Abstract
Cell migration is a highly regulated multistep process that requires the coordinated regulation of cell adhesion, protrusion, and contraction. These processes require numerous protein–protein interactions and the activation of specific signaling pathways. The Rho family of GTPases plays a key role in virtually every aspect of the cell migration cycle. The activation of Rho GTPases is mediated by a large and diverse family of proteins; the guanine nucleotide exchange factors (RhoGEFs). GEFs work immediately upstream of Rho proteins to provide a direct link between Rho activation and cell–surface receptors for various cytokines, growth factors, adhesion molecules, and G protein-coupled receptors. The regulated targeting and activation of RhoGEFs is essential to coordinate the migratory process. In this review, we summarize the recent advances in our understanding of the role of RhoGEFs in the regulation of cell migration.
Abbreviations
| bFGF | = | basic fibroblast growth factor |
| ECM | = | extracellular matrix |
| DH | = | Dbl-homology |
| DHR | = | DOCK homology region |
| DOCK | = | dedicator of cytokinesis |
| EGF | = | epidermal growth factor |
| FA | = | focal adhesion |
| FN | = | fibronectin |
| GAP | = | GTPase activating protein |
| GEF | = | guanine nucleotide exchange factor |
| GDI | = | guanine nucleotide dissociation inhibitor |
| GPCR | = | G protein-coupled receptor |
| HGF | = | hepatocyte growth factor |
| LPA | = | lysophosphatidic acid |
| MII | = | myosin II |
| PA | = | phosphatidic acid |
| PDGF | = | platelet-derived growth factor |
| PH | = | pleckstrin-homology |
| PIP2 | = | phosphatidylinositol 4, 5-bisphosphate |
| PIP3 | = | phosphatidylinositol (3, 4, 5)-trisphosphate |
Introduction
The cell migration cycle involves a series of highly coordinated steps that starts with polarization and membrane protrusion in the direction of migration.Citation1 These protrusions are then stabilized by forming adhesions that provide a link between the actin cytoskeleton and the extracellular matrix (ECM). These sites of adhesion are called focal adhesions (FA), and serve as traction points for the cell body to contract and move forward. Contraction also promotes the disassembly of the adhesions at the cell rear allowing it to detach. These processes involve hundreds of proteins forming a complex signaling network linked by multiple interactions.Citation2
At the center of this striking cytoskeleton reorganization is the Rho family of GTPases. Rho GTPases are versatile signaling molecules that regulate a diverse set of cellular functions. Rho GTPases function as molecular switches that cycle between an inactive GDP-bound and an active GTP-bound conformation. The activation of Rho proteins is mediated by guanine nucleotide exchange factors (RhoGEFs), which catalyze the exchange of GDP to GTP.Citation3 Once in the active conformation, Rho GTPases interact with one of several downstream effectors that modulate a variety of intracellular processes.Citation4 To turn the switch off, GTP has to be hydrolyzed to GDP, a reaction that is stimulated by GTPase-activating proteins (GAPs).Citation5 In addition, inactive Rho GTPases are extracted from cell membranes by Rho-specific guanine nucleotide dissociation inhibitors (RhoGDIs) to prevent their inappropriate activation and to protect them from misfolding and degradation.Citation6
RhoGEFs
There are approximately 80 RhoGEFs in the human genome, encoded by two unrelated gene families:Citation3,7 the Dbl family, which comprises 69 members in humans, and the DOCK family, with 11 members.Citation3,7 The Dbl family is characterized by the presence of a Dbl homology (DH) catalytic domain, followed by an adjacent pleckstrin homology (PH) domain, C-terminal to the DH domain.Citation3 Together, in most cases, they provide the minimal structural unit that is required to catalyze the exchange reaction in vivo.Citation3 In most GEFs, the DH–PH domains are flanked by a diverse array of protein–protein and protein–lipid interaction domains.Citation3 These domains help regulate the intrinsic catalytic activity of RhoGEFs, their intracellular localization, and their association with other proteins.
The DOCK (dedicator of cytokinesis) family of GEFs has been characterized more recently.Citation7 DOCK proteins are structurally and mechanistically unrelated to the Dbl-family, and act on Rac and/or Cdc42, but not on RhoA. The DOCK GEFs are characterized by the presence of a conserved catalytic domain, the DOCK Homology Region 2 (DHR2), and a phospholipid-binding domain (DHR1) that can target the GEFs to the membrane.Citation8 DHR2 domains share no primary sequence homology with DH domains.
Between the two RhoGEF families, there are approximately four times more RhoGEFs than Rho GTPases. This means that a single GTPase can be activated by multiple GEFs and may indicate some overlap or redundancy in their functions. Since several RhoGEFs can activate more than one GTPase, the effective number of RhoGEFs that can act on a single Rho GTPase is even higher. There are at least 25 RhoGEFs that can activate each of one of the major Rho proteins, RhoA, Rac1, and Cdc42.Citation3,9 This number is probably an underestimation, since the specificities of many RhoGEFs have not been completely characterized yet. Since most of RhoGEFs are widely expressed, most cell types usually express several RhoGEFs for each of the GTPases at any given time. The diversity in their domain structure is what allows RhoGEFs with similar specificity to be regulated by different signaling pathways.
The last decade has seen an explosion in the number of publications characterizing the functions of different RhoGEFs, and there seems to be a perception that there is little left to learn. We have made significant progress in understanding how RhoGEFs are activated and targeted to particular locations in the cell; how they interact with other proteins, and how their expression is regulated. However, we are only starting to understand the mechanisms that control this complex network of regulatory proteins. Here, we discuss recent findings on the role of RhoGEFs on the regulation of cell migration. We focus our attention on the RhoGEFs for which a significant body of evidence has been accumulated linking them directly to cell motility, and for the sake of organization, have classified them in what we believe are their main functions associated with cell migration (see ).
Table 1. RhoGEFs involved in cell migration
Polarization and Protrusion
In response to migration-inducing factors, cells polarize and form a protrusive area in the direction of migration and a retracting rear that are defined by distinct cell signaling events.Citation1 Actin polymerization at the cell front drives the extension of the lamellipodium and filopodia. At the leading edge of the lamellipodium, the cell forms adhesions that connect the ECM to the actin cytoskeleton to anchor the protrusion and contract the cell body. Rho GTPases operate at each step to promote directional migration by regulating leading edge formation. Several studies have shown that RhoA, Rac1, and Cdc42 are activated at the front of migrating cells,Citation10 and biochemical evidence suggest that extensive crosstalk between them may regulate one another.Citation11 However, their spatiotemporal regulation and coordination remains to be characterized, in particular, how the upstream (GEFs/GAPs) and downstream effectors are targeted and regulated in response to a particular stimulus.
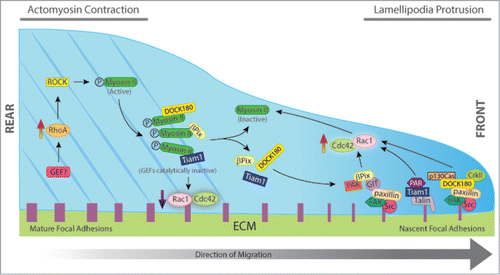
One of the key events controlling cell polarization is the localized activation of Rac at the leading edge, which is critical for both establishing and maintaining polarized protrusion.Citation1 The Rac-GEFs βPix and DOCK180 are implicated in Rac1 activation at the front of migrating cells.Citation12,13 Both βPix and DOCK180 are highly polarized and localized primarily in adhesions near the leading edge of protrusions. DOCK180 and βPix are recruited to the plasma membrane through their interaction with the paxillin-p130Cas-CrkII and paxillin-GIT-PAK complexes, respectively.Citation13,14 Non-receptor tyrosine kinases like Src or FAK phosphorylate paxillin create docking sites that recruit these complexes to adhesions.Citation15,16 Thus, it appears that the dynamics of adhesion/protrusion may be controlled by different signaling mechanisms.
Pix
βPix (also known as Cool-1 and ARHGEF7) has been initially characterized as a GEF for Cdc42 and/or Rac1, and is one of the most extensively studied RhoGEFs.Citation17 Interestingly, βPix not only interacts with Cdc42 and Rac1 through its catalytic domain, but also binds to Cdc42-GTP and to Rac1 in a nucleotide-independent manner.Citation18,19 βPix was originally identified as a binding partner to p21-activated kinase (PAK) family of Cdc42/Rac1-activated kinases.Citation17 Through the interaction with βPix, PAK is targeted to focal complexes.Citation17,20 PAK competes with Rac1 for binding to βPix, and in the absence of PAK, there is more βPix available for Rac1 binding, resulting in increased Rac1 activation and enhanced cell migration.Citation19 Moreover, FAK can bind to and phosphorylate βPix, and thereby, enhance βPix binding to Rac1 and the translocation of Rac1 to adhesions.Citation21,22
In addition to binding to PAK, βPix binds to a diverse array of adaptor and signaling proteins to modulate a complex protein–protein interaction network that regulates cell adhesion and motility.Citation23 Regulation of cell migration by βPix involves the GIT proteins, an ARF–GAP family of scaffolding proteins that bind to βPix, paxillin, and several other proteins.Citation24 The GIT family has two members, GIT1 and GIT2, and they both co-localize with βPix at focal complexes.Citation24-26 GIT may be required to target PAK and βPix to adhesions through its association with paxillin ( ).Citation25 At adhesions, PAK may regulate actin remodeling and FA turnover, whereas βPix could function as a GEF to activate Rac.Citation25,27 However, the importance of the catalytic activity of βPix is still a matter of debate, and it has been proposed it may function exclusively as a scaffold to recruit PAK to adhesions.Citation28 Several studies have also demonstrated that βPix, PAK, and GIT form a constitutively associated complex that shuttles between the endocytic compartment and the membrane.Citation29,30 Recycling the GIT/PAK/βPix may be important to target it to the edge of lamellipodia where new adhesions are being assembled. Recently, Valdes and collaborators reported the interaction of the endosomal protein sorting nexin 27 (SNX27) with βPix in complex with GIT family of proteins.Citation31 SNX27 regulates the trafficking of the βPix/GIT complex between the early endosome and FAs, and thereby, influences cell migration. Knockdown of SNX27 restricts the intracellular movement of βPix to FAs leading to defects in cell migration.Citation31 The precise function of the GIT complex in cell motility is still poorly understood, and there is conflicting evidence on whether it affects Rac-mediated protrusions positivelyCitation13 or negatively.Citation32
Figure 1. Schematic representation of the effects of RhoGEFs on focal adhesion dynamics and cell migration. Cell migration requires the coordinated spatiotemporal regulation of cell adhesion, protrusion, and contraction. Different GEFs such as βPix, DOCK180, and Tiam1 are required at the cell front for lamellipodial protrusion and rapid nascent adhesion turnover. Following stimulation, βPix, DOCK180, and Tiam1 are recruited to nascent adhesions through their interaction with kinases and adaptor proteins to form signaling complexes that control the local activation of Rac1 (GIT/βPix/PAK, p130Cas/DOCK180/CrkII, Talin/Tiam/Par complex). Rac1 activation induces the assembly of dendritic actin networks by polymerizing actin filaments at the leading edge to push the membrane forward. At the cell rear, RhoA-mediated activation of ROCK induces phosphorylation of myosin light chain, which promotes the assembly of actin-Mysoin II (MII) filaments. After MII becomes active, it interacts with βPix, DOCK180, and Tiam1 colocalizing along the actin stress fibers. When in complex with MII, all three GEFs are catalytically inactive. Thus, increased activation of MII results in large actin bundles, large and stable adhesions, decreased signaling to Rac/Cdc42, and decreased protrusion. Inactivation of MII stimulates the release of the GEFs and activation of Rac1 and Cdc42. The resultant Rac1 activation further inactivates MII, thus forming a positive feedback loop, which contributes to persistence of directional migration.
