Abstract
Cell-cell adhesion molecules play key roles at the intercellular junctions of a wide variety of cells, including interneuronal synapses and neuron-glia contacts. Functional studies suggest that adhesion molecules are implicated in many aspects of neural network formation, such as axon-guidance, synapse formation, regulation of synaptic structure, and astrocyte-synapse contacts. Some basic cell biological aspects of the assembly of junctional complexes of neurons and glial cells resemble those of epithelial cells. However, the neuron specific junctional machineries are required to exert neuronal functions, such as synaptic transmission and plasticity. In this review, we describe the distribution and function of cell adhesion molecules at synapses and at contacts between synapses and astrocytes.
Introduction
The cell-cell adhesion system is involved in many aspects of neuronal development, including neuronal cell migration, axon-bundle formation, synapse formation and formation of complex of glial networks which surround axons and synapses. These adhesion systems are important for brain morphology and highly coordinated brain functions, such as memory and learning.Citation1–Citation3 Like epithelial junctions, cell-cell junctions in the nervous systems contain a variety of transmembrane proteins, cytoskeletal elements and signaling complexes.
During early development of the nervous systems, differentiated neurons migrate to their proper positions and elongate their axons towards their targets. Growing axons are guided by various attractive or repulsive target-derived cues.Citation4 After reaching their target areas, axonal growth cones still need to recognize their appropriate target cells for the formation of synapses. Then, initial contacts are formed between axons and dendrites, and signaling through homophilic and heterophilic receptors induces differentiation of the synaptic specialization.Citation5 Most of these interactions and recognitions have been shown to be mediated by cell surface proteins. Various cell surface proteins have been identified and characterized as important regulators of axon-guidance and synapse formation (). These molecules interact with each other between the cells to activate various signaling pathways and bring the apposed cell membranes into contact. Some of these molecules are defined as adhesion molecules and others as signaling molecules. However, as certain adhesion molecules are known to have signaling functions and the signaling molecules often promote cell-cell adhesion, it might not be so easy to distinguish these membrane proteins specifically involved in adhesion or signaling.
In addition to neuron-neuron interactions, astrocyte-synapse interactions are also known to play important roles in the formation of neural networks. Astrocyte-synapse communication participates in synapse formation, synaptic transmission and axonal conduction, and perhaps modulates the activity of neuronal networks during development and throughout adult life.Citation6 Recent advances have clarified the molecular compositions of astrocyte-synapse interfaces, and have provided new insight into astrocyte-synapse communication. To date, a few adhesion molecules have been identified at the astrocyte-synapse contacts. Here, we briefly summarize the key cell adhesion molecules involved in the synapse formation and in the astrocyte-synapse interactions.
Synapses
Synapses are a specialized form of intercellular junctions where the axon terminal of a neuron comes into functional contact with a target cell ( and B). Specificity and plasticity of synapses provide neurons with a structural and functional basis for the formation of the neuronal network system. Synapses are highly asymmetrical junctions formed between two different neurons, and early ultrastructural studies showed that the synaptic junctional areas contain at least two types of adhesion structure ().Citation7,Citation8 One type of adhesion structure is the transmitter release zone associated with synaptic vesicles, termed synaptic junctions (SJs), and the other is a symmetrical junction, termed puncta adherentia junctions (PAJs), defined by the two criteria of symmetric paramembranous dense materials and the lack of association with synaptic vesicles (). SJs are regarded as sites for neurotransmission. They are associated with presynaptic active zones containing Ca2+ channels and numerous neurotransmitter-filled synaptic vesicles which are docked on the presynaptic membrane by a complex of proteins, and postsynaptic densities where the specific neurotransmitter receptors and structural scaffolding and signaling proteins are localized. PAJs are regarded as mechanical adhesion sites between axon terminals and their targets, although their exact functions remain unknown. However, PAJs are morphologically similar to adherens junctions (AJs) formed in epithelia, and several important molecular constituents of neuronal synapses are common to both neurons and epithelial cells. Thus, some basic cell biological aspects of the assembly of junctional complexes may be shared between these two cell types.Citation9,Citation10 During development, specific neuronal circuits are generated by synapse formation between the appropriate pre- and postsynaptic partners. Initial contacts between synaptic partners are frequently established between axonal growth cones and dendritic filopodia extending from dendrites in vitro.Citation11,Citation12 Once initial axon-target interactions develop, various molecules can engage in bidirectional signaling to coordinate the differentiation of synaptic membrane specializations and stabilize the synaptic contact. Several factors that may be involved in these processes are summarized below. Although electrical synapses are formed at narrow gaps between the pre- and postsynaptic neurons known as gap junctions, we describe chemical synapses.
