Abstract
Cell migration is a highly integrated, multistep process that plays an important role in physiological and pathological processes. The migrating cell is highly polarized, with complex regulatory pathways that integrate its component processes spatially and temporally.Citation1 The Drosophila tumor suppressor, Lethal (2) giant larvae (Lgl), regulates apical-basal polarity in epithelia and asymmetric cell division.Citation2 But little is known about the role of Lgl in establishing cell polarity in migrating cells. Recently, we showed that the mammalian Lgl1 interacts directly with non-muscle myosin IIA (NMIIA), inhibiting its ability to assemble into filaments in vitro.Citation3 Lgl1 also regulates the cellular localization of NMIIA, the maturation of focal adhesions, and cell migration.Citation3 We further showed that phosphorylation of Lgl1 by aPKCζ prevents its interaction with NMIIA and is important for Lgl1 and acto-NMII cytoskeleton cellular organization.Citation4 Lgl is a critical downstream target of the Par6-aPKC cell polarity complex; we showed that Lgl1 forms two distinct complexes in vivo, Lgl1-NMIIA and Lgl1-Par6-aPKCζ in different cellular compartments.Citation4 We further showed that aPKCζ and NMIIA compete to bind directly to Lgl1 through the same domain. These data provide new insights into the role of Lgl1, NMIIA, and Par6-aPKCζ in establishing front-rear polarity in migrating cells. In this commentary, I discuss the role of Lgl1 in the regulation of the acto-NMII cytoskeleton and its regulation by the Par6-aPKCζ polarity complex, and how Lgl1 activity may contribute to the establishment of front-rear polarity in migrating cells.
Cell migration is a highly integrated multistep process that orchestrates embryonic morphogenesis, contributes to tissue repair and regeneration, and drives disease progression in cancer. A cell must be polarized in order to migrate, which means that molecular processes at the front and the back of a moving cell are different. Cell polarity is important for distinguishing between random cell migration, in which cells migrate in all directions in a non-coordinated manner, and directed cell migration, in which cells respond to polarizing cues to migrate in a given direction. Although in both cases, cell polarity is required to generate a front-rear axis, polarizing cues stabilize the front–rear axis determining the extent of persistent directional cell movement.Citation1 Many different external cues initiate front-rear polarity, including growth factors and the extracellular matrix. Conversion of these cues into directional migration requires global changes in cell organization by protein complexes and signaling pathways that control the actomyosin cytoskeleton. Non-muscle myosin II (NMII) is an actin-based motor that converts chemical energy into force and movement, and thus functions as a key regulator of the eukaryotic cytoskeleton. NMII is an important motor protein present in all cell types; it participates in crucial processes, including cytokinesis, surface attachment, and cell movement.Citation5 NMII molecules are comprised of two heavy chains of 230 kDa, two 20 kDa regulatory light chains (RLCs) that regulate NMII activity, and two 17 kDa essential light chains (ELCs) that stabilize the heavy chain structure (). NMII heavy chain is composed of a globular head containing the actin-binding and force-generating ATPase domains, followed by a large coiled-coil rod that terminates with a short non-helical tailpiece. To carry out its cellular functions, NMII assembles into dimers and higher order filaments by interactions between the coiled-coil regions of the heavy chains. The assembly process is governed by electrostatic interactions between adjacent coiled-coil rods containing alternating charged regions with specific periodicity ().Citation5 The assembly process is enhanced by activation of the motor domain through RLCs phosphorylation.Citation5 Three isoforms of NMII (termed NMIIA, NMIIB, and NMIIC) have been identified in mammals.Citation6 Although NMII isoforms share somewhat overlapping roles, each isoform has distinctive tissue distribution and specific functions, which are mediated by the C-terminus of the NMII heavy chain ().Citation6 These observations point to the importance of the NMII tail in modulating the spatiotemporal localization of NMII molecules