βPix belongs to a group of GEFs, which encode a PDZ-binding motif at their C terminus, a group that also includes Syx1 and Net1 among others.Citation33 PDZ domains are protein–protein interactions domains that act as scaffolds to concentrate signaling molecules at specialized regions in the cell. We have previously proposed that the interaction between Rho-GEFs and PDZ-domain proteins can function as a general mechanism to control Rho-GEFs targeting and activation, helping to restrict and concentrate the exchange activity at appropriate subcellular destinations.Citation33 The intracellular distribution of βPix has been shown to be modulated by PDZ domain-containing proteins, including Scribble, SNX27, and Tax-interacting protein 1 (TIP-1).Citation31, Citation34-36 Scribble is a conserved protein required for maintenance of epithelial cell polarization.Citation37 In astrocytes, Scribble binds to and regulates the targeting of βPix to the leading edge during the establishment of cell polarity.Citation34,35 Scribble controls some of the same processes regulated by Cdc42, including centrosome and Golgi re-orientation, protrusion formation, and cytoskeletal polarization. Depletion of either Scribble or βPix expression inhibits Cdc42 recruitment to the leading edge, and the cells fail to form protrusion and establish a polarized phenotype.Citation34 These results indicate that, at least in astrocytes, βPix may be responsible for Cdc42 activation during polarization.Citation34 In fibroblasts, however, Cdc42 activates Pak1/2 at the leading edge of migrating cells, which promotes the recruitment of βPix and the localized activation of Rac, suggesting that different pools of βPix may independently activate Rac or Cdc42 at different cellular locations to control distinct processes during cell protrusion and polarization.Citation38
The PDZ protein TIP-1 modulates the interaction dynamics and the intracellular distribution of the Scribble/βPix complex by competing with Scribble in the selective binding to βPix.Citation36 TIP-1 knockdown enhances the protein interaction between βPix and Scribble and results in aberrant localization of βPix. In addition, Rac activation during wound healing decreases significantly and is accompanied by an increase in active RhoA. These effects are also accompanied by impaired motility.Citation36
Myosin II (MII) has been recently reported to bind to the DH domain of βPix, and is emerging as a major protein responsible for symmetry breaking and organization of the actin bundles that define the rear during the process of cell polarization.Citation39 Binding to MII inhibits βPix activity toward Rac1 and Cdc42. Accordingly, inactivation of MII activity induces the release of βPix from MII and activation of Rac1 and Cdc42 leading to lamellipodia and focal complexes formation.Citation39 In agreement with these results, when MII contractility is inhibited, βPix accumulates in assembling nascent adhesions and promotes Rac1 activation. In contrast, during MII-mediated FA maturation, βPix dissociates from FAs ( ).Citation40 Vicente-Manzanares and collaborators have also implicated βPix in MII-dependent front-back polarity in migrating cells.Citation41 They showed that both MIIA and MIIB coordinately define and assemble the rear and synergize to form actomyosin bundles. This MII-dependent bundling regulates adhesive signaling by restricting Rac activation.
In addition to βPix, MII regulates a surprising number of Dbl GEF family members through direct binding with the DH-PH module, including FGD1, Kalirin, LARG, DOCK180, Tiam1, GEF-H1, Dbl, and Trio.Citation39 Although there are some quantitative differences among these interactions, this may represent a conserved molecular mechanism for GEF regulation by MII in cell protrusion and adhesion.
DOCK180
Another key regulator of Rac activation during cell migration is DOCK180.Citation42 DOCK180 (DOCK1) is one of the best characterized members of the DOCK family of GEFs and was originally identified as a binding protein for the proto-oncogene product c-Crk.Citation43,44 The targeting and activation of DOCK180 is regulated through its interaction with different signaling and adaptor proteins.Citation45 DOCK180 forms a complex with ELMO (engulfment and cell motility) through its SH3 domain.Citation46,47 There are three ELMO proteins in mammals, and they all seem to be scaffold proteins, with no obvious catalytic activity.Citation42 The formation of an ELMO/DOCK180 complex is essential for efficient Rac1 activation during cell polarization and migration.Citation47 The initial studies on DOCK180 suggested it functions as a bipartite GEF that is catalytically active only when bound to ELMO.Citation43,47 However, it is still not clear if binding to ELMO is required for DOCK180 to be catalytically active, and there is contradictory evidence on this matter.Citation48,49 ELMO also functions as a RhoG effector, connecting RhoG with DOCK180 to regulate Rac activation.Citation50 Once activated, RhoG binds to ELMO and forms a ternary complex with ELMO and DOCK180. The interaction of RhoG with ELMO induces translocation of the ELMO/DOCK180 complex from the cytoplasm to the plasma membrane, which activates Rac and stimulates cell migration.Citation51 Thus, activation of RhoG regulates cell migration through ELMO and DOCK180-dependent activation of Rac.Citation51
DOCK180 and ELMO also function as a node between the Rho and the Arf families of small GTPases. The coordinated activation of Arf and Rac GTPases at the leading edge stimulates migration in epithelial cells.Citation52 This crosstalk depends upon the assembly of a multi-protein complex that contains the Arf-GEF ARNO/cytohesin2, DOCK180, and ELMO, which are brought together through their interaction with the scaffolding proteins GRASP/Tamalin and IPCEF/CNK3.Citation53,54 GRASP provides the physical link between Arf6 and Rac by binding both DOCK180 and ARNO.Citation53 This signaling pathway from ARNO to DOCK180 is independent of RhoG.Citation52 The precise role of ARNO/Arf-mediated Rac activation remains to be elucidated, but it probably involves the regulated targeting of DOCK180 and Rac to a particular subcellular localization where they modulate actin protrusion and cell migration.
DOCK180 activity can also be regulated downstream of chemokine receptor-mediated pathways in breast cancer and endothelial cells.Citation55,56 Binding of CXCL12 to CXCR4 induces the dissociation of heterotrimeric G-proteins into Gαi and Gβγ. The Gαi2 subunit gets activated, and associates with ELMO, which recruits DOCK180 to the membrane, where it activates Rac1 and Rac2 and promotes actin polymerization required for cell migration and invasion.Citation55
The interaction between DOCK180 with CrkII is also required for cell migration.Citation57 Upon coexpression of DOCK180, ELMO, and CrkII, cells adopt a highly elongated morphology and migration is significantly increased.Citation46 Following integrin stimulation, DOCK180 is phosphorylated and forms a complex with CrkII,Citation57 which is then recruited to FAs by binding to the tyrosine phosphorylated adaptor proteins p130Cas and paxillin.Citation12,14,57 Once at FAs, DOCK180 is activated and amplifies the downstream Rac signal ( ). Interestingly, the interaction of DOCK180 with CrkII is neither necessary for the formation and targeting of the RhoG/DOCK180/ELMO ternary complex, nor required for the downstream Rac activation associated to its formation.Citation51 The association between DOCK180 and its binding partners can also be regulated by receptor tyrosine kinases. Both EGFR and PDGFR induce the phosphorylation of DOCK180 by Src family kinases, which promotes the association between DOCK180, CrkII, and p130Cas, resulting in Rac activation and increased migration of human glioma cells.Citation58,59
DOCK180 is a versatile molecule that can be assembled into different signaling modules to regulate independent pathways that respond to different stimuli.Citation33 The interaction of DOCK180 with the adaptor protein ANKRD28 represents a good example of this scenario. ANKRD28 is a scaffolding protein comprised of 26 ankyrin repeat domains, which competes with ELMO for binding to DOCK180.Citation60 Through association with ANKRD28, DOCK180 regulates stability of FAs, via Rac, and thereby, regulates cell migration. ANKRD28 cooperates with DOCK180 to localize the FA proteins, CrkII, p130Cas, and paxillin to the peripheral region of the cells.Citation60 Depletion of ANKRD28 retards cell migration in mammalian cells, as does DOCK180.Citation60 Most of the effects observed when either ANKRD28 or ELMO expression is silenced appear to be similar. However, in cells overexpressing ANKRD28, but not ELMO, p130Cas gets hyperphosphorylated and cells have long tails that fail to retract, whereas ELMO overexpression induces the formation of lamellipodia along the circumference of the cell.Citation60 These results suggest that these two DOCK180 complexes may activate Rac at different locations of the cell to regulate lamellipodia formation and tail retraction.Citation60
DOCK180 has also been shown to couple PtdIns(3,4,5)P3 signaling to Rac activation during cell migration.Citation8 PtdIns(3,4,5)P3 is a key player in the establishment of the initial asymmetry during polarization and interacts with the DHR-1 domain of DOCK180 in vitro and in vivo.Citation61 PtdIns(3,4,5)P3 mediates the translocation of the DOCK180-CrkII-ELMO signaling complex to the leading edge.Citation61 Mutations in the DHR-1 domain of DOCK180 block Rac-dependent cell elongation and cell migration, even though Rac1 activation levels are not affected, suggesting both the activity and localization of Rac are required for proper DOCK180 signaling.Citation8
Tiam1
The Rac-specific GEF Tiam1 has been mostly associated to the development of cadherin-mediated cell–cell adhesions.Citation62 However, a series of recent studies have now shown that Tiam1 also plays a role in the development of front-rear polarity in free migrating cells.Citation63-65 In epithelial cells, cadherins signal through Cdc42 and/or Rac to activate the Par polarity complex, which in turn, stimulates the formation of tight junctions (TJ). The Par polarity complex consists of Par3, Par6, and atypical PKC (aPKC), and regulates cell polarization in many different contexts, including apico-basal, neuronal, and front-rear polarity.Citation62 Tiam1 has been shown to associate with the Par complex by binding to Par3 to regulate the assembly.Citation66,67 There is evidence that during apical-basal polarity in epithelial cells, Tiam1 promotes the assembly of TJ through the activation of Rac and the Par complex but independently of Cdc42.Citation62 Thus, by promoting the formation of cadherin-mediated adhesions and the development of an epithelial morphology, Tiam1 activity can inhibit cell migration, whereas the loss of Tiam1 can indirectly enable cell migration by promoting the disassembly of cell–cell junctions.Citation68 In contrast, in keratinocytes, which are highly polarized, the loss of Tiam1 (KO) correlates with reduced Rac activity and a complete loss of polarization.Citation63 Interestingly, random migration is only slightly affected, whereas directionality is completely lost and chemotaxis impaired.Citation63 In these cells, Tiam1 also binds to and activates the Par complex, and when Par3 is depleted directionality is also reduced. Blocking PKCζ function also inhibits directionality and chemotaxis. In cells that exhibit front-rear polarization, both Par3 and Tiam1, and to a lesser extent, PKCζ, are enriched at the leading edge. These results suggest that Tiam1 controls chemotaxis and persistence through the Par polarity complex.Citation63
A recent article has shed some light into the mechanisms by which Tiam1 regulates polarized cell migration in glioma cells.Citation64 Using TIRF microscopy, Tiam1 is found at FAs where it partially colocalizes with the integrin-binding protein talin, preferentially at the front region of the cell. Tiam1 associates directly to talin and the interaction is required to target Tiam1 to FAs.Citation64 The activation of Rac downstream of integrins is reduced in Tiam1-depleted cells and almost completely abrogated when talin is depleted. Depletion of Tiam1 or talin delays spreading, inhibits polarization, and impairs FA turnover.Citation64 These processes require the association of talin with both Tiam1 and integrins. In addition, depletion of Par3, Par6, or PKCζ expression inhibits Tiam1 recruitment to FA, which suggests the PAR complex works in concert with Tiam1 and talin to regulate FA formation and develop front-rear polarity ( ).Citation64 Interestingly, Tiam1 depletion only partially inhibits fibronectin (FN)-mediated Rac1 activation, suggesting there may be other Rac-GEFs involved. However, depletion of DOCK180 or α/βPix does not affect Rac1 activation, whereas Vav2 depletion results in a slight decrease in Rac1 activation. In addition, βPix overexpression cannot rescue Tiam1 deficiency. However, in a different glioma cell line, DOCK180 KD inhibits Rac1 activation to the same extent as Tiam1 depletion, suggesting different GEF requirements in different cell lines.Citation64
Asef
Asef1 and Asef2 are two highly homologous Rac1 and Cdc42-specific GEFs that interact with the tumor suppressor adenomatous polyposis coli (APC).Citation69-71 Binding to APC stimulates the GEF activity of Asef1/2 by releasing intramolecular inhibition.Citation70,72,73 Overexpression of Asef decreases cadherin-mediated cell–cell adhesion and promotes migration.Citation69,74 Both Asef1 and Asef2 have also been shown to regulate Rac and Cdc42 activation downstream of growth factors. Different growth factors, including EGF, HGF, and bFGF, promote the translocation of Asef to the membrane where they stimulate cell migration in a PI3K-dependent manner.Citation75,76
Intriguingly, Asef2 function has been associated with both promoting and inhibiting cell migration depending on the ECM substrate.Citation77,78 When HT1080 cells expressing GFP-Asef2 are plated on FN, Asef2 localizes to the leading edge and promotes cell migration by increasing the rapid turnover of adhesions through a mechanism that is dependent on PI3K and Akt.Citation77 In contrast, when the same cells expressing GFP-Asef2 are plated on type I collagen, migration is significantly inhibited.Citation78 The inhibition of migration requires Asef2 exchange activity, which activates Rac1, and induces MII phosphorylation. Phosphorylation of MII enhances cell contractility, and prevents FA disassembly at the leading edge, which results in impaired cell migration.Citation78 Asef2 effects on cell migration, both activation and inhibition, require the activation of Rac, but not of Cdc42.Citation77,78 On FN substrate, Asef2 also promotes the inactivation of RhoA, possibly regulated through Rac-mediated crosstalk.Citation77 How does the same GEF/GTPase module regulate a completely opposite cellular response? The mechanisms that control Asef2 in response to different substrates are currently unknown, but as we speculated for other GEFs, it is possible that by interacting with different scaffolding proteins Asef2 can be recruited to different signaling complexes that regulate distinct downstream responses.
