Abstract
Recent discoveries have unveiled the roles of a complicated network of E3 ubiquitin ligases in regulating cell migration machineries. The E3 ubiquitin ligases Smurf1 and Cul/BACURD ubiquitinate RhoA to regulate stress fiber formation and cell polarity, and ASB2α ubiquitinates filamins to modulate cytoskeletal stiffness, thus regulating cell spreading and cell migration. HACE1, XIAP, and Skp1-Cul1-F-box bind to Rac1 and cause its ubiquitination and degradation, thus suppressing lamellipodium protrusions, while PIAS3, a SUMO ligase, activates Rac1 to promote lamellipodium dynamics. Smurf1 also enhances Rac1 activation but it does not ubiquitinate Rac1. Both Smurf1 and HECTD1 regulate focal adhesion (FA) assembly and (or) disassembly through ubiquitinating the talin head domain and phosphatidylinositol 4 phosphate 5-kinase type I γ (PIPKIγ90), respectively. Thus, E3 ubiquitin ligases regulate stress fiber formation, cell polarity, lamellipodium protrusions, and FA dynamics through ubiquitinating the key proteins that control these processes.
Protein ubiquitination is a post-translational modification process that covalently conjugates single or multiple ubiquitins, an evolutionarily conserved protein with 76 amino acid residues, to target proteins.Citation1 Ubiquitinated proteins can be degraded by the 26S proteasome, fulfill a variety of signaling functions including protein kinase activation and subcellular localization, or function as a marker for endocytosis.Citation2,Citation3 Protein ubiquitination can be divided into three sequential steps: (1) ubiquitin activation by ubiquitin-activating enzymes (E1), (2) transfer of activated ubiquitin from E1 to ubiquitin-conjugating enzymes (E2), and (3) conjugation of ubiquitin to target proteins by ubiquitin ligases (E3). E3 ubiquitin ligases are the key control points for this process. So far, more than 500 E3 ubiquitin ligase genes have been identified in humans. The majority of these ubiquitin ligases can be divided into two categories based on specific structural motifs: (1) those possessing the HECT (homologous to the E6-AP carboxyl terminus) domain; (2) those containing the RING (really interesting new gene)-finger domain.Citation4
Besides regulating cell proliferation, apoptosis, cancer invasion, and neurodegenerative diseases, E3 ubiquitin ligases also regulate cell migration, a process that plays pivotal roles in wound healing, embryonic development, cancer metastasis, and inflammation. Cell migration is a cyclic process consisting of three steps: (1) the formation and protrusion of a leading lamellipodia; (2) subsequent adhesion to the substrate, followed by (3) the tail retraction.Citation5 All these steps require extensive focal adhesion dynamics and cytoskeletal reorganization. We have reviewed the roles of E3 ubiquitin ligases in cell migration previously.Citation6 In this commentary, we will highlight the new discoveries on the roles of E3 ubiquitin ligases in cell migration, focusing on how these E3 ubiquitin ligases regulate the machineries (such as focal adhesion, lamellipodia, and stress fibers) of cell migration.
E3 Ubiquitin Ligases Regulate Stress Fiber Formation, Cell Stiffness, and Cell Polarity
Ras homolog gene family, member A (RhoA), is a small GTPase protein that regulates the formation of stress fibers.Citation7 Active RhoA binds to and activates Rho-kinases, ROCKII and ROKα, which in turn, either phosphorylates and inhibits myosin phosphatase or phosphorylates myosin light chain (MLC), leading to elevated MLC phosphorylation. The posphorylated MLC then promotes the assembly of stress fibers. Stress fibers participate in FA dynamics and modulate the direction of lamellipodium protrusion,Citation8,Citation9 consequently controlling the velocity and directionality of cell migration. On the other hand, excess or dyregulated stress fibers cause a decrease in cell plasticity, thus inhibiting cell migration.Citation10,Citation11
Filamins are large actin-binding proteins that cross-link actin into a dynamic three-dimensional structure.Citation12 Filamins form homodimers and have multiple F-actin-binding domains, thus supporting orthogonal branching actin formation,Citation13 whereas the hinge regions are responsible for the intrinsic flexibility of the actin networks generated by filamins.Citation14 These properties confer filamins versatile role in regulating the elasticity and stiffness of the actin network. Filamins usually function as suppressors in cell spreading and cell migration, probably through inhibiting integrin activation or focal adhesion dynamics.Citation15,Citation16 It is likely that filamins-mediated increase in the stiffness of actin network also contributes to its suppression on cell migration.