and suggest that regulation by means of the tail could modulate NMII localization and activity. NMIIA is important for neural growth cone retraction and is distributed to the front of migrating endothelial cells; NMIIB participates in growth cone advancement and was detected in the retracting tails of migrating endothelial cells.Citation7,8 Furthermore NMIIA and NMIIB have opposite effects on motility, as depletion of NMIIA leads to increased motility, whereas NMIIB depletion hinders motility.Citation9 In migrating fibroblasts, the NMII isoforms have different roles in cell polarization. NMIIA is dynamic and assembles actomyosin bundles in protrusions. By contrast, NMIIB incorporates into preformed actin bundles and remains stationary, defining the center and rear of the migrating cell. In this manner, the cooperative functions of NMIIA and NMIIB induce big, non-dynamic actomyosin structures that define the non-protrusive parts of the cell, whereas dynamic filaments in protruding regions of the cell are comprised of NMIIA alone.Citation10,11
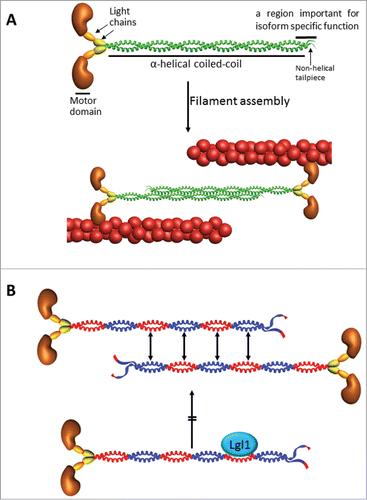
Figure 1. (A) Structure of NMII and filament formation. The subunit and domain structure of NMII, which forms a dimer through interactions between the α-helical coiled-coil rod domains. The globular head domain contains the actin-binding regions and the enzymatic Mg2+-ATPase motor domains. The essential light chains (ELCs) and the regulatory light chains (RLCs) bind to the heavy chains at the lever arms that link the head and rod domains. NMII molecules assemble into bipolar filaments through interactions between their rod domains. These filaments bind to actin through their head domains, and the ATPase activity of the head enables a conformational change that moves actin filaments in an anti-parallel manner. Bipolar NMII filaments link actin filaments together in thick bundles that form cellular structures such as stress fibers. (B) Lgl1 inhibits NMIIA filament assembly. The assembly process is governed by electrostatic interactions between adjacent α-helical coiled-coil rods containing alternating charged regions with specific periodicity (blue and red positively and negatively charged regions, respectively). Lgl1 binds to a negatively charged region of NMIIA, and conversely, NMIIA interacts with the positively charge region of Lgl1. Thus, the interaction between Lgl1 and NMIIA is electrostatic. Lgl1 binding to a NMIIA monomer masks its negatively charged region, inhibiting its ability to interact with another monomer and assemble into filaments. In addition, Lgl1 binding domain in NMIIA resides between assembly competence domains 1 and 2, that are crucial for NMII filament assembly.Citation31
The Drosophila tumor suppressor, Lgl, an evolutionarily conserved and widely expressed cytoskeletal protein, is indispensable for the establishment and maintenance of polarized epithelia and for cell polarity associated with asymmetric cell division of neuroblasts during fly development.Citation12 Lgl is implicated in cell migration, and loss of Lgl inhibits dorsal closure.Citation12 Furthermore, loss of Lgl leads to invasive cell behavior in the Drosophila follicular epithelium during border cell migration.Citation12 Conversely, in transformed human epithelial cells, overexpression of Lgl1 inhibits migration.Citation13 Lgl has also been implicated in mouse embryonic fibroblast migration.Citation14 The function of Lgl in polarized cell migration, however, has not been studied in detail. Biochemical and genetic analyses suggest that the Drosophila Lgl is the component of the cytoskeleton that interacts with NMII, and that this interaction is regulated by the phosphorylation of Lgl.Citation15 In Lgl mutant neuroblasts, the neuronal differentiation factor Miranda, did not localize asymmetrically in mitotic neurolasts, but rather distributed uniformly throughout the cortex as well as in the cytoplasm. Elimination of NMII expression restored the basal localization of Miranda.Citation16 Thus, Lgl and NMII act antagonistically in the basal targeting of cell fate determinants. It was proposed that Lgl acts to restrict NMII to the apical cortex of neuroblasts during prometaphase and metaphase of mitosis, where it acts to exclude cell fate determinants.Citation17 However, the importance of Lgl in NMII regulation and thereby for F-actin filament contractility in cell polarization remains an unresolved issue. Moreover, the role of Lgl was studied mainly in the polarity of epithelial cells, and therefore the mechanism by which Lgl contributes to the establishment of migrating cell polarity is poorly understood. In our recent studies we reported new findings on the role of Lgl1, NMII, and Par6-aPKCζ in establishing cell polarity in migrating cells.Citation3,4