Polarization in Lymphocytes and Neutrophils
During inflammation, multiple chemokines are upregulated and attract lymphocytes and myeloid cells such as neutrophils, mast cells, and macrophages. Lymphocytes are highly motile and play central roles in surveying the body's lymphoid tissues for antigens. Lymphocytes differentiate in primary lymphoid organs, and migrate into secondary lymphoid tissues such as the lymph nodes and spleen via the blood. This process of chemotaxis is guided by chemokines such as CCL21, CCL19, CXCL13, and CXCL12, which signal through G protein-coupled receptors (GPCRs) to activate Rac. In lymphocytes, chemokine-induced Rac activation is mediated by the Rac-specific GEF DOCK2. DOCK2 expression, unlike other DOCK proteins such as DOCK180, which is expressed in various tissues, is restricted to hematopoietic cells.Citation79,80 As a result, DOCK2-deficient (DOCK2 −/−) lymphocytes exhibit a severe defect in polarization and chemotactic responses.Citation79, Citation81-83
Neutrophils are also highly motile and play key roles in the innate response to invading pathogens. Bacterial proteins are translated with an N-terminal formylated methionine, and the resulting formyl-peptides (fMLP) act as potent neutrophil chemoattractants. Several lines of evidence had initially pointed to the Rac-specific GEF P-Rex1 as an important regulator of neutrophils chemotaxis.Citation84,85 However, neutrophil chemotaxis is only slightly reduced in P-Rex1-deficient (P-Rex1−/−) neutrophils with only a mild defect in cell speed, and normal polarization and directionality.Citation85,86 These results suggest the existence of another GEF responsible for Rac activation during neutrophil chemotaxis. As in lymphocytes, DOCK2 appears to be the main candidate. In the absence of DOCK2, Rac1 and Rac2 activation in response to fMLP is inhibited, and neutrophils cannot chemotact efficiently.Citation87 fMLP stimulation triggers a rapid translocation of DOCK2 to the plasma membrane in a PI3K-dependent manner, where it stimulates Rac activity and mediates the polarized accumulation of F-actin at the leading edge.Citation87,88 DOCK2 binds to PIP3 (but not to PIP2), in a process that is enhanced by binding to its effector ELMO1. Rac and PIP3 are part of a positive feedback-loop, in which PIP3 is required for DOCK2 to translocate to the membrane, where it activates Rac and further stabilizes PIP3 at the leading edge.Citation87 Surprisingly, in cells lacking PI3Kγ, the major generator of PIP3 in these cells, DOCK2 can still translocate to the plasma membrane in response to fMLP, albeit at later time points, suggesting the existence of a second mechanism of DOCK2 targeting.Citation88 This second mode of targeting requires phosphatidic acid (PA), which interacts with a stretch of basic residues at the C terminus of DOCK2.Citation88 Thus, PA functions in a second late phase, following PI3K-mediated initial recruitment, to control polarized DOCK2 localization and stabilize the leading edge. This late recruitment phase depends on the PIP3-mediated early phase, since recruitment of DOCK2 to the membrane induced by addition of PA is attenuated by inhibition of PI3K.Citation88
Finally, DOCK2 has been shown to be essential for migration of dendritic cells.Citation89 Dendritic cells (DCs) are specialized antigen-presenting cells found as sentinels in peripheral tissues and lymphoid organs. DCs are classified into two populations, myeloid DCs (mDCs) and plasmacytoid DCs (pDCs), with distinct expression patterns of costimulatory molecules and Toll-like receptors. mDCs function as most potent antigen-presenting cells, whereas pDCs function in antiviral immunity by producing interferons type I.Citation90 Interestingly, DOCK2 deficiency impairs chemokine-mediated migration in pDCs but has no effect on mDCs. DOCK2 depletion in pDCs inhibits Rac activation, polarization, and chemotactic migration. Homing of pDCs to secondary lymphoid organs is also inhibited. How can the differences between pDCs and mDCs be explained? pDCs express only DOCK2, whereas mDCs express both DOCK2 and DOCK180 (DOCK1), so it is possible than in mDC, the absence of DOCK2, can be functionally compensated by DOCK180.Citation89
RhoA and Polarity
While polarity is mostly associated with Rac and Cdc42 function, other GTPases such as RhoA can also play a role in the development of polarity. RhoA has been originally thought to be restricted to contractility and tail retraction during migration.Citation1 However, recent studies using biosensors have demonstrated that active RhoA is also found at the leading edge in migrating cells.Citation10,91
Lsc, the mouse homolog of p115RhoGEF, plays a key role in the regulation of neutrophil migration.Citation92 Following fMLP stimulation, Lsc relocates to both the leading edge and trailing edge. Neutrophils from Lsc −/− mice generate and retract their protrusions rapidly at inappropriate locations in the cell and are unable to sustain a single-dominant leading edge or pseudopod, often developing multiple pseudopodia. In addition, Lsc −/− neutrophils are significantly less adherent than their wt counterparts, and have reduced RhoA activity.Citation92 As a result, they migrate twice as fast, but with reduced directionality. Taken together, these results suggest a role for Lsc in the development of polarization.
Syx1 (PLEKHG5) is one of the RhoA-GEFs that have been recently associated with RhoA-dependent cell polarity. When Syx1 expression is silenced in breast cancer cells, cells lose their elongated morphology and display a rounded flattened shape.Citation93 In a wound healing assay, Syx1 KD cells fail to form lamellipodia in the direction of the wound and are also defective reorienting the Golgi complex in the direction of migration. Chemotactic migration in a transwell assay is also inhibited.Citation93 Similarly, silencing Syx1 in vivo in zebrafish results in inhibition of migration of endothelial cells in intersegmental vessels.Citation94 The loss in polarity is accompanied by an increase in the number and size of FA and a redistribution of actin to stress fibers and peripheral actin bundles.Citation93 The increase in stress fibers is unexpected when a RhoA-GEF is knocked down, and could reflect compensation by other RhoGEFs, as has been previously shown in other cases. For example, RhoA activity levels remain stable when p114RhoGEF is depleted, due to a compensatory activation of GEF-H1.Citation95 The fact that Syx1 KD does not change the overall RhoA activity, may also suggest a localized effect on RhoA activation. In addition to the effects on the actin cytoskeleton, Syx1 depletion also results in the dramatic reorganization of microtubules, which may also contribute to the loss of polarity.Citation93
Syx1 has been shown to localize to the membrane by interacting with PDZ domain proteins such as MUPP1/PatJ and synectin via its PDZ-binding motif.Citation94, Citation96-98 MUPP1/PatJ forms part of the Crumbs polarity complex (Crumbs-Pals-PatJ), which plays a role in the formation of cell–cell junctions.Citation37 Both the ability to bind PDZ proteins and to activate RhoA are required for Syx function in the establishment of polarity, suggesting the localized activation of RhoA is essential.Citation93 Interestingly, the Syx gene expresses two isoforms by alternative splicing; the full-length Syx1, and an almost identical isoform, Syx2, which is just two amino acids shorter and is unable to interact with PDZ domains.Citation96 Using a FRET RhoA-biosensor, Liu and colleagues showed that, in response to LPA, Syx1 stimulates RhoA activity specifically at the leading edge and promotes cell migration. Syx2 also promotes RhoA activation, but it is uniformly distributed in the cytoplasm and does not stimulate migration.Citation96 A similar series of experiments analyzed the localization of RhoA activity in the presence or absence of angiomotin (Amot), a protein that forms a ternary complex with MUPP1 and Syx.Citation94 In randomly migrating endothelial cells, RhoA activity concentrates at the leading edge of extending lamellipodia. However, in Amot-deficient cells, the localized activation of RhoA is lost and the activity redistributes evenly throughout the membrane.Citation94 The molecular mechanisms that regulate polarization through Syx-RhoA are not completely characterized, but there is evidence that the formin Dia1 may be one of the main downstream effectors.Citation93
Another RhoA-GEF that has been recently associated to the development of polarity in migrating cells is p63RhoGEF, also referred to as GEFT. p63RhoGEF was found to be required for serum-induced chemotactic migration in MDA-MB-231 breast cancer cells.Citation99 p63RhoGEF signals downstream of GPCRs and binds directly to Gαq/11, but not Gα12/13.Citation100-102 Gαq activates p63RhoGEF allosterically by relieving intramolecular inhibition.Citation103 When cells are incubated in the presence of serum, RhoA is rapidly activated, and the activation of p63RhoGEF follows a similar pattern. When p63RhoGEF is knocked down, RhoA activation in response to serum is completely abrogated, and the cells form multiple lamellipodia around the cell periphery. In contrast, control cells display a polarized cell morphology with a single lamellipodium forming in the direction of migration. Chemotactic migration toward serum is also inhibited in the absence of p63RhoGEF, but not random migration, which highlights the specificity of its function.Citation99 Supporting these results, overexpression of p63RhoGEF induces cell rounding and prevents lamellipodia formation. Taken together, these results support a role for p63RhoGEF in the development of polarity and in chemotactic migration. Although not directly addressed in these reports, the function of p63RhoGEF in cell migration is probably related to the ability of RhoA to regulate contractility through ROCK and MLC.
Cell Adhesion and Focal Adhesion Formation
The polarized protrusions at the leading edge are stabilized by adhesion to the ECM through transmembrane adhesion receptors which link to the actin cytoskeleton. The formation of such adhesion sites generate traction forces at the leading edge that the cell uses to move forward. Adhesion assembly is regulated by Rho GTPases, which in turn, are regulated by adhesion-generated signals. Focal adhesion kinase (FAK) and c-Src (Src) are both non-receptor tyrosine kinases viewed as central regulators of FA turnover.Citation104 Activation of the FAK/Src complex promotes the recruitment and phosphorylation of multiple scaffolding and signaling proteins at FA that are involved in adhesion dynamics and motility.Citation16 FAK has also been shown to bind to several RhoGEFs and plays a key role in targeting them to adhesion sites and regulating their activity. Therefore, FAK can function as a signaling hub that connects Rho GTPases to different processes associated to cell migration, including adhesion assembly and turnover, leading edge formation and tail retraction.
Most of the GEFs that bind to FAK are RhoA-specific, including Net1, LARG, PDZ-RhoGEF, and p190RhoGEF (RGNEF).Citation105-108 FAK activation promotes the phosphorylation of p190RhoGEF, which in turn, stimulates RhoA activation.Citation108 When the expression of p190RhoGEF is silenced in fibroblasts, cells form fewer FAs, which correlate with lower levels of active RhoA. KD cells also migrate at reduced speed when plated on FN, suggesting p190RhoGEF is involved in the regulation of RhoA activity and FA formation downstream of integrins.Citation109 Similar results were obtained from fibroblasts isolated from a p190RhoGEF −/− mouse, which also show reduced RhoA activity and FA number, as well as impaired migration.Citation110 Interestingly, both FAK phosphorylation and tyrosine phosphorylation decrease in the absence of p190RhoGEF, suggesting the existence of a FAK-p190RhoGEF feedback loop involved in the regulation of cell adhesion and migration.Citation109 In addition, FAK expression increases in p190RhoGEF −/− cells.Citation110 Conversely, p190RhoGEF expression is upregulated in FAK −/− cells, resulting in increased number of FA and higher RhoA-GTP levels.Citation109 These studies identify p190RhoGEF as one of the main regulators of FA formation during FN-stimulated cell motility. p190RhoGEF has also been shown to regulate RhoC activity at the lamellipodia of migrating cells.Citation111,112 In EGF-stimulated cells, p190RhoGEF is rapidly translocated to the membrane, where it promotes the localized activation of RhoC, which functions to control the activity of cofilin and the formation of actin barbed ends. The activity of RhoC is spatially and temporally regulated by the antagonistic actions of p190RhoGEF (activation) and p190RhoGAP (inactivation), which restrict the activity of RhoC to an area right behind the leading edge of the cell.Citation111,112 Although depletion of RhoC does not affect the overall migration of cells in 2D, it inhibits protrusion formation and invasion in 3D.Citation111,112
The activation of RhoA downstream of FN-adhered cells is also regulated by p115RhoGEF/Lsc and LARG (Leukemia-associated RhoGEF), two RhoA-GEFs that belong to a subfamily of GEFs known as RGS-GEFs (which also includes PDZ-RhoGEF). The RGS-GEFs have been best characterized as exchange factors responsible for RhoA activation downstream of GPCRs.Citation113 When fibroblasts are plated on FN, p115RhoGEF and LARG colocalize with paxillin-containing FAs and regulate FA and stress fiber formation.Citation114 FAK plays a key role in targeting of RGS-GEFs to FA, and it has been shown to phosphorylate LARG and PDZ-RhoGEF (but not p115RhoGEF).Citation106 The FAK-related kinase Pyk2 also phosphorylates PDZ-RhoGEF following angiotensin II stimulation and promotes RhoA activation.Citation115
In summary, p190RhoGEF, p115RhoGEF, LARG, and also Net1 (see below) seem to regulate similar processes in fibroblasts and downstream of integrins. However, it is still not known if their functions are redundant, if they act in parallel, or if they respond to different stimuli.