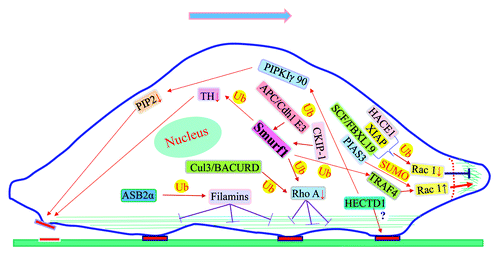
As illustrated in , both Smurf1 and Cul3/BACURD ubiquitinate RhoA to regulate stress fiber formation and cell polarity,Citation17,Citation18 whereas ASB2α ubiquitinates Filamins to modulate the elasticity of the actin network and consequently cell spreading.Citation19
Figure 1. E3 ubiquitin ligases regulate stress fiber, lamellipodium and focal adhesion dynamics. (1) Both Smurf1 and Cul3/BACURD ubiquitinate RhoA to regulate stress fiber formation and cell polarity, whereas ASB2α ubiquitinates Filamins to modulate the elasticity of the actin network and consequently cell spreading. The activity of Smurf1 is regulated by APC/Cdh1 E3 and CKIP-1. (2) Rac1 is ubiquitinated by a number of E3 ubiquitin ligases, including HACE1, XIAP, and SCF/FBX19, resulting in Rac1 degradation and reduction in lamellipodium extension. On the other hand, sumoylation of Rac1 by PIAS3, a SUMO ligase, stimulates its activation and lamellipodium protrusions. Smurf1-mediated ubiquitination of TRAF4 also promotes Rac1 activation. (3) PIPKIγ90 is ubiquitinated at K97 by HECTD1, resulting its degradation and FA assembly/disassembly during cell migration. Smurf1 binds to TH and causes TH ubiquitination and degradation, consequently leading to FA disassembly.
RhoA ubiquitination
SMURF1
RhoA ubiquitination by Smurf1 has been reviewed previously.Citation6 However, because this discovery has inspired many new studies (see the following paragraphs), it deserves to be mentioned again. It is well known that migratory cells are polarized with low RhoA activity at the leading edge and higher at the rear and sides.Citation20 The underlying molecular mechanism was unknown before Wang’s discovery. Wang et al.Citation17 reported that Smurf1 (Smad ubiquitin regulatory factor 1), a Nedd4 family ubiquitin ligase, was localized to lamellipodia, where it targeted RhoA for ubiquitination and degradation, thus inhibiting stress fiber formation to facilitate protrusive activity and polarity.Citation17 Thus, Smurf1-mediated ubiquitination of RhoA is responsible for precise temporal and spatial regulation of RhoA, which is required for optimal cell migration.
Smurf1 features an N-terminal C2 domain and two WW domains that are responsible for cellular localization and substrate recognition, and a catalytic carboxyl terminal HECT domain.Citation21 SMURF1 amplification was detected in four of 95 cases (4.2%) in primary human pancreatic cancers.Citation22 The protein levels of Smurf1 are downregulated by SCF/FBXL15 ubiquitin ligase complex,Citation23 whereas it is upregulated by deubiquitinase FAM/USP9X.Citation24
Many proteins, including RhoA, talin head, hPEM-2, Smad1, Smad5, Runx2, and Axin, were identified as Smurf1 substrates.Citation17,Citation25-Citation30 It is likely that the roles of Smurf1 in regulating different pathways are controlled by its binding proteins. CCM2 (Cerebral Cavernous Malformations 2) binds Smurf1 through its phosphotyrosine binding (PTB) domain, and promotes Smurf1-mediated degradation of RhoA.Citation31 APC/Cdh1 E3 and CKIP-1 promote the activity of Smurf1, thus downregulating multiple downstream targets that control osteoblast differentiation.Citation32,Citation33 The estrogen receptor α (ERα) forms a protein complex with Smurf and Smad, and enhances Smad ubiquitination and degradation in an estrogen-dependent manner.Citation34 The roles of Smurf1 in regulating different pathways are also changed by protein kinases. For instance, protein kinase A-mediated phosphorylation of Smurf1 at T306 reduces ubiquitination of polarity protein Par6 while increases RhoA ubiquitination, thus elevating the Par6/RhoA ratio and consequently promoting axon growth in hippocampal neurons.Citation35
Cul3/BACURD
BACURDs are a family of RhoA-binding BTB domain (for BR-C, ttk and bab, a domain present near the N terminus of zinc finger proteins) adaptors conserved from insects to mammals.Citation18 BACURDs and Cul3, a Cullin family scaffold protein, assemble to SCF (Skp1-Cullin1-F-box protein)-like ubiquitin ligase complexes through BTB domain. Chen et al. reported that Cul3/BACURD complex specifically ubiquitinated RhoA, and that dysfunction of the Cul3/BACURD complex caused profound stress fiber accumulation, thus inhibiting cell migration and impairing RhoA-mediated convergent extension movements during Xenopus gastrulation.Citation18
Filamin ubiquitination
ASB2α (Ankyrin repeat-containing protein with a Suppressor of cytokine signaling Box 2α) is a subunit of an active Cullin 5-RING E3 ubiquitin ligase complex. The ASB2α Cullin 5-ring E3 ubiquitin ligase is highly expressed in immature dendritic cells (DCs) and is downregulated after DC maturation. It was reported that ASB2α ubiquitin ligase mediated the ubiquitination and degradation of filamins, a suppressor of cell motility, and regulated cell spreading and migration in immature DCs but not in mature DCs.Citation19 Moreover, ASB2α−/− immature DCs showed defects in cell spreading, podosome rosette formation, and cell migration. Furthermore, ASB2−/− immature DCs exhibited reduced matrix-degrading function leading to defective migration, indicating that ASB2 ubiquitinates filamins to modulate cytoskeletal dynamics, thus regulating cell spreading and cell migration.
E3 Ubiquitin Ligases Regulate Lamellipodium Protrusions
Rac1 is a member of the Rac subfamily of the Rho family of GTPases. Although Rac1 regulates a variety of signaling events, the main function of Rac1 is to control lamellipodium protrusions.Citation36 As a central regulator of cell migration, Rac1 activates the WAVE protein complex, which in turn activates Arp2/3 complex to stimulate the assembly of branching actin filament network. The new polymerized actin network then generates pushing force to promote lamellipodium protrusions.