Cadherins
Cadherins are Ca2+-dependent cell-cell adhesion molecules that constitute a superfamily comprised of more than 100 members in vertebrates, and are grouped into subfamilies that are designated as classic cadherins and protocadherins.Citation13 Classic cadherins are single-pass transmembrane proteins and have five extracellular cadherin repeat (EC) domains (EC1 to EC5). All classic cadherins are homophilic adhesion molecules that function with their cytoplasmic (CP) partners, catenins ().Citation14 Catenins are cadherin-binding proteins that connect cadherins to the actin cytoskeleton. These include α-catenin, β-catenin and p120 catenin. The cadherin-catenin complexes are known to regulate actin polymerization, a property important for maintaining the cell-cell adhesion. Cadherins and their associated catenins have been observed in many neuronal populations in the central nervous systems (CNS). At the ultrastructural level, these proteins were found in synaptic junctions of most regions of the nervous systems, forming a symmetrical adhesion structure in the PAJs.Citation9 During development, the cadherin-catenin complexes accumulate at early axo-dendritic filopodial contacts, and are retained in many of the mature synapses.Citation9,Citation15–Citation18 A fragment of N-cadherin lacking its extracellular region serves as a dominant negative mutant of cadherins and inhibits their cell-cell adhesion activity. Expression of this mutant results in the appearance of filopodia-like spines, an increase in the spine length, and a decrease in the spine head width, and affects the organization of synapses in the cultured hippocampal neurons.Citation18,Citation19 Despite the evidence that cadherins are involved in the formation of synapses, they are not sufficient to form them in vitro, because expression of N-cadherin in non-neuronal cells fails to induce pre-synaptic differentiation in axons at the sites of contact.Citation20 Recent studies also implicate catenins in the control of spine structure and synaptic organization in cultured hippocampal neurons. Deletion of β-catenin affects localization of synaptic vesicles along the axon,Citation21 and loss of p120 catenin affects Rho-family small G-protein signaling, which results in a reduced spine density.Citation22 A remarkable feature of classic cadherins is its binding specificity and region-specific distribution. In the brain, many subtypes of classic cadherins are expressed by restricted groups of functionally connected nuclei and laminas.Citation23 Whether cadherin-mediated adhesion contributes to the formation of selective inter-neuronal connections during neural network formation remains unknown.
Protocadherins
Protocadherins are a group of transmembrane proteins that belong to the cadherin superfamily, and have varying numbers of the EC domains but divergent cytoplasmic domains that do not appear to signal through catenins.Citation24,Citation25 Various protocadherins are expressed in the nervous systems, and some of them are localized at synapses. Multiple α- and γ-protocadherin isoforms are highly expressed in distinct, although partially overlapping, sets of neurons and concentrated at synapses. The complex genomic organization and alternative splicing of protocadherins have led to the speculation that their diversity underlies synaptic specificity.Citation26 γ-protocadherins are required for survival of specific neuronal typesCitation27 and arcadlin is required for activity-dependent synaptic morphogenesis.Citation28 However, the biological functions of most protocadherins are unknown.