Microtubules and Polarization
The crosstalk between the actin cytoskeleton and microtubules contributes to the establishment of cell polarity and the directional movement of migrating cells.Citation116 Microtubule polymerization at the leading edge stimulates Rac1 activation, which in turn, drives actin polymerization and lamellipodial protrusions.Citation117 In contrast, microtubule disassembly results in activation of RhoA, which promotes actomyosin contractility.Citation118-121 The activation of RhoA following microtubule depolymerization is mediated by GEF-H1, a microtubule-associated RhoA-specific GEF that is inactive when bound to microtubules.Citation122-126 Once GEF-H1 is released, RhoA activation triggers a signaling cascade composed of ROCK/MLC that promotes contractility.Citation124,126 Silencing GEF-H1 in HeLa cells inhibits FA turnover, perturbs protrusion dynamics, and decreases migration.Citation126
Recent studies using FRET-based biosensors revealed that RhoA is active at the leading edge during migration, and its activation pattern increases and decreases in synchrony with protrusion and retraction.Citation10 This RhoA active zone at the leading edge of migrating cells is severely disrupted in GEF-H1-depleted cells.Citation126 The molecular mechanisms that regulate GEF-H1 targeting and activation are still being characterized, but one of the favored models is that localized microtubule depolymerization events control the temporal and spatial activation of RhoA.Citation127 Two recent studies have shown that GEF-H1 binding to microtubules may be regulated by Par1b, which plays a role in the regulation of cell polarity.Citation128,129 Different studies have shown that Par1b binds to and phosphorylates GEF-H1 at different sites in the C and N terminus, and that phosphorylation can affect its exchange activity and regulates its binding to microtubules.Citation129-131 Par1b and other kinases such as PAK1,4 and Aurora A/B, which also phosphorylate GEF-H1, may help regulate the localized binding and release of GEF-H1 from microtubules, as well as its catalytic activity in response to different stimuli.Citation132-134 These results suggest GEF-H1 is a critical component of the locomotory machinery, coordinating multiple RhoA-dependent signaling pathways during the migration of cells.
Contractility and Tail Retraction
The migration cycle is completed with the disassembly and release of the rear adhesions and the retraction of the tail, which results in a net translocation of the cell body forward. The retraction of the tail requires RhoA-mediated activation of ROCK, which phosphorylates myosin phosphatase and MLC, increasing contractility, and promoting FA disassembly.
The regulation of tail retraction and the role of the Rho GTPases in this process are still poorly understood. There are only a few RhoGEFs, mostly RhoA-specific, that have been associated with the regulation of contractility at the cell rear. The RGS-GEF PDZ-RhoGEF, in conjunction with FAK, has been shown to play a role in regulating adhesion dynamics at the trailing edge in migrating fibroblasts. Knockdown of PDZ-RhoGEF, or FAK, inhibits tail retraction downstream of LPA, in a process that also requires ROCKII, but not Dia1.Citation107 FAK binds to, and phosphorylates PDZ-RhoGEF, and they both colocalize at FAs, which suggest that activation of FAK and PDZ-RhoGEF are closely coupled.Citation106,107 The effect of PDZ-RhoGEF depletion can be attributed to impaired FA turnover, and/or a decrease in Rho-generated contractility in these cells.Citation107 These results suggest that FAK and PDZ-RhoGEF stimulate RhoA-dependent contractility at the trailing edge in response to LPA. There is evidence that another RGS-GEF, p115RhoGEF/Lsc, also plays a role in tail retraction, as Lsc −/− marginal-zone B (MZB) cells are unable to release properly from integrin-mediated adhesions. As a result, they develop long trailing tails and migration to sphingosine 1-phosphate is severely impaired.Citation135
In an interesting story that highlights another layer of the intricate relationship between Rac and Rho, Frost and colleagues have shown that Net1 plays a role in the regulation of adhesion formation and contractility.Citation105,136 Net1 is one of only two RhoGEFs that localize to the nucleus at steady-state and must be relocated to the cytosol in order to activate RhoA.Citation137 A recent report found that active Rac1 promotes the translocation of Net1 out of the nucleus and the subsequent activation of RhoA in the cytosol, and that Net1 relocalization is necessary for efficient adhesion and spreading.Citation136 In the absence of Net1, cells are more elongated and spindly and have decreased levels of pMLC, suggesting a role for Net1 in contractility and tail retraction.Citation105,136 Net1 is also required for LPA-mediated RhoA activation and regulates cell migration in breast and gastric cancer cells.Citation105,138,139 Net1 localizes in puncta that co-localize with active FAK at FA along the leading and trailing edges.Citation105 Targeting to FAs is mediated through direct association with FAK, and when Net1 is silenced, cells have spreading defects with a reduced number and size of FAs. Net1 binding to FAK is enhanced by RhoA activation and inhibited when FAK, Src, or contractility is inhibited.Citation105 This suggests a feedback loop mechanism in which Net1 is recruited to FA by FAK, where it activates RhoA and stimulates contractility, which in turn, results in additional Net1 recruitment and enhanced FAK activation. Net1 is not the only RhoA-GEF that responds to LPA, as several others have been described, including p115-RhoGEF, PDZ-RhoGEF, and LARG.Citation140,141 Thus, it is likely that LPA controls different aspects of cell migration by simultaneously activating multiple RhoA-GEFs.
Migration in 3D
One of the limitations in the migration field is that many of the key concepts about cell migration were established by studying cells on rigid two-dimensional surfaces (2D). Over the past decade, there has been a shift to study cell motility in three-dimensional (3D) environments, which represent more physiological in vivo conditions. These studies have revealed substantial differences between 2D and 3D migration. Cells migrating on 2D use actin polymerization to extend the leading edge of the plasma membrane during lamellipodia-based migration. This mesenchymal-type movement can also be seen in 3D environments and is characterized by an elongated morphology, resulting from Rac-dependent protrusions at the leading edge. In a 3D environment, cells can utilize other mechanisms of migration that are usually not observed in 2D. Studies in normal and tumor cells have shown that cells can switch between different modes of migration depending on the properties of the extracellular environment.Citation142,143 Tumor cells can move in 3D in either mesenchymal or amoeboid fashion in a process controlled mainly by Rac and RhoA, respectively.Citation142,144 Amoeboid cells are characterized by a rounded, less adhesive phenotype, which displays high levels of actomyosin contractility downstream of RhoA-ROCK, and can squeeze through the matrix by deforming the cell body, in a proteolysis-independent manner.Citation142,144,145 Importantly, these two types of movement are interconvertible or plastic, and cells can undergo amoeboid-to-mesenchymal and mesenchymal-to-amoeboid transitions.
The regulation of Rac1 activity is central to regulate the switch between amoeboid and mesenchymal movement. Activation of Rac promotes mesenchymal movement and suppresses the amoeboid phenotype.Citation146,147 In A375M2 cells, Rac1 is activated by DOCK3.Citation147,148 Silencing DOCK3 expression (but not DOCK180) inhibits the mesenchymal phenotype.Citation147 DOCK3 association with NEDD9, a member of the p130Cas family, is required to activate Rac, which signals through WAVE2 to drive mesenchymal movement.Citation146,147 WAVE2 is a member of the WASP family of proteins, which regulates actin assembly through the activation of the Arp2/3 complex ( ).
Figure 2. RhoGEF signaling pathways involved in 3D migration. During mesenchymal movement, Rac1 is activated by DOCK3. DOCK3 association with NEDD9 is required to activate Rac1, which signals through WAVE2 to drive mesenchymal movement and regulates actin assembly through the activation of the Arp2/3 complex. Twist1 and BMI1 negatively regulate the expression of the miRNA let-7i, which results in NEDD9 and DOCK3 upregulation and Rac1 activation. Activation of Rac1 suppresses amoeboid movement by inhibiting actomyosin contractility in a WAVE-dependent manner. Integrin-mediated Src activation also inhibits contractility and amoeboid migration by phosphorylating and inhibiting ROCK. In contrast, during amoeboid movement contractility activates a Rac1-GAP, ARHGAP22, which inhibits Rac and suppresses mesenchymal movement. Amoeboid migration is characterized by rounded cells, and high levels of actomyosin contractility downstream of RhoA-ROCK. The RhoA-GEFs Net1, GEF-H1, and LARG have all been associated with amoeboid migration. A positive feedack loops involving Net1 and LARG reinforces the amoeboid signaling pathway. Cdc42 also plays a role in amoeboid migration downstream of DOCK10, in a pathway that involves both PAK2 and WASP.
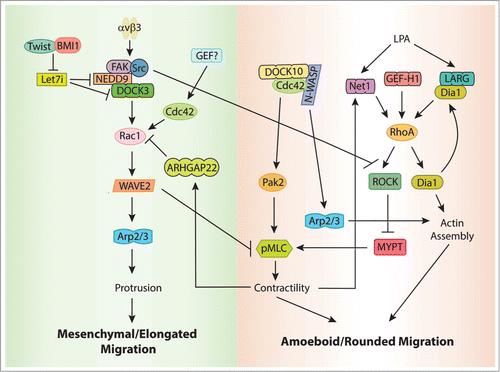
Besides promoting mesenchymal movement through actin assembly, Rac suppresses amoeboid movement by inhibiting actomyosin contractility. Silencing Rac expression leads to increased phosphorylation of MLC and faster amoeboid movement.Citation147 This requires DOCK3, NEDD9, and the Rac effector WAVE2, but not PAK. WAVE2 acts downstream of Rac to suppress MLC phosphorylation, and thus, inhibit actomysoin contractility. NEDD9 also contributes to inhibiting contractility by inactivating ROCKII in a Src and integrin β3-dependent manner.Citation149 Conversely, in rounded cells, Rac activity is kept low through the activation of a Rac-specific GAP, ARHGAP22, in a process that depends on actin contractility.Citation147 Activation of ARHGAP22 is RhoA and ROCK-dependent, but it does not seem to involve direct phosphorylation of the GAP by ROCK ( ).Citation147
Very little is known about the identity of the GEFs that regulate RhoA during amoeboid migration, but a recent report suggests that Net1 may be important.Citation105 In the absence of Net1, amoeboid invasion is inhibited in MDA-MB-231 cells, and the majority of the cells switch to a mesenchymal morphology. The increase in mesenchymal phenotype is accompanied by the upregulation of MT1-MMP, which is characteristic of this type of migration.Citation105 GEF-H1 has also been associated to the regulation of RhoA during amoeboid migration. In the absence of GEF-H1, cell migration and invasion in 3D are inhibited.Citation126,150 Supporting these results, Eitaki and colleagues have shown that, when they treat human gastric adenocarcinoma cells with the anti-cancer drug vincristine, a microtubule depolymerization agent, GEF-H1 is activated and promotes amoeboid migration in a RhoA-ROCK-dependent manner.Citation151 GEF-H1 has been recently shown to respond to cellular tension and ECM stiffness,Citation150,152 suggesting that different matrix environments may control its activity and determine the type of migration utilized. Finally, LARG has been associated to amoeboid migration in 3D matrices.Citation153 In this study, the RhoA effector Dia1 was found to associate and activate LARG downstream of LPA, but not other RGS-GEFs. Both LARG and Dia1 are required for LPA-mediated RhoA activation, and silencing LARG or Dia1 expression decreases ROCK activity, pMLC, and invasion of MDA-MB-435 cells through matrigel containing LPA. LARG or Dia1 knockdown has no effect on 2D migration, which is slightly increased compared with control cells.Citation153 These results describe a novel role for Dia1 functioning upstream of RhoA through its interaction with LARG, in addition to its role as a RhoA downstream effector, and establish a positive feedback loop that may play a role in the regulation of tumor cell invasion ( ).