Rac1 is ubiquitinated by a number of E3 ubiquitin ligases, including HACE1, XIAP, and Skp1-Cul1-F-box, resulting in Rac1 degradation and reduction in lamellipodium extension ().Citation37-Citation40 On the other hand, sumoylation of Rac1 by PIAS3, a SUMO ligase, stimulates its activation and lamellipodium protrusions.Citation41 Smurf1-mediated ubiquitination of TRAF4 also promotes Rac1 activation.Citation42
HACE1
The gene of HACE1 (HECT domain and ankyrin repeat-containing E3 ubiquitin-protein ligase 1) is mapped to a region of chromosome 6q21 implicated in multiple human malignancies.Citation43 HACE1 encodes a 102 kDa protein, featuring six ankyrin protein–protein interaction motifs with sequence similarity to those of INK4A, and a C-terminal HECT ubiquitin-protein ligase domain.Citation44
The active small GTPase Rac1 is a substrate for HACE1.Citation37,Citation38 HACE1 binds preferentially active (GTP-bound) Rac1. The binding of HACE1 to Rac1 is stimulated by hepatocyte growth factor (HGF) signaling, resulting in the poly-ubiquitylation of Rac1 at lysine 147, and consequently, its proteasomal degradation. Overexpression of HACE1 stimulates Rac1 ubiquitylation, whereas depletion of HACE1 by RNAi blocks the ubiquitylation of active Rac1 and increases GTP-bound Rac1 cellular levels and accumulation of Rac1 in membrane ruffles. Furthermore, an ubiquitination-resistant Rac1 mutant rescues the migration defect of Rac1-null cells to a greater extent than wild-type Rac1. These findings indicate that HACE1 suppresses cell migration through ubiquitinating active Rac1.
HACE1 is widely expressed in human tissues, with strong expression in heart, brain, placenta, kidney, and pancreas. It localizes predominantly in the endoplasmic reticulum (ER) and the cytoplasm, although it also presents in other fractions of cells.
HACE1 is epigenetically inactivated in human Wilms' tumors.Citation44 The expression of HACE1 is markedly downregulated in a variety of human cancers.Citation43 HACE1 also inhibits the Rac1-dependent DNA damage.Citation45 Genetic deletion of HACE1 in mice or HACE1 inactivation in human tumor cell lines result in an increase in Rac1 levels, consequently leading to an increase in the NADPH oxidase-dependent reactive oxygen species and DNA damage responses. Also, genetic deletion of HACE1 in mice results in spontaneous tumor development or renders mice susceptible to environmental and genetic cancer triggers,Citation43 probably through Rac1-mediated increase in DNA damage and tumor growth. These studies indicate that HACE1 is a tumor suppressor. However, it remains to be determined whether HACE1-regulated cell migration is involved tumor progression and metastasis.
XIAP
XIAP is a member of the inhibitor of apoptosis family of proteins (IAP). XIAP contains three N-terminal baculoviral IAP repeat (BIR) domains, followed by an UBA domain and a C-terminal RING finger.Citation46 The well-known function of XIAP is to inhibit caspases and prevent cell death, but it has been shown that it suppresses cell migration through causing Rac1 ubiquitination.Citation46 Oberoi et al. reported that XIAP bound to Rac1 and ubiquitinated it at K147, leading to its degradation.Citation39 Depletion of XIAP caused an increase in Rac1 protein levels and promoted cell migration, indicating that XIAP suppresses cell migration through mediating Rac1 ubiquitination. On the other hand, it has also been reported that either knockout or knockdown of XIAP significantly inhibits cell migration.Citation47 Thus, the role of XIAP in cell migration remains to be clarified.
Skp1-Cul1-F-box
Skp1-Cullin-F-box (SCF)-containing complex is a multi-protein E3 ubiquitin ligase complex.Citation48 SCF contains three core subunits: Skip1, Cullin, and F-box protein. Skp1 contributes to the recognition and binding of the F-box. Cullin (CUL1), as the major structural scaffold of the SCF complex, links the skp1 domain with the Rbx1 domain. The F-box protein functions as the bridge linking the ligase complex and specific substrates via its F-box domain and substrate-binding motif. FBXL19, an orphan member of the Skp1-Cullin-F-box family of E3 ubiquitin ligases, ubiquitinates Rac1 at Lys166, resulting in its proteasomal degradation.Citation40 Overexpression of FBXL19 caused a reduction in Rac1 levels and lemellipodium formation and inhibited cell migration. Phosphorylation of Rac1 at Ser71 by Akt was required for FBXL19-mediated Rac1 ubiquitination and degradation. Substitution of either Ser71 with Ala or Lys166 with Arg blocked FBXL19-mediated Rac1 ubiquitination and degradation. Furthermore, expression of FBXL19 inhibited migration of the cells expressing the WT Rac1, but not that of the cells that express mutant Rac1K166R. Thus, SCF/FBXL19 targets Rac1 for ubiquitination and degradation, which is in turn regulated by AKT, thus inhibiting lamellipodium protrusions and cell migration.
PIAS3
PIAS3, an E3 SUMO ligase, bound to Rac1 and caused sumoylation within the polybasic regions of Rac1 in response to hepatocyte growth factor (HGF) stimulation.Citation41 Furthermore, PIAS3-mediated SUMOylation was essential for Rac1 activation and Rac1-mediated lamellipodium extension, cell migration, and invasion.