Nectins
Nectins represent a family of Ca2+-independent immunoglobulin (Ig)-like cell-cell adhesion molecules, which consist of four members ().Citation29 At the CA3 region of hippocampus, nectin-1 and nectin-3 asymmetrically localize at the pre-and post-synaptic sides, respectively, of the PAJs, but not at SJs.Citation10 Nectins form homo- or hetero-trans-dimers in a Ca2+-independent manner, where heterotypic binding leads to stronger adhesion than homotypic binding.Citation30–Citation32 In epithelial cells in culture, nectins first form cell-cell adhesion and then recruit cadherin to the nectin-based cell-cell adhesion sites to cooperatively form AJs.Citation33,Citation34 Afadin, an actin-filament binding protein that connects nectins to the actin cytoskeleton, is also present at PAJs. Disruption of nectin-based cell-cell adhesion in cultured hippocampal neurons decreases the size of synapses but increases their number,Citation10 and a nectin-1 mutant causes human cleft lip/palateectodermal dysplasia, Margarita island ectodermal dysplasia, and Zlotogora-Ogür syndrome, characterized by mental retardation, cleft lip/palate, syndactyly and ectodermal dysplasia.Citation35 In both nectin-1 and -3-deficient mice, the number of PAJs at the synapses between the mossy fiber terminals and the dendrites of the CA3 pyramidal cells in the hippocampus is reduced. In addition, the abnormal mossy fiber trajectory is observed, suggesting that nectins are involved in the formation of PAJs, which maintain the proper mossy fiber trajectory in the CA3 region of the hippocampus.Citation36 In afadin-deficient mice, perforated synapses in the hippocampus are observed. Reduction in the number of PAJs is likely to be further enhanced in afadin-deficient mice than in nectin-1 or -3-deficient mice. The observation of loss of PAJs in the nectin and afadin-deficient mice suggests the possibility that the localization of the cadherin/catenin complex is regulated by the nectin/afadin system, as for epithelial adherens junctions. The recruitment of afadin (AF-6) to postsynapse is regulated by small G-protein Rap1, and is involved in spine formation.Citation37
The axon-biased localization of nectin-1 and its trans-interaction with nectin-3 in cooperation with the cadherin machinery is critical for the ordered association of axons and dendrites.Citation38 However, the sorting signal of nectin-1 to axons has not been identified.Citation39 The genetic deletion of nectin-1 loosens the contacts between axons and dendritic spines, while the overexpression of nectin-1, causing mislocalization of nectin-1 to dendrites, induces atypical dendro-dendritic as well as excessive axo-dendritic contacts. These actions of nectins require cadherin-catenin complexes suggesting that the two adhesion systems cooperate.Citation38 These data suggest that localized cadherin activity may be achieved by cooperative heterophilic nectin interactions. It is also likely that mechanisms work for restricting adhesion activity at specific cell-cell contact sites. These data are consistent with those obtained in epithelial cells, suggesting that nectins form initial cell-cell adhesion and recruit cadherins to the nectin-based cell-cell adhesion sites to form AJs, and suggest that nectins play similar roles in the formation of PAJs.
Other Ig Superfamily CAMs
Other Ig superfamily CAMs, which have varying numbers of Ig-like domains, have been identified at synapses and have been shown to be involved in synaptic formation and plasticity. For example, neural cell adhesion molecule (NCAM), which contains five Ig-like domains and two fibronectin type III repeats, is engaged in homophilic and heterophilic interactions with a variety of ligands at synapses, such as fibroblast growth factor receptor (FGFR), L1, TAG-1/axonin-1 and heparan sulfate proteoglycans ().Citation40,Citation41 NCAM is widely expressed in the developing and adult brains and plays crucial roles in migration, pathfinding of axons, and synaptic plasticity. It is involved in both early synaptogenesis and subsequent synaptic maturation.Citation42,Citation43 NCAM is unique among adhesion molecules in that it carries a large amount of the negatively charged sugar, polysialic acid (PSA) (Bonfanti et al., in this issue). Poor axonal fasciculation is observed in the hippocampus of NCAM-deficient mice, resulting in an impaired synapse formation in the CA3 region.Citation44 Mossy fibers also appear defasciculated in mice with the NCAM-180 isoform.Citation45 These functions of NCAM appear to be mediated by primarily by presence of the PSA moiety.Citation46 Neurofascin 186 (NF186), an L1 family Ig-like cell adhesion molecule, is implicated in the subcellular organization of GABAergic synapses between basket interneurons and Purkinje cells in the cerebellum.Citation47
SYG-1/SYG-2
SYG-1/SYG-2 are specific adhesion molecules that determine synaptic specificity in a lock-and-key manner. SYG-1, a four Ig-like domain-containing protein, and SYG-2, a seven Ig-like domain- and one fibronectin type III repeat-containing protein, were isolated in a genetic screen for C. elegans mutants that exhibit defective synaptic positioning.Citation48,Citation49 Interactions between SYG-1 and SYG-2 induce formation of synapses at appropriate synaptic targets. The Drosophila orthologues of roughest(rst) have been implicated in axon fasciculation and layer targeting in the fly visual system.Citation50 Moreover, SYG-1 and SYG-2 share significant homology with the mice and human proteins NEPH and Nephrin, which are expressed in the CNS,Citation51 although their roles in the CNS remain unknown.