Cdc42 has been typically associated with mesenchymal-type migration in 2D, where it regulates cell polarization in the direction of migration.Citation154 However, Gadea and colleagues have recently shown that Cdc42 also plays a role in amoeboid migration.Citation155 Using a siRNA library, they identified DOCK10 as a GEF involved in the regulation of amoeboid migration in A375M2 cells, a melanoma cell line which exhibits predominantly a rounded phenotype.Citation155 DOCK10 is a Cdc42-specific GEF that is expressed in several tissues.Citation155,156 When DOCK10 expression is silenced, a significant number of cells switch to an elongated, polarized morphology that moves in a mesenchymal fashion. These phenotypic changes correlated with a decrease in RhoA activity and contractility, and higher levels of active Rac1, an antagonistic effect that has been extensively documented.Citation11
The signaling pathway downstream DOCK10 and Cdc42 that regulates contractility involves PAK2 and MLC. Silencing the Cdc42 effectors PAK2 and N-WASP (but not others) phenocopies the effects of DOCK10 KD, with formation of elongated cells.Citation155 Conversely, overexpression of both PAK2 and N-WASP stimulates mesenchymal to amoeboid transition. However, only PAK2 induce pMLC phosphorylation. PAK2 has been previously shown to phosphorylate MLC in a Cdc42-dependent manner to induce contractility.Citation157 The role of N-WASP during amoeboid migration is still not clear, but N-WASP was found in a complex with DOCK10 supporting the idea that a signaling complex that includes the RhoGEF and the effector may function to confer selectivity to a particular GTPase pathway.Citation33,155 These results suggest that both PAK2 and N-WASP function downstream of Cdc42 and regulate parallel pathways, the former involved in phosphorylation of MLC and contractility, and the latter probably regulating actin assembly. As expected, this study shows that interfering with Cdc42 also affects mesenchymal-type movement, and suggests that Cdc42 is required for both amoeboid and mesenchymal morphology.Citation155 However, the activation of Cdc42 by DOCK10 functions specifically during amoeboid movement and the Cdc42-GEFs required for mesenchymal morphology and migration have not been identified yet ( ).
Who Controls Cdc42?
Cdc42 has been recognized as the master of polarity, so it is really surprising that the identity of the RhoGEFs that control its localized activation during polarization has remained elusive.Citation154 There are some isolated examples of Cdc42-specific GEFs that regulate migration, which include βPix, Tuba, FGD1, FGD4, Ect2, and the Cdc42-specific DOCK proteins (DOCK6–11).Citation7,34, Citation158-161 However, most of these reports describe a specific situation, cell type, or a particular type of migration that has not been yet validated as a conserved mechanism.
Conclusions and Future Directions
As we make progress in the understanding of these processes, we seem to have gotten to a point in which there are more new questions than answers. It appears that several GEFs can control the same processes and we still don't understand the subtleties of the system. Are all the GEFs associated with contractility required to control tail retraction? Do they act in different pathways converging into the same process? Do they synergize or act redundantly? Does the loss of one induce the compensation by others? What are the differences between 3D and 2D migration? Maybe our readouts are not sensitive enough to distinguish the specificity of each GEF. Maybe there are cell-specific, substrate-specific, or context-specific effects that we don't understand. Maybe the conditions used in vitro eliminate some of the differences that occur in a more complex in vivo environment. We are only starting to shed light on some of these questions. Some of these questions can be addressed by analyzing several GEFs simultaneously and trying to dissect both their temporal and spatial regulation in relation to each other. Another aspect that needs to be addressed is the fact that many of these proteins function in “signaling units” that comprise GEFs, GTPases, effectors, adaptor proteins, and sometimes even GAPs. These signaling units can recruit different components dependent on the stimulus. For example, the same GEF may bind a different scaffold to associate with a different GTPase and effector (see βPix/Scribble/Cdc42, βPix/GIT/PAK/Rac above). That means that in order to understand the role of a particular GEF, we need to identify the components of its particular signal unit that are associated with a specific function. Our future efforts should be focused on trying to address some of these exciting challenges and will enhance our understanding of this complex network of interactions that control cell migration.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We apologize to colleagues whose work on important aspects of RhoGEF functions were not discussed due to space limitations.
Funding
This work was supported by an OCRA grant to Garcia-Mata R.
References
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 2003; 302:1704-9; PMID:14657486; http://dx.doi.org/10.1126/science.1092053
- Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol 2007; 9:858-67; PMID:17671451; http://dx.doi.org/10.1038/ncb0807-858
- Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 2005; 6:167-80; PMID:15688002; http://dx.doi.org/10.1038/nrm1587
- Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays 2007; 29:356-70; PMID:17373658; http://dx.doi.org/10.1002/bies.20558
- Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell 2007; 99:67-86; PMID:17222083; http://dx.doi.org/10.1042/BC20060086
- Garcia-Mata R, Boulter E, Burridge K. The ‘invisible hand’: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol 2011; 12:493-504; PMID:21779026; http://dx.doi.org/10.1038/nrm3153
- Meller N, Merlot S, Guda C. CZH proteins: a new family of Rho-GEFs. J Cell Sci 2005; 118:4937-46; PMID:16254241; http://dx.doi.org/10.1242/jcs.02671
- Côté JF, Motoyama AB, Bush JA, Vuori K. A novel and evolutionarily conserved PtdIns(3,4,5)P3-binding domain is necessary for DOCK180 signalling. Nat Cell Biol 2005; 7:797-807; PMID:16025104; http://dx.doi.org/10.1038/ncb1280
- Cook DR, Rossman KL, Der CJ. Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease. Oncogene 2013; (Forthcoming); PMID:24037532; http://dx.doi.org/10.1038/onc.2013.362
- Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature 2009; 461:99-103; PMID:19693013; http://dx.doi.org/10.1038/nature08242
- Guilluy C, Garcia-Mata R, Burridge K. Rho protein crosstalk: another social network? Trends Cell Biol 2011; 21:718-26; PMID:21924908; http://dx.doi.org/10.1016/j.tcb.2011.08.002
- Kiyokawa E, Hashimoto Y, Kobayashi S, Sugimura H, Kurata T, Matsuda M. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev 1998; 12:3331-6; PMID:9808620; http://dx.doi.org/10.1101/gad.12.21.3331
- Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J Cell Biol 2006; 173:587-9; PMID:16717130; http://dx.doi.org/10.1083/jcb.200509075
- Vallés AM, Beuvin M, Boyer B. Activation of Rac1 by paxillin-Crk-DOCK180 signaling complex is antagonized by Rap1 in migrating NBT-II cells. J Biol Chem 2004; 279:44490-6; PMID:15308668; http://dx.doi.org/10.1074/jbc.M405144200
- Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev 2004; 84:1315-39; PMID:15383653; http://dx.doi.org/10.1152/physrev.00002.2004
- Tomar A, Schlaepfer DD. Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr Opin Cell Biol 2009; 21:676-83; PMID:19525103; http://dx.doi.org/10.1016/j.ceb.2009.05.006
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell 1998; 1:183-92; PMID:9659915; http://dx.doi.org/10.1016/S1097-2765(00)80019-2
- Feng Q, Baird D, Peng X, Wang J, Ly T, Guan JL, Cerione RA. Cool-1 functions as an essential regulatory node for EGF receptor- and Src-mediated cell growth. Nat Cell Biol 2006; 8:945-56; PMID:16892055; http://dx.doi.org/10.1038/ncb1453
- ten Klooster JP, Jaffer ZM, Chernoff J, Hordijk PL. Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J Cell Biol 2006; 172:759-69; PMID:16492808; http://dx.doi.org/10.1083/jcb.200509096
- Stofega MR, Sanders LC, Gardiner EM, Bokoch GM. Constitutive p21-activated kinase (PAK) activation in breast cancer cells as a result of mislocalization of PAK to focal adhesions. Mol Biol Cell 2004; 15:2965-77; PMID:15047871; http://dx.doi.org/10.1091/mbc.E03-08-0604
- Chang F, Lemmon CA, Park D, Romer LH. FAK potentiates Rac1 activation and localization to matrix adhesion sites: a role for betaPIX. Mol Biol Cell 2007; 18:253-64; PMID:17093062; http://dx.doi.org/10.1091/mbc.E06-03-0207
- Lee J, Jung ID, Chang WK, Park CG, Cho DY, Shin EY, Seo DW, Kim YK, Lee HW, Han JW, et al. p85 beta-PIX is required for cell motility through phosphorylations of focal adhesion kinase and p38 MAP kinase. Exp Cell Res 2005; 307:315-28; PMID:15893751; http://dx.doi.org/10.1016/j.yexcr.2005.03.028
- Frank SR, Hansen SH. The PIX-GIT complex: a G protein signaling cassette in control of cell shape. Semin Cell Dev Biol 2008; 19:234-44; PMID:18299239; http://dx.doi.org/10.1016/j.semcdb.2008.01.002
- Hoefen RJ, Berk BC. The multifunctional GIT family of proteins. J Cell Sci 2006; 119:1469-75; PMID:16598076; http://dx.doi.org/10.1242/jcs.02925
- Turner CE, Brown MC, Perrotta JA, Riedy MC, Nikolopoulos SN, McDonald AR, Bagrodia S, Thomas S, Leventhal PS. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling. J Cell Biol 1999; 145:851-63; PMID:10330411; http://dx.doi.org/10.1083/jcb.145.4.851
- Bagrodia S, Bailey D, Lenard Z, Hart M, Guan JL, Premont RT, Taylor SJ, Cerione RA. A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J Biol Chem 1999; 274:22393-400; PMID:10428811; http://dx.doi.org/10.1074/jbc.274.32.22393
- Premont RT, Perry SJ, Schmalzigaug R, Roseman JT, Xing Y, Claing A. The GIT/PIX complex: an oligomeric assembly of GIT family ARF GTPase-activating proteins and PIX family Rac1/Cdc42 guanine nucleotide exchange factors. Cell Signal 2004; 16:1001-11; PMID:15212761; http://dx.doi.org/10.1016/j.cellsig.2004.02.002
- Rosenberger G, Kutsche K. AlphaPIX and betaPIX and their role in focal adhesion formation. Eur J Cell Biol 2006; 85:265-74; PMID:16337026; http://dx.doi.org/10.1016/j.ejcb.2005.10.007
- Di Cesare A, Paris S, Albertinazzi C, Dariozzi S, Andersen J, Mann M, Longhi R, de Curtis I. p95-APP1 links membrane transport to Rac-mediated reorganization of actin. Nat Cell Biol 2000; 2:521-30; PMID:10934473; http://dx.doi.org/10.1038/35019561