Smurf1 and TRAF4
TRAF4 is a member of the TNF receptor-associated factor (TRAF) family. However, unlike other family members that function as scaffolds for TNF receptor, Toll-like receptor and IL-1 receptor signaling complexes, TRAF4 does not interact with TNF receptors.Citation49 Instead, TRAF4 bound the nicotinamide adenine dinucleotide phosphate reduced (NADPH) oxidase adaptor p47phox and localized to nascent, focal complex-like structures in motile endothelial cells.Citation50 Active TRAF4 initiated robust membrane ruffling through activating Rac1, PAK1, and the oxidase, thus promoting cell migration. Most recently, Zhang’s group reported that TRAF4 was ubiquitinated at K190 by Smurf1. TRAF4 ubiquitination by Smurf1 was essential for TRAF4-depended rac1 activation and migration of both normal mammary epithelial and breast cancer cells.Citation42 However, the molecular mechanism by which TRAF4 ubiquitination regulates Rac1 activation remains to be elucidated.
E3 Ubiquitin Ligases Regulate Cell Adhesion Dynamics
Cell migration is a dynamic process that requires focal adhesion (FA) assembly at the front of cells with concomitant disassembly at the trailing edges of cells.Citation20,Citation51 Talin, an actin and β-integrin tail-binding protein, is essential for integrin activation.Citation52 Talin is cleaved by calpain into an N-terminal head domain and a C-terminal rod domain.Citation53 The talin head domain contains a FERM (band fourpoint-one, ezrin, radixin, moesin homology) domain, and is responsible for the binding of talin to β-integrin tail and integrin activation.Citation54 Phosphatidylinositol 4 phosphate 5-kinase type I γ (PIPKIγ90) is an enzyme that catalyzes the production of phosphatidylinositol 4,5-bisphosphate (PIP2), a molecule that is well implicated in FA formation.Citation55 PIPKIγ90 interacts with talin and localizes at FAs.Citation56,Citation57 Both talin and PIPKIγ90 are essential for FA dynamics and cell migration.Citation25,Citation55,Citation58,Citation59 We recently showed that E3 ubiquitin ligases Smurf1 and HECTD1 regulate FA assembly and disassembly during cell migration ().Citation25,Citation59
Smurf1
The role of Smurf1 in regulating FA dynamics has been reviewed previously.Citation6 Briefly, the talin head (TH) domain, when released by calpain, stimulates integrin activation. Smurf1 binds to TH and causes TH ubiquitination and degradation, consequently leading to FA disassembly.Citation25 Thus, Smurf1 in concert with calpain mediates FA disassembly during cell migration. This action of Smurf1 likely occurs during the tail retraction, a migration step where calpain is required, because the role of Smurf1 at the leading edges is prevented by Cdk5-mediated phosphorylation of talin. Since this function of Smurf1 is dependent of calpain, we expect it mainly plays a role in FA disassembly in those cells where calpain prevails.
HECTD1
The gene of HECTD1 (HECT domain containing 1) is mapped to a region of chromosome 14q12 (www.genecards.org). Deletion or duplication in this region is associated with severe mental retard and Rett-like syndrome.Citation60 The human HECTD1 consists of 2610 amino acid residues, with apparent molecular weight 270 kDa, containing four N-terminal ankyrin repeats, a central MIB/HERC2 domain, and an unusual HECT ubiquitin-protein ligase domain at the C terminus. The HECTD1 protein is expressed in human blood cells, liver, and heart (from MOPED and PaxDb). Its presence in other tissues remains to be determined. HECTD1 has a cytoplasmic localization when it is epitopically expressed HEK293 cells.Citation61 It has been reported that HECTD1 is localized to the leading edge in some cells. In fact, we found that HECTD1 partially co-localized with talin at the actin arcs immediately behind the leading edges,Citation59 where FA in association with actin filaments and myosin, implying a role of HECTD1 in cell migration.
It has been well demonstrated that PIPKIγ90 plays a central role in focal adhesion assembly and disassembly, a key step in cell migration, but the underlying mechanism was unknown. We showed that PIPKIγ90 was ubiquitinated at K97 by HECTD1, resulting in its degradation, which was prevented by proteasome inhibitors.Citation59 Depletion of HECTD1 by shRNAs resulted in an increase in endogenous PIPKIγ90 levels, indicating that HECTD1 is responsible for the ubiquitination of PIPKIγ90.
HECTD1-mediated ubiquitination of PIPKIγ90 is required for focal adhesion dynamics and cell migration.Citation59,Citation62 Expression of PIPKIγ90K97R, an ubiquitination-resistant mutant that retains its ability for talin-binding, inhibited both FA assembly and disassembly. Also, depletion of HECTD1 significantly suppressed both FA assembly and disassembly. In contrast, expression of PIPKIγ90K97R,W647F, which is resistant to degradation but has a reduced binding capacity for talin, did not significantly impair FA assembly and disassembly rates. Based on these results, we conclude that PIPKIγ90 ubiquitination by HECTD1 and subsequently degradation cause a reduction in the production of PIP2, which is required for talin and vinculin activation, thus reducing the binding of the β integrin tail to talin and consequently leading to FA disassembly.