Sidekicks
Sidekicks, which have six Ig-like domains and thirteen fibronectin type III repeats, have been implicated in selective synapse formation in the chicken retina.Citation52 Sidekick-1 and -2 are differentially expressed among subsets of retinal ganglion cells in a non-overlapping manner. Sidekicks act as homophilic adhesion molecules in vitro, and are highly concentrated at synapses of restricted regions in vivo. Ectopic expression of Sidekick in Sidekick-negative cells induces mistargeting. These data suggest that sidekick interactions may promote lamina-specific connectivity.
Neuroligin
Neuroligin is an esterase-like domain-containing protein and localizes at the post-synaptic side of SJs, whereas β-neurexin is a laminin-globular-domain-containing protein and localizes at the pre-synaptic side of SJs. These two molecules interact with one another and this interaction induces the formation of synapses in vitro (). The neurexin family was first identified as receptors for alpha-latrotoxin, which acts presynaptically to release neurotransmitters from sensory and motor neurons.Citation53 More than 1,000 neurexin isoforms are generated by alternative splicing, which are differentially expressed in the nervous systems.Citation54 Neuroligins is a β-neurexin binding partner.Citation55,Citation56 β-Neurexin binds neuroligins trans-synaptically and induces formation of glutamatergic and GABAergic presynaptic specializations in vitro.Citation20,Citation57,Citation58 However, neuroligins are indispensable for synapse maturation and synaptic transmission, but not for triggering initial synapse formation from the phenotypes of knockout mice.Citation59
Necl-2
Necl-2 was previously characterized as a tumor suppressor gene, and is also termed TSLC1/SgIGSF/RA175/IGSF4/SynCAM1. Necl-2 is a homophilic adhesion molecule, but also shows heterophilic cell-cell adhesion activity with Necl-1 and nectin-3.Citation60 Necl-2 is widely expressed in various tissues and localizes at the basolateral plasma membrane in epithelial cells, not in the specialized cell-cell junctions such as AJs, TJs and desmosomes.Citation61 Necl-2 localizes at synapses and induces presynaptic differentiation and stabilization, at least in vitro.Citation62
Eph Receptor
Eph receptor tyrosine kinases and their ephrin ligands are grouped into two families: ephrinA ligands are tethered to the plasma membrane by a GPI linkage and bind to EphA receptors, whereas ephrinB ligands are transmembrane proteins that bind preferentially to EphB receptors ().Citation63 EphB receptors localize to synapses, where they can bind the NMDA-type glutamate receptor subunit NR1 via the extracellular domain.Citation64 Stimulation of EphB receptors by ephrin ligands results in increased synaptic density and in NMDA receptor-mediated calcium influx and gene expression.Citation65 EphBs multiple mutant mice develop abnormal spines in the hippocampus both in vitro and in vivo.Citation66 However, the molecular mechanisms of many ephrin/Eph-related synaptic functions and their roles in the initial steps of synapse assembly are still largely unknown.
Axon-Astrocyte Contacts
Astrocytes are characteristic star-shaped glial cells, and their many processes ensheath synaptic junctions in the brain, but do not form myelin (). Astrocytes are also known to regulate synaptic transmission by uptake of neurotransmitters, such as glutamate, ATP and GABA, from the synaptic cleft through membrane transporters, and release of glutamate upon reversal of the transporter.Citation6 Other substances released by astrocytes can strengthen synaptic transmission by co-activating NMDA receptors in the postsynaptic membrane (e.g., D-serine) or can reduce it by binding to neurotransmitters.Citation67,Citation68 Synapse formation may also be regulated by factors produced by astrocytes.Citation69 The co-culture of purified neurons with astrocytes can facilitate synaptogenesis.Citation70 For example, an astrocyte-derived factor induces the maturation of retinal ganglion cells Citation71; this diffusible factor has been identified as cholesterol complexed with apolipoprotein E-containing lipoproteins.Citation69 A recent study showed that thrombospondin-1 and -2, astrocyte-secreted proteins, promote CNS synaptogenesis in vitro and in vivo.Citation72 On the other hand, integrins localize at contacts between neurons and astrocytes promote synaptogenesis.Citation73 The interaction between neurons and astrocytes is important for synaptogenesis. However, a few adhesion molecules have been identified at contacts between neurons and astrocytes.