- Matafora V, Paris S, Dariozzi S, de Curtis I. Molecular mechanisms regulating the subcellular localization of p95-APP1 between the endosomal recycling compartment and sites of actin organization at the cell surface. J Cell Sci 2001; 114:4509-20; PMID:11792816
- Valdes JL, Tang J, McDermott MI, Kuo JC, Zimmerman SP, Wincovitch SM, Waterman CM, Milgram SL, Playford MP. Sorting nexin 27 protein regulates trafficking of a p21-activated kinase (PAK) interacting exchange factor (β-Pix)-G protein-coupled receptor kinase interacting protein (GIT) complex via a PDZ domain interaction. J Biol Chem 2011; 286:39403-16; PMID:21926430; http://dx.doi.org/10.1074/jbc.M111.260802
- Nishiya N, Kiosses WB, Han J, Ginsberg MH. An alpha4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat Cell Biol 2005; 7:343-52; PMID:15793570; http://dx.doi.org/10.1038/ncb1234
- García-Mata R, Burridge K. Catching a GEF by its tail. Trends Cell Biol 2007; 17:36-43; PMID:17126549; http://dx.doi.org/10.1016/j.tcb.2006.11.004
- Osmani N, Vitale N, Borg JP, Etienne-Manneville S. Scrib controls Cdc42 localization and activity to promote cell polarization during astrocyte migration. Curr Biol 2006; 16:2395-405; PMID:17081755; http://dx.doi.org/10.1016/j.cub.2006.10.026
- Audebert S, Navarro C, Nourry C, Chasserot-Golaz S, Lécine P, Bellaiche Y, Dupont JL, Premont RT, Sempéré C, Strub JM, et al. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr Biol 2004; 14:987-95; PMID:15182672; http://dx.doi.org/10.1016/j.cub.2004.05.051
- Wang H, Han M, Whetsell W Jr., Wang J, Rich J, Hallahan D, Han Z. Tax-interacting protein 1 coordinates the spatiotemporal activation of Rho GTPases and regulates the infiltrative growth of human glioblastoma. Oncogene 2013; 33:1558-69; PMID:23563176
- Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol 2008; 9:846-59; PMID:18946474; http://dx.doi.org/10.1038/nrm2521
- Cau J, Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J Cell Sci 2005; 118:2579-87; PMID:15928049; http://dx.doi.org/10.1242/jcs.02385
- Lee CS, Choi CK, Shin EY, Schwartz MA, Kim EG. Myosin II directly binds and inhibits Dbl family guanine nucleotide exchange factors: a possible link to Rho family GTPases. J Cell Biol 2010; 190:663-74; PMID:20713598; http://dx.doi.org/10.1083/jcb.201003057
- Kuo JC, Han X, Yates JR 3rd, Waterman CM. Isolation of focal adhesion proteins for biochemical and proteomic analysis. Methods Mol Biol 2012; 757:297-323; PMID:21909920; http://dx.doi.org/10.1007/978-1-61779-166-6_19
- Vicente-Manzanares M, Newell-Litwa K, Bachir AI, Whitmore LA, Horwitz AR. Myosin IIA/IIB restrict adhesive and protrusive signaling to generate front-back polarity in migrating cells. J Cell Biol 2011; 193:381-96; PMID:21482721; http://dx.doi.org/10.1083/jcb.201012159
- Côté JF, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol 2007; 17:383-93; PMID:17765544; http://dx.doi.org/10.1016/j.tcb.2007.05.001
- Brugnera E, Haney L, Grimsley C, Lu M, Walk SF, Tosello-Trampont AC, Macara IG, Madhani H, Fink GR, Ravichandran KS. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol 2002; 4:574-82; PMID:12134158
- Hasegawa H, Kiyokawa E, Tanaka S, Nagashima K, Gotoh N, Shibuya M, Kurata T, Matsuda M. DOCK180, a major CRK-binding protein, alters cell morphology upon translocation to the cell membrane. Mol Cell Biol 1996; 16:1770-6; PMID:8657152
- deBakker CD, Haney LB, Kinchen JM, Grimsley C, Lu M, Klingele D, Hsu PK, Chou BK, Cheng LC, Blangy A, et al. Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr Biol 2004; 14:2208-16; PMID:15620647; http://dx.doi.org/10.1016/j.cub.2004.12.029
- Gumienny TL, Brugnera E, Tosello-Trampont AC, Kinchen JM, Haney LB, Nishiwaki K, Walk SF, Nemergut ME, Macara IG, Francis R, et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell 2001; 107:27-41; PMID:11595183; http://dx.doi.org/10.1016/S0092-8674(01)00520-7
- Grimsley CM, Kinchen JM, Tosello-Trampont AC, Brugnera E, Haney LB, Lu M, Chen Q, Klingele D, Hengartner MO, Ravichandran KS. Dock180 and ELMO1 proteins cooperate to promote evolutionarily conserved Rac-dependent cell migration. J Biol Chem 2004; 279:6087-97; PMID:14638695; http://dx.doi.org/10.1074/jbc.M307087200
- Komander D, Patel M, Laurin M, Fradet N, Pelletier A, Barford D, Côté JF. An α-helical extension of the ELMO1 pleckstrin homology domain mediates direct interaction to DOCK180 and is critical in Rac signaling. Mol Biol Cell 2008; 19:4837-51; PMID:18768751; http://dx.doi.org/10.1091/mbc.E08-04-0345
- Lu M, Kinchen JM, Rossman KL, Grimsley C, deBakker C, Brugnera E, Tosello-Trampont AC, Haney LB, Klingele D, Sondek J, et al. PH domain of ELMO functions in trans to regulate Rac activation via Dock180. Nat Struct Mol Biol 2004; 11:756-62; PMID:15247908; http://dx.doi.org/10.1038/nsmb800
- Katoh H, Negishi M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature 2003; 424:461-4; PMID:12879077; http://dx.doi.org/10.1038/nature01817
- Katoh H, Hiramoto K, Negishi M. Activation of Rac1 by RhoG regulates cell migration. J Cell Sci 2006; 119:56-65; PMID:16339170; http://dx.doi.org/10.1242/jcs.02720
- Santy LC, Ravichandran KS, Casanova JE. The DOCK180/Elmo complex couples ARNO-mediated Arf6 activation to the downstream activation of Rac1. Curr Biol 2005; 15:1749-54; PMID:16213822; http://dx.doi.org/10.1016/j.cub.2005.08.052
- Attar MA, Santy LC. The scaffolding protein GRASP/Tamalin directly binds to Dock180 as well as to cytohesins facilitating GTPase crosstalk in epithelial cell migration. BMC Cell Biol 2013; 14:9; PMID:23441967; http://dx.doi.org/10.1186/1471-2121-14-9
- White DT, McShea KM, Attar MA, Santy LC. GRASP and IPCEF promote ARF-to-Rac signaling and cell migration by coordinating the association of ARNO/cytohesin 2 with Dock180. Mol Biol Cell 2010; 21:562-71; PMID:20016009; http://dx.doi.org/10.1091/mbc.E09-03-0217
- Li H, Yang L, Fu H, Yan J, Wang Y, Guo H, Hao X, Xu X, Jin T, Zhang N. Association between Gαi2 and ELMO1/Dock180 connects chemokine signalling with Rac activation and metastasis. Nat Commun 2013; 4:1706; PMID:23591873; http://dx.doi.org/10.1038/ncomms2680
- Sanematsu F, Hirashima M, Laurin M, Takii R, Nishikimi A, Kitajima K, Ding G, Noda M, Murata Y, Tanaka Y, et al. DOCK180 is a Rac activator that regulates cardiovascular development by acting downstream of CXCR4. Circ Res 2010; 107:1102-5; PMID:20829512; http://dx.doi.org/10.1161/CIRCRESAHA.110.223388
- Kiyokawa E, Hashimoto Y, Kurata T, Sugimura H, Matsuda M. Evidence that DOCK180 up-regulates signals from the CrkII-p130(Cas) complex. J Biol Chem 1998; 273:24479-84; PMID:9733740; http://dx.doi.org/10.1074/jbc.273.38.24479
- Feng H, Hu B, Jarzynka MJ, Li Y, Keezer S, Johns TG, Tang CK, Hamilton RL, Vuori K, Nishikawa R, et al. Phosphorylation of dedicator of cytokinesis 1 (Dock180) at tyrosine residue Y722 by Src family kinases mediates EGFRvIII-driven glioblastoma tumorigenesis. Proc Natl Acad Sci U S A 2012; 109:3018-23; PMID:22323579; http://dx.doi.org/10.1073/pnas.1121457109
- Feng H, Hu B, Liu KW, Li Y, Lu X, Cheng T, Yiin JJ, Lu S, Keezer S, Fenton T, et al. Activation of Rac1 by Src-dependent phosphorylation of Dock180(Y1811) mediates PDGFRα-stimulated glioma tumorigenesis in mice and humans. J Clin Invest 2011; 121:4670-84; PMID:22080864; http://dx.doi.org/10.1172/JCI58559
- Tachibana M, Kiyokawa E, Hara S, Iemura S, Natsume T, Manabe T, Matsuda M. Ankyrin repeat domain 28 (ANKRD28), a novel binding partner of DOCK180, promotes cell migration by regulating focal adhesion formation. Exp Cell Res 2009; 315:863-76; PMID:19118547; http://dx.doi.org/10.1016/j.yexcr.2008.12.005
- Franca-Koh J, Kamimura Y, Devreotes PN. Leading-edge research: PtdIns(3,4,5)P3 and directed migration. Nat Cell Biol 2007; 9:15-7; PMID:17199126; http://dx.doi.org/10.1038/ncb0107-15
- Mertens AE, Pegtel DM, Collard JG. Tiam1 takes PARt in cell polarity. Trends Cell Biol 2006; 16:308-16; PMID:16650994; http://dx.doi.org/10.1016/j.tcb.2006.04.001
- Pegtel DM, Ellenbroek SI, Mertens AE, van der Kammen RA, de Rooij J, Collard JG. The Par-Tiam1 complex controls persistent migration by stabilizing microtubule-dependent front-rear polarity. Curr Biol 2007; 17:1623-34; PMID:17825562; http://dx.doi.org/10.1016/j.cub.2007.08.035
- Wang S, Watanabe T, Matsuzawa K, Katsumi A, Kakeno M, Matsui T, Ye F, Sato K, Murase K, Sugiyama I, et al. Tiam1 interaction with the PAR complex promotes talin-mediated Rac1 activation during polarized cell migration. J Cell Biol 2012; 199:331-45; PMID:23071154; http://dx.doi.org/10.1083/jcb.201202041
- Hamelers IH, Olivo C, Mertens AE, Pegtel DM, van der Kammen RA, Sonnenberg A, Collard JG. The Rac activator Tiam1 is required for (alpha)3(beta)1-mediated laminin-5 deposition, cell spreading, and cell migration. J Cell Biol 2005; 171:871-81; PMID:16330714; http://dx.doi.org/10.1083/jcb.200509172
- Mertens AE, Rygiel TP, Olivo C, van der Kammen R, Collard JG. The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J Cell Biol 2005; 170:1029-37; PMID:16186252; http://dx.doi.org/10.1083/jcb.200502129
- Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol 2005; 7:262-9; PMID:15723052; http://dx.doi.org/10.1038/ncb1226
- Hordijk PL, ten Klooster JP, van der Kammen RA, Michiels F, Oomen LC, Collard JG. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science 1997; 278:1464-6; PMID:9367959; http://dx.doi.org/10.1126/science.278.5342.1464
- Kawasaki Y, Senda T, Ishidate T, Koyama R, Morishita T, Iwayama Y, Higuchi O, Akiyama T. Asef, a link between the tumor suppressor APC and G-protein signaling. Science 2000; 289:1194-7; PMID:10947987; http://dx.doi.org/10.1126/science.289.5482.1194
- Hamann MJ, Lubking CM, Luchini DN, Billadeau DD. Asef2 functions as a Cdc42 exchange factor and is stimulated by the release of an autoinhibitory module from a concealed C-terminal activation element. Mol Cell Biol 2007; 27:1380-93; PMID:17145773; http://dx.doi.org/10.1128/MCB.01608-06
- Kawasaki Y, Sagara M, Shibata Y, Shirouzu M, Yokoyama S, Akiyama T. Identification and characterization of Asef2, a guanine-nucleotide exchange factor specific for Rac1 and Cdc42. Oncogene 2007; 26:7620-267; PMID:17599059; http://dx.doi.org/10.1038/sj.onc.1210574
- Mitin N, Betts L, Yohe ME, Der CJ, Sondek J, Rossman KL. Release of autoinhibition of ASEF by APC leads to CDC42 activation and tumor suppression. Nat Struct Mol Biol 2007; 14:814-23; PMID:17704816; http://dx.doi.org/10.1038/nsmb1290