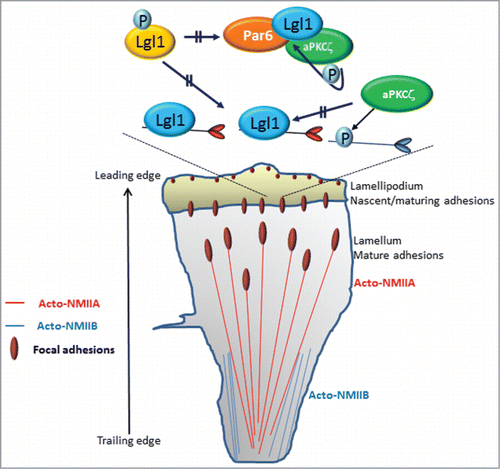
Front-back polarization of migrating cells results in two defined regions: a protrusive area in the direction of migration and a retracting rear ().Citation1 NMIIA and NMIIB reside outside of protrusions and are largely absent from the lamellipodiuma, acting at a distance to regulate cell protrusion, signaling, and maturation of nascent adhesions.Citation6 MIIA also controls the dynamics and size of adhesions in central regions of the cell and contributes to retraction and adhesion disassembly at the rear. In contrast, MIIB establishes front-back polarity ().Citation6 Our studies provide a clue to the differential roles played by NMIIA and NMIIB in establishing front-back polarity in migrating cells. We showed that Lgl1 interacts directly with NMIIA both in vivo and in vitro, inhibiting its filament assembly in vitro ().Citation3 The binding site of Lgl1 to NMIIA is localized to the tail coiled-coil region, between the domains that are critical for NMII filament assembly ().Citation3 Ectopic expression of Lgl1 decreased the amount of NMIIA associated with the cytoskeleton, reflecting a decrease in NMIIA filaments.Citation4 Furthermore, Lgl1 localization to the leading edge of the cella and depletion of Lgl1 expression result in the unexpected presence of NMIIA in the lamellipodium and the leading edge of the cell. This is consistent with the findings that asymmetric segregation in Drosophila neuroblasts is achieved in part by the restriction of NMII to the apical cortex by Lgl.Citation17 Recently we found that Lgl1 did not interact with NMIIB, indicating that NMIIB regulation with regard to Lgl1 is different from that of NMIIA (Dahan and Ravid, unpublished data). Based on these data we propose that Lgl1 interacts with NMIIA in the lamellipodium inhibiting NMIIA filament assembly in this region, thereby confining its activity to the lamella (). Lgl1 also affects the size and number of focal adhesions as well as cell polarity, membrane dynamics, and the rate of migrating cells.Citation3 NMIIA mediates several important component processes that drive migration, including the initiation and maturation of adhesion sites.Citation6 We showed that in cells depleted of Lgl1 there was a marked increase in the number of small nascent focal adhesions and a decrease in the number of large mature focal adhesions.Citation3 NMII is dispensable for the assembly and disassembly of nascent adhesions inside the lamellipodium.Citation18 Actomyosin bundles containing only MIIA mediate initial adhesion maturation, whereas the incorporation of MIIB enlarges and stabilizes them.Citation18 The maturation of adhesions in the lamellipodium, seems to depend on the level of active NMIIA.Citation18 But the mechanism by which NMIIA mediates focal adhesion maturation is not completely understood. We propose that during protrusion, adhesions initially assemble as puncta in the lamellipodia; their formation is driven by actin polymerization (). Following assembly, these nascent adhesions remain small and stable within the lamellipodium. In the lamella, the activity of both actin and NMIIA is necessary for the initial elongation and maturation of adhesions. Restricting NMIIA by Lgl1 to the lamelluma allows nascent focal adhesion assembly that is linked to actin polymerization in the lamellipodium. These focal adhesions then undergo NMIIA-dependent maturation near the lamellum (). Depletion of Lgl1, which allows NMIIA localization in the lamellipodium, prevents focal adhesion assembly and maturation. The inability of Lgl1-depleted cells to mature focal adhesions may also provide an explanation for the findings that these cells have high levels of membrane activity at the cell periphery, characterized by the rapid formation and retraction of small, short-lived protrusions. It is possible that this behavior is the result of aberrant inefficient cell adhesion due to the formation of small nascent focal adhesion.