AMOG (Adhesion Molecule on Glia)
AMOG (Adhesion molecule on glia), the β2-subunit of the Na+/K+ ATPase, is a membrane glycoprotein and localizes at contacts between neurons and astrocytes. AMOG is implicated in neurite outgrowth and neuronal migration.Citation74–Citation76 AMOG associates with the catalytic α-subunit of the Na+/K+ ATPase and forms a functional ion channel.Citation77 This unique molecule serves as a cell adhesion molecule and a subunit of ion channel. AMOG is firstly expressed in the brain shortly before granule cell migration. The expression level of AMOG increases during early postnatal development and reaches the highest expression level in adult.Citation78 AMOG-deficient mice present motor incoordination and paralysis in early postnatal life and die shortly after birth.Citation79 The exact functions of AMOG still remain elusive.
Integrins
Integrins are cell surface receptors that interact with the extracellular matrix (ECM) and transduce the signal from the ECM to the cell. Integrins consist of two distinct chains, α- and β-subunits. Integrins at contacts between neurons and astrocytes activates protein kinase C (PKC) signaling and promotes synaptogenesis in vitro.Citation73 However, the involvement of the integrins-dependent PKC signaling in synaptogenesis still remains elusive in vivo.
Necl-1/TSLL1/SynCAM3
Necl-1/TSLL1/SynCAM3, which has a domain structure similar to those of nectins, localizes at axon-astrocyte contacts ().Citation80 Necl-1 shows Ca2+-independent homophilic cell-cell adhesion activity and heterophilic cell-cell adhesion activity with Necl-2, nectin-1 and nectin-3, but not Necl-5 or nectin-2. Necl-1 does not bind afadin, but binds Dlg3/MPP3, a membrane-associated guanylate kinase family member, Pals2 and CASK. Necl-1 is specifically expressed in neural tissue, and localizes to contact sites along axons, nerve terminals, glial cell processes, axon bundles and myelinated axons. However, the exact functions of Necl-1 remain unknown.
Conclusions and Perspectives
Herein, we described the roles of the various adhesion molecules in synapse formation, and neuron-glia interactions. Functional studies of individual cell adhesion molecules have provided a wealth of information on their roles in synapse assembly, spine morphogenesis and synaptic plasticity. Although the various adhesion systems can mediate adhesive interactions, individually, they probably control specific aspects of synapse formation. Because multiple systems appear to cooperate at individual synapse, it will be of great interest to determine whether they act in a parallel or in a hierarchical manner. For most of the functions of neuron-glia contacts, we still lack sufficient information on their functions at both cellular and molecular levels. Future research on the mechanisms of neuron-glia interactions will lead to greater insight into the mechanisms underlying the formation of complex neural circuitries.
Figures and Tables
Figure 1 (A) Synapses are formed at the contact points between axons and dendrites of their target neurons. (B) At synapses, at least two types of intercellular junctions, synaptic and puncta adherentia junctions, have been recognized. Synaptic junctions are regarded as sites of neurotransmission, associated with synaptic vesicles at the presynaptic active zone where Ca2+ channels localize, and postsynaptic densities (PSDs), where neurotransmitter receptors localize. Puncta adherentia junctions, which are not associated with synaptic vesicles or PSDs, appear to be ultrastructurally similar to adherence junctions of epithelial cells. (C) Electron microscopic morphology of the synapses between the mossy fiber terminals and the dendrites of pyramidal cells in the CA3 area of the hippocampus. Arrows indicate PAJs. Arrow heads indicate SJs. D: dendrite. S: dendritic spine. MT: mossy fiber terminal. Scale bar, 200 nm. (D) Molecular composition of the synapse. Many of these adhesion molecules possess a binding motif that binds to PDZ proteins. These interactions associate with each other and lead to the formation of a multi-molecular scaffold beneath both the pre- and post-synaptic membranes. (E) Astrocytes have many characteristic processes and ensheath synaptic junctions in the brain, but do not form myelin. Necl-1 localizes at the contact sites between axon terminals and glia cell processes and interacts homophilically.