- Murayama K, Shirouzu M, Kawasaki Y, Kato-Murayama M, Hanawa-Suetsugu K, Sakamoto A, Katsura Y, Suenaga A, Toyama M, Terada T, et al. Crystal structure of the rac activator, Asef, reveals its autoinhibitory mechanism. J Biol Chem 2007; 282:4238-42; PMID:17190834; http://dx.doi.org/10.1074/jbc.C600234200
- Kawasaki Y, Sato R, Akiyama T. Mutated APC and Asef are involved in the migration of colorectal tumour cells. Nat Cell Biol 2003; 5:211-5; PMID:12598901; http://dx.doi.org/10.1038/ncb937
- Itoh RE, Kiyokawa E, Aoki K, Nishioka T, Akiyama T, Matsuda M. Phosphorylation and activation of the Rac1 and Cdc42 GEF Asef in A431 cells stimulated by EGF. J Cell Sci 2008; 121:2635-42; PMID:18653540; http://dx.doi.org/10.1242/jcs.028647
- Kawasaki Y, Tsuji S, Sagara M, Echizen K, Shibata Y, Akiyama T. Adenomatous polyposis coli and Asef function downstream of hepatocyte growth factor and phosphatidylinositol 3-kinase. J Biol Chem 2009; 284:22436-43; PMID:19525225; http://dx.doi.org/10.1074/jbc.M109.020768
- Bristow JM, Sellers MH, Majumdar D, Anderson B, Hu L, Webb DJ. The Rho-family GEF Asef2 activates Rac to modulate adhesion and actin dynamics and thereby regulate cell migration. J Cell Sci 2009; 122:4535-46; PMID:19934221; http://dx.doi.org/10.1242/jcs.053728
- Jean L, Majumdar D, Shi M, Hinkle LE, Diggins NL, Ao M, Broussard JA, Evans JC, Choma DP, Webb DJ. Activation of Rac by Asef2 promotes myosin II-dependent contractility to inhibit cell migration on type I collagen. J Cell Sci 2013; 126:5585-97; PMID:24144700; http://dx.doi.org/10.1242/jcs.131060
- Fukui Y, Hashimoto O, Sanui T, Oono T, Koga H, Abe M, Inayoshi A, Noda M, Oike M, Shirai T, et al. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature 2001; 412:826-31; PMID:11518968; http://dx.doi.org/10.1038/35090591
- Nishihara H, Kobayashi S, Hashimoto Y, Ohba F, Mochizuki N, Kurata T, Nagashima K, Matsuda M. Non-adherent cell-specific expression of DOCK2, a member of the human CDM-family proteins. Biochim Biophys Acta 1999; 1452:179-87; PMID:10559471; http://dx.doi.org/10.1016/S0167-4889(99)00133-0
- Nombela-Arrieta C, Lacalle RA, Montoya MC, Kunisaki Y, Megías D, Marqués M, Carrera AC, Mañes S, Fukui Y, Martínez-A C, et al. Differential requirements for DOCK2 and phosphoinositide-3-kinase gamma during T and B lymphocyte homing. Immunity 2004; 21:429-41; PMID:15357953; http://dx.doi.org/10.1016/j.immuni.2004.07.012
- Nombela-Arrieta C, Mempel TR, Soriano SF, Mazo I, Wymann MP, Hirsch E, Martínez-A C, Fukui Y, von Andrian UH, Stein JV. A central role for DOCK2 during interstitial lymphocyte motility and sphingosine-1-phosphate-mediated egress. J Exp Med 2007; 204:497-510; PMID:17325199; http://dx.doi.org/10.1084/jem.20061780
- Shulman Z, Pasvolsky R, Woolf E, Grabovsky V, Feigelson SW, Erez N, Fukui Y, Alon R. DOCK2 regulates chemokine-triggered lateral lymphocyte motility but not transendothelial migration. Blood 2006; 108:2150-8; PMID:16772603; http://dx.doi.org/10.1182/blood-2006-04-017608
- Zhao T, Nalbant P, Hoshino M, Dong X, Wu D, Bokoch GM. Signaling requirements for translocation of P-Rex1, a key Rac2 exchange factor involved in chemoattractant-stimulated human neutrophil function. J Leukoc Biol 2007; 81:1127-36; PMID:17227822; http://dx.doi.org/10.1189/jlb.0406251
- Dong X, Mo Z, Bokoch G, Guo C, Li Z, Wu D. P-Rex1 is a primary Rac2 guanine nucleotide exchange factor in mouse neutrophils. Curr Biol 2005; 15:1874-9; PMID:16243036; http://dx.doi.org/10.1016/j.cub.2005.09.014
- Welch HC, Condliffe AM, Milne LJ, Ferguson GJ, Hill K, Webb LM, Okkenhaug K, Coadwell WJ, Andrews SR, Thelen M, et al. P-Rex1 regulates neutrophil function. Curr Biol 2005; 15:1867-73; PMID:16243035; http://dx.doi.org/10.1016/j.cub.2005.09.050
- Kunisaki Y, Nishikimi A, Tanaka Y, Takii R, Noda M, Inayoshi A, Watanabe K, Sanematsu F, Sasazuki T, Sasaki T, et al. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J Cell Biol 2006; 174:647-52; PMID:16943182; http://dx.doi.org/10.1083/jcb.200602142
- Nishikimi A, Fukuhara H, Su W, Hongu T, Takasuga S, Mihara H, Cao Q, Sanematsu F, Kanai M, Hasegawa H, et al. Sequential regulation of DOCK2 dynamics by two phospholipids during neutrophil chemotaxis. Science 2009; 324:384-7; PMID:19325080; http://dx.doi.org/10.1126/science.1170179
- Gotoh K, Tanaka Y, Nishikimi A, Inayoshi A, Enjoji M, Takayanagi R, Sasazuki T, Fukui Y. Differential requirement for DOCK2 in migration of plasmacytoid dendritic cells versus myeloid dendritic cells. Blood 2008; 111:2973-6; PMID:18198348; http://dx.doi.org/10.1182/blood-2007-09-112169
- Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol 2002; 2:151-61; PMID:11913066; http://dx.doi.org/10.1038/nri746
- Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature 2006; 440:1069-72; PMID:16547516; http://dx.doi.org/10.1038/nature04665
- Francis SA, Shen X, Young JB, Kaul P, Lerner DJ. Rho GEF Lsc is required for normal polarization, migration, and adhesion of formyl-peptide-stimulated neutrophils. Blood 2006; 107:1627-35; PMID:16263795; http://dx.doi.org/10.1182/blood-2005-03-1164
- Dachsel JC, Ngok SP, Lewis-Tuffin LJ, Kourtidis A, Geyer R, Johnston L, Feathers R, Anastasiadis PZ. The RhoGEF Syx regulates the balance of Dia and ROCK activities to promote polarized cancer cell migration. Mol Cell Biol 2013; 33:4909-18; http://dx.doi.org/10.1128/MCB.00565-13
- Ernkvist M, Luna Persson N, Audebert S, Lecine P, Sinha I, Liu M, Schlueter M, Horowitz A, Aase K, Weide T, et al. The Amot/Patj/Syx signaling complex spatially controls RhoA GTPase activity in migrating endothelial cells. Blood 2009; 113:244-53; PMID:18824598; http://dx.doi.org/10.1182/blood-2008-04-153874
- Terry SJ, Zihni C, Elbediwy A, Vitiello E, Leefa Chong San IV, Balda MS, Matter K. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol 2011; 13:159-66; PMID:21258369; http://dx.doi.org/10.1038/ncb2156
- Liu M, Horowitz A. A PDZ-binding motif as a critical determinant of Rho guanine exchange factor function and cell phenotype. Mol Biol Cell 2006; 17:1880-7; PMID:16467373; http://dx.doi.org/10.1091/mbc.E06-01-0002
- Ngok SP, Geyer R, Liu M, Kourtidis A, Agrawal S, Wu C, Seerapu HR, Lewis-Tuffin LJ, Moodie KL, Huveldt D, et al. VEGF and Angiopoietin-1 exert opposing effects on cell junctions by regulating the Rho GEF Syx. J Cell Biol 2012; 199:1103-15; PMID:23253477; http://dx.doi.org/10.1083/jcb.201207009
- Estévez MA, Henderson JA, Ahn D, Zhu XR, Poschmann G, Lübbert H, Marx R, Baraban JM. The neuronal RhoA GEF, Tech, interacts with the synaptic multi-PDZ-domain-containing protein, MUPP1. J Neurochem 2008; 106:1287-97; PMID:18537874; http://dx.doi.org/10.1111/j.1471-4159.2008.05472.x
- Hayashi A, Hiatari R, Tsuji T, Ohashi K, Mizuno K. p63RhoGEF-mediated formation of a single polarized lamellipodium is required for chemotactic migration in breast carcinoma cells. FEBS Lett 2013; 587:698-705; PMID:23380069; http://dx.doi.org/10.1016/j.febslet.2013.01.043
- Lutz S, Freichel-Blomquist A, Yang Y, Rümenapp U, Jakobs KH, Schmidt M, Wieland T. The guanine nucleotide exchange factor p63RhoGEF, a specific link between Gq/11-coupled receptor signaling and RhoA. J Biol Chem 2005; 280:11134-9; PMID:15632174; http://dx.doi.org/10.1074/jbc.M411322200
- Lutz S, Shankaranarayanan A, Coco C, Ridilla M, Nance MR, Vettel C, Baltus D, Evelyn CR, Neubig RR, Wieland T, et al. Structure of Galphaq-p63RhoGEF-RhoA complex reveals a pathway for the activation of RhoA by GPCRs. Science 2007; 318:1923-7; PMID:18096806; http://dx.doi.org/10.1126/science.1147554
- Rojas RJ, Yohe ME, Gershburg S, Kawano T, Kozasa T, Sondek J. Galphaq directly activates p63RhoGEF and Trio via a conserved extension of the Dbl homology-associated pleckstrin homology domain. J Biol Chem 2007; 282:29201-10; PMID:17606614; http://dx.doi.org/10.1074/jbc.M703458200
- Shankaranarayanan A, Boguth CA, Lutz S, Vettel C, Uhlemann F, Aittaleb M, Wieland T, Tesmer JJ. Galpha q allosterically activates and relieves autoinhibition of p63RhoGEF. Cell Signal 2010; 22:1114-23; PMID:20214977; http://dx.doi.org/10.1016/j.cellsig.2010.03.006
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol 2004; 6:154-61; PMID:14743221; http://dx.doi.org/10.1038/ncb1094
- Carr HS, Zuo Y, Oh W, Frost JA. Regulation of focal adhesion kinase activation, breast cancer cell motility, and amoeboid invasion by the RhoA guanine nucleotide exchange factor Net1. Mol Cell Biol 2013; 33:2773-86; PMID:23689132; http://dx.doi.org/10.1128/MCB.00175-13
- Chikumi H, Fukuhara S, Gutkind JS. Regulation of G protein-linked guanine nucleotide exchange factors for Rho, PDZ-RhoGEF, and LARG by tyrosine phosphorylation: evidence of a role for focal adhesion kinase. J Biol Chem 2002; 277:12463-73; PMID:11799111; http://dx.doi.org/10.1074/jbc.M108504200
- Iwanicki MP, Vomastek T, Tilghman RW, Martin KH, Banerjee J, Wedegaertner PB, Parsons JT. FAK, PDZ-RhoGEF and ROCKII cooperate to regulate adhesion movement and trailing-edge retraction in fibroblasts. J Cell Sci 2008; 121:895-905; PMID:18303050; http://dx.doi.org/10.1242/jcs.020941
- Zhai J, Lin H, Nie Z, Wu J, Cañete-Soler R, Schlaepfer WW, Schlaepfer DD. Direct interaction of focal adhesion kinase with p190RhoGEF. J Biol Chem 2003; 278:24865-73; PMID:12702722; http://dx.doi.org/10.1074/jbc.M302381200
- Lim Y, Lim ST, Tomar A, Gardel M, Bernard-Trifilo JA, Chen XL, Uryu SA, Canete-Soler R, Zhai J, Lin H, et al. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol 2008; 180:187-203; PMID:18195107; http://dx.doi.org/10.1083/jcb.200708194
- Miller NL, Lawson C, Chen XL, Lim ST, Schlaepfer DD. Rgnef (p190RhoGEF) knockout inhibits RhoA activity, focal adhesion establishment, and cell motility downstream of integrins. PLoS One 2012; 7:e37830; PMID:22649559; http://dx.doi.org/10.1371/journal.pone.0037830
- Bravo-Cordero JJ, Sharma VP, Roh-Johnson M, Chen X, Eddy R, Condeelis J, Hodgson L. Spatial regulation of RhoC activity defines protrusion formation in migrating cells. J Cell Sci 2013; 126:3356-69; PMID:23704350; http://dx.doi.org/10.1242/jcs.123547
- Bravo-Cordero JJ, Oser M, Chen X, Eddy R, Hodgson L, Condeelis J. A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr Biol 2011; 21:635-44; PMID:21474314; http://dx.doi.org/10.1016/j.cub.2011.03.039
- Fukuhara S, Chikumi H, Gutkind JS. RGS-containing RhoGEFs: the missing link between transforming G proteins and Rho? Oncogene 2001; 20:1661-8; PMID:11313914; http://dx.doi.org/10.1038/sj.onc.1204182
- Dubash AD, Wennerberg K, García-Mata R, Menold MM, Arthur WT, Burridge K. A novel role for Lsc/p115 RhoGEF and LARG in regulating RhoA activity downstream of adhesion to fibronectin. J Cell Sci 2007; 120:3989-98; PMID:17971419; http://dx.doi.org/10.1242/jcs.003806