Figure 2. Model summarizing the role of Lgl1, NMIIA, NMIIB, and Par6-aPKCζ in establishing front-back polarity in migrating cells. NMIIA and NMIIB reside outside of protrusions and are absent from the lamellipodium. MIIA controls cell protrusion and the dynamics and size of adhesions. In contrast, MIIB establishes front-back polarity. Lgl1 interacts with NMIIA in the lamellipodium, inhibiting NMIIA filament assembly at this region, thereby confining NMIIA activity to the lamellum. During protrusion, adhesions initially assemble as puncta in the lamellipodia; their formation is driven by actin polymerization. In the lamellum, the activities of both actin and NMIIA are necessary for the initial elongation and maturation of the adhesions. The complex Lgl1-Par6-aPKCζ resides in the leading edge of the cell. Upon phosphorylation by aPKCζ, Lgl1 is released from the cell leading edge to the lamellipodium, where it undergoes dephosphorylation, allowing its interaction with NMIIA and inhibiting NMIIA filament assembly. Lgl1 forms in two distinct complexes, NMIIA-Lgl1 and Par6-aPKCζ-Lgl1, because NMIIA and aPKCζ compete to bind to the same region on Lgl1. The lamellipodial aPKCζ phosphorylates NMIIB, preventing its assembly in this region, thus restricting its activity to the cell trailing edge.
Lgl1-depleted cells present three unique characterizations during migration: elongated shape, detachment from the cell sheet, and migration in different directions – in contrast with control cells that move as one sheet in one direction.Citation3 This behavior of loss of cell-cell contact and independent migration of detached cells into the wound space is characteristic of tumor cells.Citation19 Indeed, Lgl mutant cells form metastatic tumors in Drosophila.Citation20 We propose that cell polarity of migrating cells and maintenance of cell-cell contact are achieved at least in part by exclusion of NMIIA from the leading edge of the cell by Lgl1. Failure to exclude NMIIA from the leading edge may result in the appearance of non-polarized cells, detachment from the cell sheet, and independent cell migration.
The aPKC-Par6 complex is involved in establishing the apical-basal polarity of epithelial cells.Citation21 A direct interaction between basolateral Lgl and the apical Par6-aPKC complex has been demonstrated in Drosophila and mammalian epithelial cells.Citation14,22,23 Lgl can be phosphorylated in the Lgl-Par6-aPKC complex by a process that requires five closely spaced serine residues located in aPKC consensus phosphorylation sites.Citation24 The functional significance of phosphorylation of Lgl by the Par6-aPKC complex is suggested by the observation that a mutant of Lgl lacking the five serine residues reduces the polarization of cells in response to wounding.Citation14 Furthermore, phosphorylation of the Drosophila Lgl by aPKC is required for the exclusion of Lgl from the apical region in epithelial cells.Citation21 In a genetic study in Drosophila, reduction in aPKC levels suppressed the development of Lgl mutant phenotypes, such as cell polarity defects and tumorigenesis.Citation25 In human cancers, overexpression of aPKC at the membrane results in a cytosolic accumulation of Lgl, suggesting that membrane-bound Lgl is necessary for tissue homeostasis.Citation26,27 Moreover, studies using Drosophila or Xenopus embryos indicate that mutual inhibition between apical aPKC and basolateral Lgl is important for maintaining epithelial membrane polarity.Citation23 These observations suggest the importance of an antagonistic interaction of Lgl with the apical Par6-aPKC complex for the development of polarized membrane domains in epithelial cells. The mechanisms by which Lgl phosphorylation modifies its function remain to be established. Our recent observation may shed light on the role of Lgl phosphorylation by aPKC in establishing cell polarity of migrating cells. We found that aPKCζ is necessary for proper cellular localization of Lgl1.Citation4 Lgl1 expressed in aPKCζ knockout cells (aPKCζ−/− cells) presented aberrant cellular localization, and Lgl1 was confined to the cell cortex, forming a shell-like structure around the cell. Similarly, expression of an unphosphorylatable form of Lg1 (Lgl1Ala) was restricted mainly to the cell cortex. In contrast, a phosphorylation-regulated form of Lgl1 (Lgl1WT) localized to the leading edge of the cell. Furthermore, a phosphomimetic form of Lgl1 (Lgl1Aap) diffused throughout the cell and was completely absent from the leading edge.Citation4 These cellular localization properties were also reflected by the degree of Lgl1 phospho-mutant association with the cytoskeleton.Citation4 Furthermore, the extent of Lgl1 phosphorylation also affects the level of NMIIA association with the cytoskeleton. Ectopic expression of Lgl1WT decreased the amount of NMIIA associated with the cytoskeleton, and expression of Lgl1Ala decreased it further.Citation4 We conclude that the effect of the decreased amounts of cytoskeletal NMIIA by expression of Lgl1WT or Lgl1Ala reflects the increased amounts of non-filamentous NMIIA. We propose that the phosphorylation of Lgl1 by aPKCζ regulates its binding to NMIIA. The localization properties of Lgl1 in aPKCζ−/− cells and of Lgl1Ala may indicate that the phosphorylation of Lgl1 by aPKCζ takes place in the leading edge of the cell, where the complex Lgl1-Par6-aPKCζ is localized (). We propose that upon phosphorylation by aPKCζ, Lgl1 is released from the leading edge of the cell to the lamellipodium, and therefore phospho-Lgl1 is unable to bind NMIIA. In the lamellipodium, phospho-Lgl1 undergoes dephosphorylation by unknown phosphatase, allowing the interaction between Lgl1 and NMIIA, and inhibiting NMIIA filament assembly ().
Lgl interacts directly with both Par6 and aPKC to form a multiprotein complex.Citation14 Par6 can associate with Lgl both through its PDZ domain and through an N-terminal segment.Citation14 This association may be bridged by aPKC, which binds directly to both Par6Citation28,29 and Lgl.Citation14 Although the importance of the Lgl-Par6–aPKC complex in the formation of apical-basal polarity in Drosophila is well established,Citation21 its importance in establishing front-rear cell polarization is not understood. We showed that in polarized migrating cells the complex Par6-aPKCζ-Lgl1 localized to the leading edge of the cell.Citation4 We further showed that Par6-aPKCζ-Lgl1 complex formation is regulated by aPKCζ phosphorylation of Lgl1.Citation4 We propose that the interaction between Lgl1 and the asymmetrically localized Par6–aPKCζ complex may localize and phosphorylate a subpopulation of Lgl1, thereby creating a gradient of Lgl1 activity in the cell (). In vivo Lgl1 exists in two distinct complexes, NMIIA-Lgl1 and Par6-aPKCζ-Lgl1, which are affected by the state of Lgl1 phosphorylation.Citation4 The formation of two discrete complexes is explained by our finding that NMIIA and aPKCζ compete to bind to the same region on Lgl1.Citation4 This behavior may ensure the cellular localization of the two complexes to different cellular compartments, thereby establishing front-rear polarization in migrating cells. We propose that Lgl1 in the leading edge of the cell is in an unphosphorylated state and therefore forms a complex with Par6-aPKCζ in this region. It is possible that non-filamentous NMIIA forms a complex with unphosphorylated Lgl1 at the lamellipodium, preventing NMIIA from assembling into filaments, thus allowing polymerization of F-actin in that region. In contrast, filamentous NMIIA that is unbound to Lgl1 is found in the lamellum, where together with F-actin it forms the stress fibers required for attachment/detachment of migrating cells. Several years ago we showed that upon EGF stimulation, aPKCζ phosphorylates NMIIB but not NMIIA, leading to slower filament assembly of NMIIB. Furthermore, a decrease in aPKCζ expression alters the localization and organization of NMIIB after EGF stimulation, causing it to become more diffuse and not localized to the cell cortex.Citation30 These results were confirmed using aPKCζ−/− cells, we found that the amount of NMIIB that is associated with the cytoskeleton is higher than that in control cells (Dahan and Ravid, unpublished data). These results indicate that aPKCζ regulates NMIIA filament assembly indirectly by phosphorylation of Lgl1 and that it regulates NMIIB directly. Furthermore, aPKCζ has a positive effect on NMIIA assembly because phosphorylation of Lgl1 results in Lgl1 inhibition and NMIIA filament assembly. We propose a model for the role of Lgl1-NMIIA and Lgl1-Par6-aPKCζ in establishing front-rear polarization in migrating cells ().