The role of PIPKIγ90 ubiquitination in focal adhesion assembly is less clear.Citation59 Either expression of ubiquitination-resistent PIPKIγ90K97R or knockdown of HECTD1 inhibited focal adhesion assembly; also overexpression of PIPKIγ90 suppressed integrin activation. Since PIPKIγ90 and the β integrin tail compete for the same site on talin, PIPKIγ90 ubiquitination and degradation might facilitate the talin-integrin interaction. However, it has been shown that talin-PIPKIγ90-β1 integrin exist as a complex in migrating cells. Thus, the mechanisms by which PIPKIγ90 ubiquitination regulates FA assembly remain to be determined. Nevertheless, PIPKIγ90 ubiquitination by HECTD1 and consequent degradation modulate the on-site production of PIP2, thus regulating focal adhesion dynamics and cell migration. The study provides new insights into the molecular mechanisms regulating cell adhesion and migration.
On the other hand, Zohn’s group reported that HECTD1 bound and ubiquitinated Hsp90 to form K63-linked poly-ubiquitin chains, thus inhibiting the secretion of Hsp90, and consequently, cell migration.Citation61 However, this seems to be inconsistent with the role of HECTD1 in the neural tube closure,Citation63 a cell migration-depended developmental process, but could be the results of cell-type difference. Thus, the role of HECTD1 in regulating Hsp90 secretion and cell migration deserves further investigation.
Conclusions and Future Prospects
More and more E3 ubiquitin ligases have been shown to modulate migration machineries to regulate cell migration. They regulate stress fiber formation, cell stiffness and polarity, lamellipodium protrusions, and FA assembly and disassembly to govern cell migration through ubiquitinating the key molecules that control these processes. These molecules include RhoA, Rac1, talin, PIPKIγ, and filamin. Future studies will focus on determining how these E3 ubiquitin ligases are regulated by different signaling pathways. It is of great importance to examine how ubiquitin ligases temporally and spatially regulate the degradation of their substrates using advanced photo-manipulated methods. For example, photo convertible fluorescence protein could be used to determine the temporal and spatial protein degradation in live cells. For full understanding of the roles of E3 ubiquitin ligases, it is important to identify other E3 ubiquitin ligases and novel substrates that regulate these processes, particularly FA dynamics. Finally, identification of specific E3 ubiquitin ligase inhibitors will benefit basic and clinical studies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work is supported by an American Cancer Society Research Scholar Grant (RSG-13-184-01-CSM), an American Cancer Society Institutional Research Grant (IRG 85-001-22), and a start-up fund from Markey Cancer Center, University of Kentucky (to C Huang). S Deng is a visiting scholar from North Sichuan Medical College supported by China Scholarship council.
References
- Finley D, Chau V. Ubiquitination. Annu Rev Cell Biol 1991; 7:25 - 69; http://dx.doi.org/10.1146/annurev.cb.07.110191.000325; PMID: 1667082
- Pickart CM. Ubiquitin in chains. Trends Biochem Sci 2000; 25:544 - 8; http://dx.doi.org/10.1016/S0968-0004(00)01681-9; PMID: 11084366
- Sun L, Chen ZJ. The novel functions of ubiquitination in signaling. Curr Opin Cell Biol 2004; 16:119 - 26; http://dx.doi.org/10.1016/j.ceb.2004.02.005; PMID: 15196553
- Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem 2001; 70:503 - 33; http://dx.doi.org/10.1146/annurev.biochem.70.1.503; PMID: 11395416
- Lee J, Ishihara A, Jacobson K. How do cells move along surfaces?. Trends Cell Biol 1993; 3:366 - 70; http://dx.doi.org/10.1016/0962-8924(93)90084-E; PMID: 14731652
- Huang C. Roles of E3 ubiquitin ligases in cell adhesion and migration. Cell Adh Migr 2010; 4:10 - 8; http://dx.doi.org/10.4161/cam.4.1.9834; PMID: 20009572
- Schoenwaelder SM, Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr Opin Cell Biol 1999; 11:274 - 86; http://dx.doi.org/10.1016/S0955-0674(99)80037-4; PMID: 10209151
- Tojkander S, Gateva G, Lappalainen P. Actin stress fibers--assembly, dynamics and biological roles. J Cell Sci 2012; 125:1855 - 64; http://dx.doi.org/10.1242/jcs.098087; PMID: 22544950
- Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, Ingber DE. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J 2002; 16:1195 - 204; http://dx.doi.org/10.1096/fj.02-0038com; PMID: 12153987
- Mizutani T, Haga H, Koyama Y, Takahashi M, Kawabata K. Diphosphorylation of the myosin regulatory light chain enhances the tension acting on stress fibers in fibroblasts. J Cell Physiol 2006; 209:726 - 31; http://dx.doi.org/10.1002/jcp.20773; PMID: 16924661