- Ohtsu H, Mifune M, Frank GD, Saito S, Inagami T, Kim-Mitsuyama S, Takuwa Y, Sasaki T, Rothstein JD, Suzuki H, et al. Signal-crosstalk between Rho/ROCK and c-Jun NH2-terminal kinase mediates migration of vascular smooth muscle cells stimulated by angiotensin II. Arterioscler Thromb Vasc Biol 2005; 25:1831-6; PMID:15994438; http://dx.doi.org/10.1161/01.ATV.0000175749.41799.9b
- Li R, Gundersen GG. Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol 2008; 9:860-73; PMID:18946475; http://dx.doi.org/10.1038/nrm2522
- Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat Cell Biol 1999; 1:45-50; PMID:10559863; http://dx.doi.org/10.1038/9018
- Danowski BA. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J Cell Sci 1989; 93:255-66; PMID:2482296
- Enomoto T. Microtubule disruption induces the formation of actin stress fibers and focal adhesions in cultured cells: possible involvement of the rho signal cascade. Cell Struct Funct 1996; 21:317-26; PMID:9118237; http://dx.doi.org/10.1247/csf.21.317
- Liu BP, Chrzanowska-Wodnicka M, Burridge K. Microtubule depolymerization induces stress fibers, focal adhesions, and DNA synthesis via the GTP-binding protein Rho. Cell Adhes Commun 1998; 5:249-55; PMID:9762466; http://dx.doi.org/10.3109/15419069809040295
- Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J 1999; 18:578-85; PMID:9927417; http://dx.doi.org/10.1093/emboj/18.3.578
- Ren Y, Li R, Zheng Y, Busch H. Cloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPases. J Biol Chem 1998; 273:34954-60; PMID:9857026; http://dx.doi.org/10.1074/jbc.273.52.34954
- Glaven JA, Whitehead I, Bagrodia S, Kay R, Cerione RA. The Dbl-related protein, Lfc, localizes to microtubules and mediates the activation of Rac signaling pathways in cells. J Biol Chem 1999; 274:2279-85; PMID:9890991; http://dx.doi.org/10.1074/jbc.274.4.2279
- Chang YC, Nalbant P, Birkenfeld J, Chang ZF, Bokoch GM. GEF-H1 couples nocodazole-induced microtubule disassembly to cell contractility via RhoA. Mol Biol Cell 2008; 19:2147-53; PMID:18287519; http://dx.doi.org/10.1091/mbc.E07-12-1269
- Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol 2002; 4:294-301; PMID:11912491; http://dx.doi.org/10.1038/ncb773
- Nalbant P, Chang YC, Birkenfeld J, Chang ZF, Bokoch GM. Guanine nucleotide exchange factor-H1 regulates cell migration via localized activation of RhoA at the leading edge. Mol Biol Cell 2009; 20:4070-82; PMID:19625450; http://dx.doi.org/10.1091/mbc.E09-01-0041
- Birkenfeld J, Nalbant P, Yoon SH, Bokoch GM. Cellular functions of GEF-H1, a microtubule-regulated Rho-GEF: is altered GEF-H1 activity a crucial determinant of disease pathogenesis? Trends Cell Biol 2008; 18:210-9; PMID:18394899; http://dx.doi.org/10.1016/j.tcb.2008.02.006
- Tonami K, Kurihara Y, Arima S, Nishiyama K, Uchijima Y, Asano T, Sorimachi H, Kurihara H. Calpain-6, a microtubule-stabilizing protein, regulates Rac1 activity and cell motility through interaction with GEF-H1. J Cell Sci 2011; 124:1214-23; PMID:21406564; http://dx.doi.org/10.1242/jcs.072561
- Yamahashi Y, Saito Y, Murata-Kamiya N, Hatakeyama M. Polarity-regulating kinase partitioning-defective 1b (PAR1b) phosphorylates guanine nucleotide exchange factor H1 (GEF-H1) to regulate RhoA-dependent actin cytoskeletal reorganization. J Biol Chem 2011; 286:44576-84; PMID:22072711; http://dx.doi.org/10.1074/jbc.M111.267021
- Nie M, Aijaz S, Leefa Chong San IV, Balda MS, Matter K. The Y-box factor ZONAB/DbpA associates with GEF-H1/Lfc and mediates Rho-stimulated transcription. EMBO Rep 2009; 10:1125-31; PMID:19730435; http://dx.doi.org/10.1038/embor.2009.182
- Yoshimura Y, Miki H. Dynamic regulation of GEF-H1 localization at microtubules by Par1b/MARK2. Biochem Biophys Res Commun 2011; 408:322-8; PMID:21513698; http://dx.doi.org/10.1016/j.bbrc.2011.04.032
- Birkenfeld J, Nalbant P, Bohl BP, Pertz O, Hahn KM, Bokoch GM. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev Cell 2007; 12:699-712; PMID:17488622; http://dx.doi.org/10.1016/j.devcel.2007.03.014
- Callow MG, Zozulya S, Gishizky ML, Jallal B, Smeal T. PAK4 mediates morphological changes through the regulation of GEF-H1. J Cell Sci 2005; 118:1861-72; PMID:15827085; http://dx.doi.org/10.1242/jcs.02313
- Zenke FT, Krendel M, DerMardirossian C, King CC, Bohl BP, Bokoch GM. p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J Biol Chem 2004; 279:18392-400; PMID:14970201; http://dx.doi.org/10.1074/jbc.M400084200
- Rubtsov A, Strauch P, Digiacomo A, Hu J, Pelanda R, Torres RM. Lsc regulates marginal-zone B cell migration and adhesion and is required for the IgM T-dependent antibody response. Immunity 2005; 23:527-38; PMID:16286020; http://dx.doi.org/10.1016/j.immuni.2005.09.018
- Carr HS, Morris CA, Menon S, Song EH, Frost JA. Rac1 controls the subcellular localization of the Rho guanine nucleotide exchange factor Net1A to regulate focal adhesion formation and cell spreading. Mol Cell Biol 2013; 33:622-34; PMID:23184663; http://dx.doi.org/10.1128/MCB.00980-12
- Schmidt A, Hall A. The Rho exchange factor Net1 is regulated by nuclear sequestration. J Biol Chem 2002; 277:14581-8; PMID:11839749; http://dx.doi.org/10.1074/jbc.M111108200
- Leyden J, Murray D, Moss A, Arumuguma M, Doyle E, McEntee G, O’Keane C, Doran P, MacMathuna P. Net1 and Myeov: computationally identified mediators of gastric cancer. Br J Cancer 2006; 94:1204-12; PMID:16552434; http://dx.doi.org/10.1038/sj.bjc.6603054
- Murray D, Horgan G, Macmathuna P, Doran P. NET1-mediated RhoA activation facilitates lysophosphatidic acid-induced cell migration and invasion in gastric cancer. Br J Cancer 2008; 99:1322-9; PMID:18827818; http://dx.doi.org/10.1038/sj.bjc.6604688
- Wang Q, Liu M, Kozasa T, Rothstein JD, Sternweis PC, Neubig RR. Thrombin and lysophosphatidic acid receptors utilize distinct rhoGEFs in prostate cancer cells. J Biol Chem 2004; 279:28831-4; PMID:15143072; http://dx.doi.org/10.1074/jbc.C400105200
- Yamada T, Ohoka Y, Kogo M, Inagaki S. Physical and functional interactions of the lysophosphatidic acid receptors with PDZ domain-containing Rho guanine nucleotide exchange factors (RhoGEFs). J Biol Chem 2005; 280:19358-63; PMID:15755723; http://dx.doi.org/10.1074/jbc.M414561200
- Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, Strongin AY, Bröcker EB, Friedl P. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol 2003; 160:267-77; PMID:12527751; http://dx.doi.org/10.1083/jcb.200209006
- Petrie RJ, Gavara N, Chadwick RS, Yamada KM. Nonpolarized signaling reveals two distinct modes of 3D cell migration. J Cell Biol 2012; 197:439-55; PMID:22547408; http://dx.doi.org/10.1083/jcb.201201124
- Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol 2003; 5:711-9; PMID:12844144; http://dx.doi.org/10.1038/ncb1019
- Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol 2006; 16:1515-23; PMID:16890527; http://dx.doi.org/10.1016/j.cub.2006.05.065
- Yamazaki D, Kurisu S, Takenawa T. Involvement of Rac and Rho signaling in cancer cell motility in 3D substrates. Oncogene 2009; 28:1570-83; PMID:19234490; http://dx.doi.org/10.1038/onc.2009.2
- Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell 2008; 135:510-23; PMID:18984162; http://dx.doi.org/10.1016/j.cell.2008.09.043
- Namekata K, Enokido Y, Iwasawa K, Kimura H. MOCA induces membrane spreading by activating Rac1. J Biol Chem 2004; 279:14331-7; PMID:14718541; http://dx.doi.org/10.1074/jbc.M311275200
- Ahn J, Sanz-Moreno V, Marshall CJ. The metastasis gene NEDD9 product acts through integrin β3 and Src to promote mesenchymal motility and inhibit amoeboid motility. J Cell Sci 2012; 125:1814-26; PMID:22328516; http://dx.doi.org/10.1242/jcs.101444
- Heck JN, Ponik SM, Garcia-Mendoza MG, Pehlke CA, Inman DR, Eliceiri KW, Keely PJ. Microtubules regulate GEF-H1 in response to extracellular matrix stiffness. Mol Biol Cell 2012; 23:2583-92; PMID:22593214; http://dx.doi.org/10.1091/mbc.E11-10-0876
- Eitaki M, Yamamori T, Meike S, Yasui H, Inanami O. Vincristine enhances amoeboid-like motility via GEF-H1/RhoA/ROCK/Myosin light chain signaling in MKN45 cells. BMC Cancer 2012; 12:469; PMID:23057787; http://dx.doi.org/10.1186/1471-2407-12-469
- Guilluy C, Swaminathan V, Garcia-Mata R, O’Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol 2011; 13:722-7; PMID:21572419; http://dx.doi.org/10.1038/ncb2254
- Kitzing TM, Sahadevan AS, Brandt DT, Knieling H, Hannemann S, Fackler OT, Grosshans J, Grosse R. Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion. Genes Dev 2007; 21:1478-83; PMID:17575049; http://dx.doi.org/10.1101/gad.424807
- Etienne-Manneville S. Cdc42–the centre of polarity. J Cell Sci 2004; 117:1291-300; PMID:15020669; http://dx.doi.org/10.1242/jcs.01115
- Gadea G, Sanz-Moreno V, Self A, Godi A, Marshall CJ. DOCK10-mediated Cdc42 activation is necessary for amoeboid invasion of melanoma cells. Curr Biol 2008; 18:1456-65; PMID:18835169; http://dx.doi.org/10.1016/j.cub.2008.08.053
- Nishikimi A, Meller N, Uekawa N, Isobe K, Schwartz MA, Maruyama M. Zizimin2: a novel, DOCK180-related Cdc42 guanine nucleotide exchange factor expressed predominantly in lymphocytes. FEBS Lett 2005; 579:1039-46; PMID:15710388; http://dx.doi.org/10.1016/j.febslet.2005.01.006
- Zeng Q, Lagunoff D, Masaracchia R, Goeckeler Z, Côté G, Wysolmerski R. Endothelial cell retraction is induced by PAK2 monophosphorylation of myosin II. J Cell Sci 2000; 113:471-82; PMID:10639334
- Qin Y, Meisen WH, Hao Y, Macara IG. Tuba, a Cdc42 GEF, is required for polarized spindle orientation during epithelial cyst formation. J Cell Biol 2010; 189:661-9; PMID:20479467; http://dx.doi.org/10.1083/jcb.201002097
- Liu HP, Chen CC, Wu CC, Huang YC, Liu SC, Liang Y, Chang KP, Chang YS. Epstein-Barr virus-encoded LMP1 interacts with FGD4 to activate Cdc42 and thereby promote migration of nasopharyngeal carcinoma cells. PLoS Pathog 2012; 8:e1002690; PMID:22589722; http://dx.doi.org/10.1371/journal.ppat.1002690
- Fortin SP, Ennis MJ, Schumacher CA, Zylstra-Diegel CR, Williams BO, Ross JT, Winkles JA, Loftus JC, Symons MH, Tran NL. Cdc42 and the guanine nucleotide exchange factors Ect2 and trio mediate Fn14-induced migration and invasion of glioblastoma cells. Mol Cancer Res 2012; 10:958-68; PMID:22571869; http://dx.doi.org/10.1158/1541-7786.MCR-11-0616
- Oshima T, Fujino T, Ando K, Hayakawa M. Role of FGD1, a Cdc42 guanine nucleotide exchange factor, in epidermal growth factor-stimulated c-Jun NH2-terminal kinase activation and cell migration. Biol Pharm Bull 2011; 34:54-60; PMID:21212517; http://dx.doi.org/10.1248/bpb.34.54
