with breast cancer or neuroblastoma. Blood 1991; 77:1717 - 22; PMID: 1707696
- Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 1996; 273:242 - 5; http://dx.doi.org/10.1126/science.273.5272.242; PMID: 8662508
- Yabe H, Yabe M, Hattori K, Hinohara T, Morimoto T, Nakamura Y, Noma M, Takei M, Kobayashi N, Tsuji K, et al. Successful engraftment of allogeneic CD34-enriched marrow cell transplantation from HLA-mismatched parental donors. Bone Marrow Transplant 1996; 17:985 - 91; PMID: 8807104
- Cheng J, Baumhueter S, Cacalano G, Carver-Moore K, Thibodeaux H, Thomas R, Broxmeyer HE, Cooper S, Hague N, Moore M, et al. Hematopoietic defects in mice lacking the sialomucin CD34. Blood 1996; 87:479 - 90; PMID: 8555469
- Suzuki A, Andrew DP, Gonzalo JA, Fukumoto M, Spellberg J, Hashiyama M, Takimoto H, Gerwin N, Webb I, Molineux G, et al. CD34-deficient mice have reduced eosinophil accumulation after allergen exposure and show a novel crossreactive 90-kD protein. Blood 1996; 87:3550 - 62; PMID: 8611677
- Drew E, Merzaban JS, Seo W, Ziltener HJ, McNagny KM. CD34 and CD43 inhibit mast cell adhesion and are required for optimal mast cell reconstitution. Immunity 2005; 22:43 - 57; http://dx.doi.org/10.1016/j.immuni.2004.11.014; PMID: 15664158
- Healy L, May G, Gale K, Grosveld F, Greaves M, Enver T. The stem cell antigen CD34 functions as a regulator of hemopoietic cell adhesion. Proc Natl Acad Sci USA 1995; 92:12240 - 4; http://dx.doi.org/10.1073/pnas.92.26.12240; PMID: 8618877
- Lanza F, Healy L, Sutherland DR. Structural and functional features of the CD34 antigen: an update. J Biol Regul Homeost Agents 2001; 15:1 - 13; PMID: 11388737
- Delia D, Lampugnani MG, Resnati M, Dejana E, Aiello A, Fontanella E, Soligo D, Pierotti MA, Greaves MF. CD34 expression is regulated reciprocally with adhesion molecules in vascular endothelial cells in vitro. Blood 1993; 81:1001 - 8; PMID: 7679004
- Nielsen JS, Graves ML, Chelliah S, Vogl AW, Roskelley CD, McNagny KM. The CD34-related molecule podocalyxin is a potent inducer of microvillus formation. PLoS One 2007; 2:e237; http://dx.doi.org/10.1371/journal.pone.0000237; PMID: 17311105
- Nielsen JS, McNagny KM. Novel functions of the CD34 family. J Cell Sci 2008; 121:3683 - 92; http://dx.doi.org/10.1242/jcs.037507; PMID: 18987355
- Niggli V, Rossy J. Ezrin/radixin/moesin: versatile controllers of signaling molecules and of the cortical cytoskeleton. Int J Biochem Cell Biol 2008; 40:344 - 9; http://dx.doi.org/10.1016/j.biocel.2007.02.012; PMID: 17419089
- Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol 2007; 9:1110 - 21; http://dx.doi.org/10.1038/ncb1007-1110; PMID: 17909522
- Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol 1998; 140:647 - 57; http://dx.doi.org/10.1083/jcb.140.3.647; PMID: 9456324
- Simmons DL, Satterthwaite AB, Tenen DG, Seed B. Molecular cloning of a cDNA encoding CD34, a sialomucin of human hematopoietic stem cells. J Immunol 1992; 148:267 - 71; PMID: 1370171
- Sutherland DR, Marsh JCW, Davidson J, Baker MA, Keating A, Mellors A. Differential sensitivity of CD34 epitopes to cleavage by Pasteurella haemolytica glycoprotease: implications for purification of CD34-positive progenitor cells. Exp Hematol 1992; 20:590 - 9; PMID: 1375160
- Kunda P, Pelling AE, Liu T, Baum B. Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Curr Biol 2008; 18:91 - 101; http://dx.doi.org/10.1016/j.cub.2007.12.051; PMID: 18207738
- Carreno S, Kouranti I, Glusman ES, Fuller MT, Echard A, Payre F. Moesin and its activating kinase Slik are required for cortical stability and microtubule organization in mitotic cells. J Cell Biol 2008; 180:739 - 46; http://dx.doi.org/10.1083/jcb.200709161; PMID: 18283112
- Arpin M, Chirivino D, Naba A, Zwaenepoel I. Emerging role for ERM proteins in cell adhesion and migration. Cell Adh Migr 2011; 5:199 - 206; http://dx.doi.org/10.4161/cam.5.2.15081; PMID: 21343695
- Hao J-J, Liu Y, Kruhlak M, Debell KE, Rellahan BL, Shaw S. Phospholipase C-mediated hydrolysis of PIP2 releases ERM proteins from lymphocyte membrane. J Cell Biol 2009; 184:451 - 62; http://dx.doi.org/10.1083/jcb.200807047; PMID: 19204146
- Takeuchi K, Sato N, Kasahara H, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J Cell Biol 1994; 125:1371 - 84; http://dx.doi.org/10.1083/jcb.125.6.1371; PMID: 8207064
- Yonemura S, Hirao M, Doi Y, Takahashi N, Kondo T, Tsukita S, Tsukita S. Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J Cell Biol 1998; 140:885 - 95; http://dx.doi.org/10.1083/jcb.140.4.885; PMID: 9472040
- Brown MJ, Nijhara R, Hallam JA, Gignac M, Yamada KM, Erlandsen SL, Delon J, Kruhlak M, Shaw S. Chemokine stimulation of human peripheral blood T lymphocytes induces rapid dephosphorylation of ERM proteins, which facilitates loss of microvilli and polarization. Blood 2003; 102:3890 - 9; http://dx.doi.org/10.1182/blood-2002-12-3807; PMID: 12907449
- Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells 2005; 23:879 - 94; http://dx.doi.org/10.1634/stemcells.2004-0342; PMID: 15888687
- Lapidot T, Dar A, Kollet O. How do stem cells find their way home?. Blood 2005; 106:1901 - 10; http://dx.doi.org/10.1182/blood-2005-04-1417; PMID: 15890683
- Kamiguchi K, Tachibana K, Iwata S, Ohashi Y, Morimoto C. Cas-L is required for beta 1 integrin-mediated costimulation in human Tcells. J Immunol 1999; 163:563 - 8; PMID: 10395641