- Nagayama M, Haga H, Kawabata K. Drastic change of local stiffness distribution correlating to cell migration in living fibroblasts. Cell Motil Cytoskeleton 2001; 50:173 - 9; http://dx.doi.org/10.1002/cm.10008; PMID: 11807938
- Nakamura F, Stossel TP, Hartwig JH. The filamins: organizers of cell structure and function. Cell Adh Migr 2011; 5:160 - 9; http://dx.doi.org/10.4161/cam.5.2.14401; PMID: 21169733
- Nakamura F, Osborn TM, Hartemink CA, Hartwig JH, Stossel TP. Structural basis of filamin A functions. J Cell Biol 2007; 179:1011 - 25; http://dx.doi.org/10.1083/jcb.200707073; PMID: 18056414
- Gardel ML, Nakamura F, Hartwig JH, Crocker JC, Stossel TP, Weitz DA. Prestressed F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc Natl Acad Sci U S A 2006; 103:1762 - 7; http://dx.doi.org/10.1073/pnas.0504777103; PMID: 16446458
- Xu Y, Bismar TA, Su J, Xu B, Kristiansen G, Varga Z, Teng L, Ingber DE, Mammoto A, Kumar R, et al. Filamin A regulates focal adhesion disassembly and suppresses breast cancer cell migration and invasion. J Exp Med 2010; 207:2421 - 37; http://dx.doi.org/10.1084/jem.20100433; PMID: 20937704
- Kiema T, Lad Y, Jiang P, Oxley CL, Baldassarre M, Wegener KL, Campbell ID, Ylänne J, Calderwood DA. The molecular basis of filamin binding to integrins and competition with talin. Mol Cell 2006; 21:337 - 47; http://dx.doi.org/10.1016/j.molcel.2006.01.011; PMID: 16455489
- Wang HR, Zhang Y, Ozdamar B, Ogunjimi AA, Alexandrova E, Thomsen GH, Wrana JL. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science 2003; 302:1775 - 9; http://dx.doi.org/10.1126/science.1090772; PMID: 14657501
- Chen Y, Yang Z, Meng M, Zhao Y, Dong N, Yan H, Liu L, Ding M, Peng HB, Shao F. Cullin mediates degradation of RhoA through evolutionarily conserved BTB adaptors to control actin cytoskeleton structure and cell movement. Mol Cell 2009; 35:841 - 55; http://dx.doi.org/10.1016/j.molcel.2009.09.004; PMID: 19782033
- Lamsoul I, Métais A, Gouot E, Heuzé ML, Lennon-Duménil A-M, Moog-Lutz C, Lutz PG. ASB2α regulates migration of immature dendritic cells. Blood 2013; 122:533 - 41; http://dx.doi.org/10.1182/blood-2012-11-466649; PMID: 23632887
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 2003; 302:1704 - 9; http://dx.doi.org/10.1126/science.1092053; PMID: 14657486
- Zhu H, Kavsak P, Abdollah S, Wrana JL, Thomsen GH. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature 1999; 400:687 - 93; http://dx.doi.org/10.1038/23293; PMID: 10458166
- Kwei KA, Shain AH, Bair R, Montgomery K, Karikari CA, van de Rijn M, Hidalgo M, Maitra A, Bashyam MD, Pollack JR. SMURF1 amplification promotes invasiveness in pancreatic cancer. PLoS One 2011; 6:e23924; http://dx.doi.org/10.1371/journal.pone.0023924; PMID: 21887346
- Cui Y, He S, Xing C, Lu K, Wang J, Xing G, Meng A, Jia S, He F, Zhang L. SCFFBXL¹⁵ regulates BMP signalling by directing the degradation of HECT-type ubiquitin ligase Smurf1. EMBO J 2011; 30:2675 - 89; http://dx.doi.org/10.1038/emboj.2011.155; PMID: 21572392
- Xie Y, Avello M, Schirle M, McWhinnie E, Feng Y, Bric-Furlong E, Wilson C, Nathans R, Zhang J, Kirschner MW, et al. Deubiquitinase FAM/USP9X interacts with the E3 ubiquitin ligase SMURF1 protein and protects it from ligase activity-dependent self-degradation. J Biol Chem 2013; 288:2976 - 85; http://dx.doi.org/10.1074/jbc.M112.430066; PMID: 23184937
- Huang C, Rajfur Z, Yousefi N, Chen Z, Jacobson K, Ginsberg MH. Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration. Nat Cell Biol 2009; 11:624 - 30; http://dx.doi.org/10.1038/ncb1868; PMID: 19363486
- Yamaguchi K, Ohara O, Ando A, Nagase T. Smurf1 directly targets hPEM-2, a GEF for Cdc42, via a novel combination of protein interaction modules in the ubiquitin-proteasome pathway. Biol Chem 2008; 389:405 - 13; http://dx.doi.org/10.1515/BC.2008.036; PMID: 18208356
- Sapkota G, Alarcón C, Spagnoli FM, Brivanlou AH, Massagué J. Balancing BMP signaling through integrated inputs into the Smad1 linker. Mol Cell 2007; 25:441 - 54; http://dx.doi.org/10.1016/j.molcel.2007.01.006; PMID: 17289590
- Ying SX, Hussain ZJ, Zhang YE. Smurf1 facilitates myogenic differentiation and antagonizes the bone morphogenetic protein-2-induced osteoblast conversion by targeting Smad5 for degradation. J Biol Chem 2003; 278:39029 - 36; http://dx.doi.org/10.1074/jbc.M301193200; PMID: 12871975
- Shen R, Chen M, Wang YJ, Kaneki H, Xing L, O’keefe RJ, Chen D. Smad6 interacts with Runx2 and mediates Smad ubiquitin regulatory factor 1-induced Runx2 degradation. J Biol Chem 2006; 281:3569 - 76; http://dx.doi.org/10.1074/jbc.M506761200; PMID: 16299379
- Fei C, Li Z, Li C, Chen Y, Chen Z, He X, Mao L, Wang X, Zeng R, Li L. Smurf1-mediated Lys29-linked nonproteolytic polyubiquitination of axin negatively regulates Wnt/β-catenin signaling. Mol Cell Biol 2013; 33:4095 - 105; http://dx.doi.org/10.1128/MCB.00418-13; PMID: 23959799