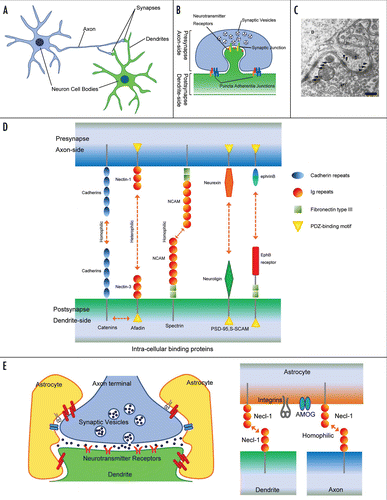
Table 1 Lists of the neuron-neuron and neuron-glia interactions in the nervous systems
Acknowledgements
We are grateful to Dr. Yoshihisa Kudo at Tokyo University of Pharmacy and Life Sciences and colleagues at Kobe University for comments on the manuscript. This work was supported by grants-in-aid for Scientific Research and for Cancer Research from Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- Sanes JR, Yamagata M. Formation of lamina-specific synaptic connections. Curr Opin Neurobiol 1999; 9:79 - 87
- Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol 2003; 15:621 - 632
- Washbourne P, Dityatev A, Scheiffele P, Biederer T, Weiner JA, Christopherson KS, et al. Cell adhesion molecules in synapse formation. J Neurosci 2004; 24:9244 - 9249
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science 1996; 274:1123 - 1133
- Scheiffele P. Cell-cell signaling during synapse formation in the CNS. Annu Rev Neurosci 2003; 26:485 - 508
- Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science 2002; 298:556 - 562
- Spacek J, Lieberman AR. Three dimensional reconstruction in electron microscopy of the central nervous system. Sb Ved Pr Lek Fak Karlovy Univerzity Hradci Kralove 1974; 17:203 - 222
- Peters A, Palay SL, Webster HD. The fine structure of the nervous system: neurons and their supporting cells 1991; New York Oxford University Press
- Uchida N, Honjo Y, Johnson KR, Wheelock MJ, Takeichi M. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J Cell Biol 1996; 135:767 - 779
- Mizoguchi A, Nakanishi H, Kimura K, Matsubara K, Ozaki-Kuroda K, Katata T, et al. Nectin: an adhesion molecule involved in formation of synapses. J Cell Biol 2002; 156:555 - 565
- Jontes JD, Smith SJ. Filopodia, spines and the generation of synaptic diversity. Neuron 2000; 27:11 - 14
- Ziv NE, Garner CC. Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci 2004; 5:385 - 399
- Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci 2007; 8:11 - 20
- Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol 2003; 19:207 - 235
- Yamagata M, Herman JP, Sanes JR. Lamina-specific expression of adhesion molecules in developing chick optic tectum. J Neurosci 1995; 15:4556 - 4571
- Fannon AM, Colman DR. A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron 1996; 17:423 - 434
- Benson DL, Tanaka H. N-cadherin redistribution during synaptogenesis in hippocampal neurons. J Neurosci 1998; 18:6892 - 6904
- Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron 2002; 35:77 - 89
- Bozdagi O, Valcin M, Poskanzer K, Tanaka H, Benson DL. Temporally distinct demands for classic cadherins in synapse formation and maturation. Mol Cell Neurosci 2004; 27:509 - 521
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 2000; 101:657 - 669
- Bamji SX, Shimazu K, Kimes N, Huelsken J, Birchmeier W, Lu B, et al. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron 2003; 40:719 - 731
- Elia LP, Yamamoto M, Zang K, Reichardt LF. p120 catenin regulates dendritic spine and synapse development through Rho-family GTPases and cadherins. Neuron 2006; 51:43 - 56
- Suzuki SC, Inoue T, Kimura Y, Tanaka T, Takeichi M. Neuronal circuits are subdivided by differential expression of type-II classic cadherins in postnatal mouse brains. Mol Cell Neurosci 1997; 9:433 - 447
- Yagi T, Takeichi M. Cadherin superfamily genes: functions, genomic organization and neurologic diversity. Genes Dev 2000; 14:1169 - 1180
- Hirano S, Suzuki ST, Redies C. The cadherin superfamily in neural development: diversity, function and interaction with other molecules. Front Biosci 2003; 8:306 - 355
- Wu Q, Maniatis T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell 1999; 97:779 - 790
- Weiner JA, Wang X, Tapia JC, Sanes JR. Gamma protocadherins are required for synaptic development in the spinal cord. Proc Natl Acad Sci USA 2005; 102:8 - 14
- Yasuda S, Tanaka H, Sugiura H, Okamura K, Sakaguchi T, Tran U, et al. Activity-induced protocadherin arcadlin regulates dendritic spine number by triggering N-cadherin endocytosis via TAO2beta and p38 MAP kinases. Neuron 2007; 56:456 - 471
- Takai Y, Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci 2003; 116:17 - 27