aCell leading edge, the very front region of the cell; lamellipodium, a broad, flat cell protrusion containing a branched dendritic actin-network; lamellum, localizes behind the lamellipodium and contains acto-NMII stress fibers.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
This study was supported by the Israel Science Foundation (Grant No. 1174/12), Israel Cancer Research Foundation and Israel Cancer Association (Grant No. 20140082). S.R. holds the Dr. Daniel G. Miller Chair in Cancer Research.
References
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 2003; 302:1704-9; PMID:14657486; http://dx.doi.org/10.1126/science.1092053
- Etienne-Manneville S. Polarity proteins in migration and invasion. Oncogene 2008; 27:6970-80; PMID:19029938; http://dx.doi.org/10.1038/onc.2008.347
- Dahan I, Yearim A, Touboul Y, Ravid S. The tumor suppressor Lgl1 regulates NMII-A cellular distribution and focal adhesion morphology to optimize cell migration. Mol Biol Cell 2012; 23:591-601; PMID:22219375; http://dx.doi.org/10.1091/mbc.E11-01-0015
- Dahan I, Petrov D, Cohen-Kfir E, Ravid S. The tumor suppressor Lgl1 forms discrete complexes with NMII-A and Par6α-aPKCζ that are affected by Lgl1 phosphorylation. J Cell Sci 2014; 127:295-304; PMID:24213535; http://dx.doi.org/10.1242/jcs.127357
- Conti MA, Adelstein RS. Nonmuscle myosin II moves in new directions. J Cell Sci 2008; 121:11-8; PMID:18096687; http://dx.doi.org/10.1242/jcs.007112
- Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 2009; 10:778-90; PMID:19851336; http://dx.doi.org/10.1038/nrm2786
- Wylie SR, Chantler PD. Myosin IIA drives neurite retraction. Mol Biol Cell 2003; 14:4654-66; PMID:12960431; http://dx.doi.org/10.1091/mbc.E03-03-0187
- Martins GG, Kolega J. Endothelial cell protrusion and migration in three-dimensional collagen matrices. Cell Motil Cytoskeleton 2006; 63:101-15; PMID:16395720; http://dx.doi.org/10.1002/cm.20104
- Sandquist JC, Swenson KI, Demali KA, Burridge K, Means AR. Rho kinase differentially regulates phosphorylation of nonmuscle myosin II isoforms A and B during cell rounding and migration. J Biol Chem 2006; 281:35873-83; PMID:17020881; http://dx.doi.org/10.1074/jbc.M605343200
- Vicente-Manzanares M, Koach MA, Whitmore L, Lamers ML, Horwitz AF. Segregation and activation of myosin IIB creates a rear in migrating cells. J Cell Biol 2008; 183:543-54; PMID:18955554; http://dx.doi.org/10.1083/jcb.200806030
- Vicente-Manzanares M, Newell-Litwa K, Bachir AI, Whitmore LA, Horwitz AR. Myosin IIA/IIB restrict adhesive and protrusive signaling to generate front-back polarity in migrating cells. J Cell Biol 2011; 193:381-96; PMID:21482721; http://dx.doi.org/10.1083/jcb.201012159
- Assémat E, Bazellières E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta 2008; 1778:614-30; PMID:18005931; http://dx.doi.org/10.1016/j.bbamem.2007.08.029
- Schimanski CC, Schmitz G, Kashyap A, Bosserhoff AK, Bataille F, Schäfer SC, Lehr HA, Berger MR, Galle PR, Strand S, et al. Reduced expression of Hugl-1, the human homologue of Drosophila tumour suppressor gene lgl, contributes to progression of colorectal cancer. Oncogene 2005; 24:3100-9; PMID:15735678; http://dx.doi.org/10.1038/sj.onc.1208520
- Plant PJ, Fawcett JP, Lin DC, Holdorf AD, Binns K, Kulkarni S, Pawson T. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat Cell Biol 2003; 5:301-8; PMID:12629547; http://dx.doi.org/10.1038/ncb948