- Crose LES, Hilder TL, Sciaky N, Johnson GL. Cerebral cavernous malformation 2 protein promotes smad ubiquitin regulatory factor 1-mediated RhoA degradation in endothelial cells. J Biol Chem 2009; 284:13301 - 5; http://dx.doi.org/10.1074/jbc.C900009200; PMID: 19318350
- Wan L, Zou W, Gao D, Inuzuka H, Fukushima H, Berg AH, Drapp R, Shaik S, Hu D, Lester C, et al. Cdh1 regulates osteoblast function through an APC/C-independent modulation of Smurf1. Mol Cell 2011; 44:721 - 33; http://dx.doi.org/10.1016/j.molcel.2011.09.024; PMID: 22152476
- Lu K, Yin X, Weng T, Xi S, Li L, Xing G, Cheng X, Yang X, Zhang L, He F. Targeting WW domains linker of HECT-type ubiquitin ligase Smurf1 for activation by CKIP-1. Nat Cell Biol 2008; 10:994 - 1002; http://dx.doi.org/10.1038/ncb1760; PMID: 18641638
- Ito I, Hanyu A, Wayama M, Goto N, Katsuno Y, Kawasaki S, Nakajima Y, Kajiro M, Komatsu Y, Fujimura A, et al. Estrogen inhibits transforming growth factor β signaling by promoting Smad2/3 degradation. J Biol Chem 2010; 285:14747 - 55; http://dx.doi.org/10.1074/jbc.M109.093039; PMID: 20207742
- Cheng PL, Lu H, Shelly M, Gao H, Poo MM. Phosphorylation of E3 ligase Smurf1 switches its substrate preference in support of axon development. Neuron 2011; 69:231 - 43; http://dx.doi.org/10.1016/j.neuron.2010.12.021; PMID: 21262463
- Ridley AJ. Life at the leading edge. Cell 2011; 145:1012 - 22; http://dx.doi.org/10.1016/j.cell.2011.06.010; PMID: 21703446
- Torrino S, Visvikis O, Doye A, Boyer L, Stefani C, Munro P, Bertoglio J, Gacon G, Mettouchi A, Lemichez E. The E3 ubiquitin-ligase HACE1 catalyzes the ubiquitylation of active Rac1. Dev Cell 2011; 21:959 - 65; http://dx.doi.org/10.1016/j.devcel.2011.08.015; PMID: 22036506
- Castillo-Lluva S, Tan CT, Daugaard M, Sorensen PHB, Malliri A. The tumour suppressor HACE1 controls cell migration by regulating Rac1 degradation. Oncogene 2013; 32:1735 - 42; http://dx.doi.org/10.1038/onc.2012.189; PMID: 22614015
- Oberoi TK, Dogan T, Hocking JC, Scholz R-P, Mooz J, Anderson CL, Karreman C, Meyer zu Heringdorf D, Schmidt G, Ruonala M, et al. IAPs regulate the plasticity of cell migration by directly targeting Rac1 for degradation. EMBO J 2012; 31:14 - 28; http://dx.doi.org/10.1038/emboj.2011.423; PMID: 22117219
- Zhao J, Mialki RK, Wei J, Coon TA, Zou C, Chen BB, Mallampalli RK, Zhao Y. SCF E3 ligase F-box protein complex SCF(FBXL19) regulates cell migration by mediating Rac1 ubiquitination and degradation. FASEB J 2013; 27:2611 - 9; http://dx.doi.org/10.1096/fj.12-223099; PMID: 23512198
- Castillo-Lluva S, Tatham MH, Jones RC, Jaffray EG, Edmondson RD, Hay RT, Malliri A. SUMOylation of the GTPase Rac1 is required for optimal cell migration. Nat Cell Biol 2010; 12:1078 - 85; http://dx.doi.org/10.1038/ncb2112; PMID: 20935639
- Wang X, Jin C, Tang Y, Tang L-Y, Zhang YE. Ubiquitination of tumor necrosis factor receptor-associated factor 4 (TRAF4) by Smad ubiquitination regulatory factor 1 (Smurf1) regulates motility of breast epithelial and cancer cells. J Biol Chem 2013; 288:21784 - 92; http://dx.doi.org/10.1074/jbc.M113.472704; PMID: 23760265
- Zhang L, Anglesio MS, O’Sullivan M, Zhang F, Yang G, Sarao R, Mai PN, Cronin S, Hara H, Melnyk N, et al. The E3 ligase HACE1 is a critical chromosome 6q21 tumor suppressor involved in multiple cancers. Nat Med 2007; 13:1060 - 9; http://dx.doi.org/10.1038/nm1621; PMID: 17694067
- Anglesio MS, Evdokimova V, Melnyk N, Zhang L, Fernandez CV, Grundy PE, Leach S, Marra MA, Brooks-Wilson AR, Penninger J, et al. Differential expression of a novel ankyrin containing E3 ubiquitin-protein ligase, Hace1, in sporadic Wilms’ tumor versus normal kidney. Hum Mol Genet 2004; 13:2061 - 74; http://dx.doi.org/10.1093/hmg/ddh215; PMID: 15254018
- Daugaard M, Nitsch R, Razaghi B, McDonald L, Jarrar A, Torrino S, Castillo-Lluva S, Rotblat B, Li L, Malliri A, et al. Hace1 controls ROS generation of vertebrate Rac1-dependent NADPH oxidase complexes. Nat Commun 2013; 4:2180; http://dx.doi.org/10.1038/ncomms3180; PMID: 23864022
- Galbán S, Duckett CS. XIAP as a ubiquitin ligase in cellular signaling. Cell Death Differ 2010; 17:54 - 60; http://dx.doi.org/10.1038/cdd.2009.81; PMID: 19590513
- Liu J, Zhang D, Luo W, Yu Y, Yu J, Li J, Zhang X, Zhang B, Chen J, Wu X-R, et al. X-linked inhibitor of apoptosis protein (XIAP) mediates cancer cell motility via Rho GDP dissociation inhibitor (RhoGDI)-dependent regulation of the cytoskeleton. J Biol Chem 2011; 286:15630 - 40; http://dx.doi.org/10.1074/jbc.M110.176982; PMID: 21402697
- Jia L, Sun Y. SCF E3 ubiquitin ligases as anticancer targets. Curr Cancer Drug Targets 2011; 11:347 - 56; http://dx.doi.org/10.2174/156800911794519734; PMID: 21247385
- Kedinger V, Rio M-C. TRAF4, the Unique Family Member. In: Wu H, ed. TNF Receptor Associated Factors (TRAFs): Springer New York, 2007:60-71.