- Fabre S, Reymond N, Cocchi F, Menotti L, Dubreuil P, Campadelli-Fiume G, et al. Prominent role of the Ig-like V domain in trans-interactions of nectins. Nectin3 and nectin 4 bind to the predicted C-C′-C″-D beta-strands of the nectin1 V domain. J Biol Chem 2002; 277:27006 - 27013
- Yasumi M, Shimizu K, Honda T, Takeuchi M, Takai Y. Role of each immunoglobulin-like loop of nectin for its cell-cell adhesion activity. Biochem Biophys Res Commun 2003; 302:61 - 66
- Martinez-Rico C, Pincet F, Perez E, Thiery JP, Shimizu K, Takai Y, et al. Separation force measurements reveal different types of modulation of E-cadherin-based adhesion by nectin-1 and -3. J Biol Chem 2005; 280:4753 - 4760
- Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, et al. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol 2000; 150:1161 - 1176
- Honda T, Shimizu K, Kawakatsu T, Yasumi M, Shingai T, Fukuhara A, et al. Antagonistic and agonistic effects of an extracellular fragment of nectin on formation of E-cadherin-based cell-cell adhesion. Genes Cells 2003; 8:51 - 63
- Suzuki K, Hu D, Bustos T, Zlotogora J, Richieri-Costa A, Helms JA, et al. Mutations of PVRL1, encoding a cell-cell adhesion molecule/herpesvirus receptor, in cleft lip/palate-ectodermal dysplasia. Nat Genet 2000; 25:427 - 430
- Honda T, Sakisaka T, Yamada T, Kumazawa N, Hoshino T, Kajita M, et al. Involvement of nectins in the formation of puncta adherentia junctions and the mossy fiber trajectory in the mouse hippocampus. Mol Cell Neurosci 2006; 31:315 - 325
- Xie Z, Huganir RL, Penzes P. Activity-dependent dendritic spine structural plasticity is regulated by small GTPase Rap1 and its target AF-6. Neuron 2005; 48:605 - 618
- Togashi H, Miyoshi J, Honda T, Sakisaka T, Takai Y, Takeichi M. Interneurite affinity is regulated by heterophilic nectin interactions in concert with the cadherin machinery. J Cell Biol 2006; 174:141 - 151
- Lim ST, Lim KC, Giuliano RE, Federoff HJ. Temporal and spatial localization of nectin-1 and l-afadin during synaptogenesis in hippocampal neurons. J Comp Neurol 2008; 507:1228 - 1244
- Walsh FS, Doherty P. Neural cell adhesion molecules of the immunoglobulin superfamily: role in axon growth and guidance. Annu Rev Cell Dev Biol 1997; 13:425 - 456
- Kiss JZ, Muller D. Contribution of the neural cell adhesion molecule to neuronal and synaptic plasticity. Rev Neurosci 2001; 12:297 - 310
- Dityatev A, Dityateva G, Sytnyk V, Delling M, Toni N, Nikonenko I, et al. Polysialylated neural cell adhesion molecule promotes remodeling and formation of hippocampal synapses. J Neurosci 2004; 24:9372 - 9382
- Polo-Parada L, Bose CM, Landmesser LT. Alterations in transmission, vesicle dynamics and transmitter release machinery at NCAM-deficient neuromuscular junctions. Neuron 2001; 32:815 - 828
- Cremer H, Chazal G, Goridis C, Represa A. NCAM is essential for axonal growth and fasciculation in the hippocampus. Mol Cell Neurosci 1997; 8:323 - 335
- Seki T, Rutishauser U. Removal of polysialic acid-neural cell adhesion molecule induces aberrant mossy fiber innervation and ectopic synaptogenesis in the hippocampus. J Neurosci 1998; 18:3757 - 3766
- Monnier PP, Beck SG, Bolz J, Henke-Fahle S. The polysialic acid moiety of the neural cell adhesion molecule is involved in intraretinal guidance of retinal ganglion cell axons. Dev Biol 2001; 229:1 - 14
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell 2004; 119:257 - 272
- Shen K, Bargmann CI. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell 2003; 112:619 - 630
- Shen K, Fetter RD, Bargmann CI. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell 2004; 116:869 - 881
- Schneider T, Reiter C, Eule E, Bader B, Lichte B, Nie Z, et al. Restricted expression of the irreC-rst protein is required for normal axonal projections of columnar visual neurons. Neuron 1995; 15:259 - 271
- Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, et al. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol 2001; 21:4829 - 4836
- Yamagata M, Weiner JA, Sanes JR. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell 2002; 110:649 - 660
- Ushkaryov YA, Petrenko AG, Geppert M, Sudhof TC. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science 1992; 257:50 - 56