- Strand D, Jakobs R, Merdes G, Neumann B, Kalmes A, Heid HW, Husmann I, Mechler BM. The Drosophila lethal(2)giant larvae tumor suppressor protein forms homo-oligomers and is associated with nonmuscle myosin II heavy chain. J Cell Biol 1994; 127:1361-73; PMID:7962095; http://dx.doi.org/10.1083/jcb.127.5.1361
- Peng CY, Manning L, Albertson R, Doe CQ. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature 2000; 408:596-600; PMID:11117748; http://dx.doi.org/10.1038/35046094
- Barros CS, Phelps CB, Brand AH. Drosophila nonmuscle myosin II promotes the asymmetric segregation of cell fate determinants by cortical exclusion rather than active transport. Dev Cell 2003; 5:829-40; PMID:14667406; http://dx.doi.org/10.1016/S1534-5807(03)00359-9
- Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol 2008; 10:1039-50; PMID:19160484; http://dx.doi.org/10.1038/ncb1763
- Friedl P, Hegerfeldt Y, Tusch M. Collective cell migration in morphogenesis and cancer. Int J Dev Biol 2004; 48:441-9; PMID:15349818; http://dx.doi.org/10.1387/ijdb.041821pf
- Woodhouse E, Hersperger E, Shearn A. Growth, metastasis, and invasiveness of Drosophila tumors caused by mutations in specific tumor suppressor genes. Dev Genes Evol 1998; 207:542-50; PMID:9510549; http://dx.doi.org/10.1007/s004270050145
- Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci 2006; 119:979-87; PMID:16525119; http://dx.doi.org/10.1242/jcs.02898
- Betschinger J, Mechtler K, Knoblich JA. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature 2003; 422:326-30; PMID:12629552; http://dx.doi.org/10.1038/nature01486
- Hutterer A, Betschinger J, Petronczki M, Knoblich JA. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev Cell 2004; 6:845-54; PMID:15177032; http://dx.doi.org/10.1016/j.devcel.2004.05.003
- Yamanaka T, Horikoshi Y, Sugiyama Y, Ishiyama C, Suzuki A, Hirose T, Iwamatsu A, Shinohara A, Ohno S. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr Biol 2003; 13:734-43; PMID:12725730; http://dx.doi.org/10.1016/S0960-9822(03)00244-6
- Rolls MM, Albertson R, Shih HP, Lee CY, Doe CQ. Drosophila aPKC regulates cell polarity and cell proliferation in neuroblasts and epithelia. J Cell Biol 2003; 163:1089-98; PMID:14657233; http://dx.doi.org/10.1083/jcb.200306079
- Grifoni D, Garoia F, Bellosta P, Parisi F, De Biase D, Collina G, Strand D, Cavicchi S, Pession A. aPKCzeta cortical loading is associated with Lgl cytoplasmic release and tumor growth in Drosophila and human epithelia. Oncogene 2007; 26:5960-5; PMID:17369850; http://dx.doi.org/10.1038/sj.onc.1210389
- Lisovsky M, Dresser K, Baker S, Fisher A, Woda B, Banner B, Lauwers GY. Cell polarity protein Lgl2 is lost or aberrantly localized in gastric dysplasia and adenocarcinoma: an immunohistochemical study. Mod Pathol 2009; 22:977-84; PMID:19407852; http://dx.doi.org/10.1038/modpathol.2009.68
- Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol 2000; 2:531-9; PMID:10934474; http://dx.doi.org/10.1038/35019573
- Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol 2000; 2:540-7; PMID:10934475; http://dx.doi.org/10.1038/35019592
- Even-Faitelson L, Ravid S. PAK1 and aPKCzeta regulate myosin II-B phosphorylation: a novel signaling pathway regulating filament assembly. Mol Biol Cell 2006; 17:2869-81; PMID:16611744; http://dx.doi.org/10.1091/mbc.E05-11-1001
- Dulyaninova NG, Bresnick AR. The heavy chain has its day: Regulation of myosin-II assembly. Bioarchitecture 2013; 3:77-85; PMID:24002531; http://dx.doi.org/10.4161/bioa.26133