- Wu RF, Xu YC, Ma Z, Nwariaku FE, Sarosi GA Jr., Terada LS. Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol 2005; 171:893 - 904; http://dx.doi.org/10.1083/jcb.200507004; PMID: 16330715
- Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells -- over and over and over again. Nat Cell Biol 2002; 4:E97 - 100; http://dx.doi.org/10.1038/ncb0402-e97; PMID: 11944043
- Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin β tails: a final common step in integrin activation. Science 2003; 302:103 - 6; http://dx.doi.org/10.1126/science.1086652; PMID: 14526080
- Nuckolls GH, Turner CE, Burridge K. Functional studies of the domains of talin. J Cell Biol 1990; 110:1635 - 44; http://dx.doi.org/10.1083/jcb.110.5.1635; PMID: 2110569
- Calderwood DA, Zent R, Grant R, Rees DJG, Hynes RO, Ginsberg MH. The Talin head domain binds to integrin β subunit cytoplasmic tails and regulates integrin activation. J Biol Chem 1999; 274:28071 - 4; http://dx.doi.org/10.1074/jbc.274.40.28071; PMID: 10497155
- Wu Z, Li X, Sunkara M, Spearman H, Morris AJ, Huang C. PIPKIγ regulates focal adhesion dynamics and colon cancer cell invasion. PLoS One 2011; 6:e24775; http://dx.doi.org/10.1371/journal.pone.0024775; PMID: 21931851
- Di Paolo G, Pellegrini L, Letinic K, Cestra G, Zoncu R, Voronov S, Chang S, Guo J, Wenk MR, De Camilli P. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1 gamma by the FERM domain of talin. Nature 2002; 420:85 - 9; http://dx.doi.org/10.1038/nature01147; PMID: 12422219
- Ling K, Doughman RL, Firestone AJ, Bunce MW, Anderson RA. Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature 2002; 420:89 - 93; http://dx.doi.org/10.1038/nature01082; PMID: 12422220
- Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, Huttenlocher A. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol 2004; 6:977 - 83; http://dx.doi.org/10.1038/ncb1175; PMID: 15448700
- Li X, Zhou Q, Sunkara M, Kutys ML, Wu Z, Rychahou P, Morris AJ, Zhu H, Evers BM, Huang C. Ubiquitylation of phosphatidylinositol 4-phosphate 5-kinase type I γ by HECTD1 regulates focal adhesion dynamics and cell migration. J Cell Sci 2013; 126:2617 - 28; http://dx.doi.org/10.1242/jcs.117044; PMID: 23572508
- Brunetti-Pierri N, Paciorkowski AR, Ciccone R, Della Mina E, Bonaglia MC, Borgatti R, Schaaf CP, Sutton VR, Xia Z, Jelluma N, et al. Duplications of FOXG1 in 14q12 are associated with developmental epilepsy, mental retardation, and severe speech impairment. Eur J Hum Genet 2011; 19:102 - 7; http://dx.doi.org/10.1038/ejhg.2010.142; PMID: 20736978
- Sarkar AA, Zohn IE. Hectd1 regulates intracellular localization and secretion of Hsp90 to control cellular behavior of the cranial mesenchyme. J Cell Biol 2012; 196:789 - 800; http://dx.doi.org/10.1083/jcb.201105101; PMID: 22431752
- Zheng Q, Li X, Sunkara M, Morris AJ, Wu W, Huang C. Leptin Up-Regulates HECTD1 to Promote Phosphoinositide Metabolism and Cell Migration and Invasion in Breast Cancer Cells. J Pharmacol Clin Toxicol 2013; 1:1001
- Zohn IE, Anderson KV, Niswander L. The Hectd1 ubiquitin ligase is required for development of the head mesenchyme and neural tube closure. Dev Biol 2007; 306:208 - 21; http://dx.doi.org/10.1016/j.ydbio.2007.03.018; PMID: 17442300