- Missler M, Fernandez-Chacon R, Sudhof TC. The making of neurexins. J Neurochem 1998; 71:1339 - 1347
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, et al. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell 1995; 81:435 - 443
- Nguyen T, Sudhof TC. Binding properties of neuroligin 1 and neurexin 1beta reveal function as heterophilic cell adhesion molecules. J Biol Chem 1997; 272:26032 - 26039
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 2004; 119:1013 - 1026
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science 2005; 307:1324 - 1328
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, et al. Neuroligins determine synapse maturation and function. Neuron 2006; 51:741 - 754
- Sakisaka T, Takai Y. Biology and pathology of nectins and nectin-like molecules. Curr Opin Cell Biol 2004; 16:513 - 521
- Shingai T, Ikeda W, Kakunaga S, Morimoto K, Takekuni K, Itoh S, et al. Implications of nectin-like molecule-2/IGSF4/RA175/SgIGSF/TSLC1/SynCAM1 in cell-cell adhesion and transmembrane protein localization in epithelial cells. J Biol Chem 2003; 278:35421 - 35427
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, et al. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 2002; 297:1525 - 1531
- Flanagan JG, Vanderhaeghen P. The Ephrins and Eph receptors in neural development. Annu Rev of Neurosci 1998; 21:309 - 345
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, et al. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell 2000; 103:945 - 956
- Takasu MA, Dalva MB, Zigmond RE, Greenberg ME. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science 2002; 295:491 - 495
- Henkemeyer M, Itkis OS, Ngo M, Hickmott PW, Ethell IM. Multiple EphB receptor tyrosine kinases shape dendritic spines in the hippocampus. J Cell Biol 2003; 163:1313 - 1326
- Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci USA 1999; 96:13409 - 13414
- Baranano DE, Ferris CD, Snyder SH. Atypical neural messengers. Trends Neurosci 2001; 24:99 - 106
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science 2001; 294:1354 - 1357
- Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science 1997; 277:1684 - 1687
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science 2001; 291:657 - 661
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 2005; 120:421 - 433
- Hama H, Hara C, Yamaguchi K, Miyawaki A. PKC signaling mediates global enhancement of excitatory synaptogenesis in neurons triggered by local contact with astrocytes. Neuron 2004; 41:405 - 415
- Muller-Husmann G, Gloor S, Schachner M. Functional characterization of beta isoforms of murine Na,K-ATPase. The adhesion molecule on glia (AMOG/beta2), but not beta1, promotes neurite outgrowth. J Biol Chem 1993; 268:26260 - 26267
- Antonicek H, Persohn E, Schachner M. Biochemical and functional characterization of a novel neuron-glia adhesion molecule that is involved in neuronal migration. J Cell Biol 1987; 104:1587 - 1595
- Lecuona E, Luquin S, Avila J, Garcia-Segura LM, Martin-Vasallo P. Expression of the beta1 and beta2(AMOG) subunits of the Na,K-ATPase in neural tissues: cellular and developmental distribution patterns. Brain Res Bull 1996; 40:167 - 174
- Gloor S, Antonicek H, Sweadner KJ, Pagliusi S, Frank R, Moos M, Schachner M. The adhesion molecule on glia (AMOG) is a homologue of the beta subunit of the Na,K-ATPase. J Cell Biol 1990; 110:165 - 174
- Pagliusi SR, Schachner M, Seeburg PH, Shivers BD. The Adhesion Molecule on Glia (AMOG) Is Widely Expressed by Astrocytes in Developing and Adult Mouse Brain. Eur J Neurosci 1990; 2:471 - 480
- Magyar JP, Bartsch U, Wang ZQ, Howells N, Aguzzi A, Wagner EF, et al. Degeneration of neural cells in the central nervous system of mice deficient in the gene for the adhesion molecule on Glia, the beta2 subunit of murine Na,K-ATPase. J Cell Biol 1994; 127:835 - 845
- Kakunaga S, Ikeda W, Itoh S, Deguchi-Tawarada M, Ohtsuka T, Mizoguchi A, et al. Nectin-like molecule-1/TSLL1/SynCAM3: a neural tissue-specific immunoglobulin-like cell-cell adhesion molecule localizing at non-junctional contact sites of presynaptic nerve terminals, axons and glia cell processes. Cell Sci 2005; 118:1267 - 1277