Abstract
Interactions between endothelial cells and the surrounding extracellular matrix are continuously adapted during angiogenesis, from early sprouting through to lumen formation and vessel maturation. Regulated control of these interactions is crucial to sustain normal responses in this rapidly changing environment, and dysfunctional endothelial cell behaviour results in angiogenic disorders. The proteoglycan decorin, an extracellular matrix component, is upregulated during angiogenesis. While it was shown previously that the absence of decorin leads to dysregulated angiogenesis in vivo, the molecular mechanisms were not clear. These abnormal endothelial cell responses have been attributed to indirect effects of decorin; however, our recent data provides evidence that decorin directly regulates endothelial cell-matrix interactions. This data will be discussed in conjunction with findings from previous studies, to better understand the role of this proteoglycan in angiogenesis.
Led by appropriate cues, the vascular system undergoes postnatal remodelling (angiogenesis), to maintain tissue homeostasis. Thus while much of the mature endothelium is quiescent, locally activated endothelial cells re-enter the cell cycle, and assume a motile phenotype essential for sprouting and neo-vessel formation. Concomitantly, the surrounding extracellular matrix (ECM) is significantly altered through de novo protein expression, deposition of plasma components and protease-mediated degradation. The latter liberates cryptic binding sites and sequestered growth factors in addition to intact and degraded ECM components, which themselves possess pro- and anti-angiogenic signalling properties. For supported blood flow, endothelium quiescence and integrity is re-established, and the ECM is organized into mature, cross-linked networks. In short, endothelial cells regulate ECM synthesis, assembly and turnover while the structure and composition of ECM in turn influences cellular phenotype. The ECM therefore, plays a critical role in control of endothelial cell behaviour during angiogenesis.
Decorin is a member of the small leucine-rich repeat proteoglycan (SLRP) family, which was first discovered ‘decorating’ collagen I fibrils and was subsequently shown to regulate fibrillogenesis.Citation1,Citation2 Both the protein core and the single, covalently attached glycosaminoglycan (GAG) moieties of decorin are involved in this function, the relevance of which is demonstrated by the phenotype of the decorin null mouse, which exhibits loose, fragile skin due to dysregulated fibrillogenesis.Citation2 Interestingly, a role for decorin in postnatal angiogenesis was also revealed by studies in the decorin null background. Corneal neoangiogenesis was reduced.Citation3 Conversely, neo-angiogenesis was enhanced during dermal wound healing, although surprisingly this led to delayed wound closure.Citation4 In this case, skin fragility due to the absence of decorin may have hindered wound closure, despite an increased blood supply. It is apparent however, that decorin plays a role in inflammation-associated angiogenesis. Indeed, endothelial cells undergoing angiogenic morphogenesis in this environment express decorin, while quiescent endothelial cells do not,Citation3–Citation6 indicating that decorin modulates endothelial cell behaviour specifically during inflammatory-associated remodelling of the vascular system.
To understand decorin effects on angiogenic morphogenesis within a minimalist environment, various in vitro models of angiogenesis have been employed (). In three-dimensional collagen I gels, decorin expression enhanced endothelial cell survival and tube formation.Citation6 Similarly, decorin expression enhanced tube formation on matrigel,Citation8 but in other studies utilising this substrate was found to either have no influenceCitation9 or to inhibit tubulogenesis induced by growth factors.Citation10 In yet another study, decorin inhibited tube formation when presented as a substrate prior to addition of collagen I.Citation7 These contrasting observations may reflect the importance of the micro-environment within which decorin is presented. Alternatively, controversial results could result from different sources of decorin since cell types differ in their post-translational modifications of the GAG moiety. Hence, varying length or sulfation patterns of GAG chains may account for different biological activities of decorin. Discrepancies can also be explained as artefacts due to different purification protocols, such as when denaturing conditions are used to extract decorin from tissue. Taken together however, these observations suggest that decorin is neither a pro- nor an anti-angiogenic factor per se, but rather a regulator of angiogenesis, dependent on local cues for different activities. Further, that decorin is capable of both enhancing and inhibiting tubulogenesis may suggest a role in balancing vessel regression versus persistence. Immature vessels have a period of plasticity prior to maturation, during which they can be remodelled, and either regress, or given the appropriate signals, proceed to maturity.Citation11 As a modulator of tube formation, it is tempting to speculate that decorin could influence the switch from immature to mature vessels, favouring one or the other in conjunction with signals from the local environment.
What are the molecular mechanisms by which decorin influences tubulogenesis? Since endothelial cell-matrix interactions control all aspects of angiogenesis, from motility, sprouting and lumen formation, to survival and proliferation, the role of decorin should be considered in this regard. Indirectly, decorin could quite feasibly modulate cell-matrix interactions through regulation of matrix structure and organisationCitation2,Citation12 and growth factor activity.Citation13 However in vitro studies have begun to unravel rather more direct mechanisms. Studies on fibroblasts indicate that decorin can inhibit cell-matrix interactions by binding to and masking integrin attachment sites in matrix substrates. For instance, decorin inhibits fibroblast adhesion by competing with cell-surface GAG-containing CD44 for GAG binding sites on collagen XIV;Citation14 similarly, decorin inhibits fibroblast adhesion to thrombospondin by interacting with the cell-binding domain of this substrateCitation15 and may compete with fibroblast cell-surface heparin sulphate proteoglycans for binding to fibronectin.Citation16 While such studies are rather lacking in endothelial cell systems, any one of these interactions could be relevant to endothelial cells. However, that decorin slightly enhanced endothelial cell attachment to fibronectin and collagen I in our system points to the existence of alternative mechanisms.Citation17
Indeed, a recent study demonstrated that decorin is an important signalling molecule in endothelial cells, where it both signals through the insulin-like growth factor I receptor (IGF-IR) and competes with the natural ligand for interaction.Citation18 Further, decorin appears to be biologically available and relevant for interaction with this receptor in vivo. Increased receptor expression was observed in both native and neo-vessels in decorin knockout mouse cornea in conjunction with reduced neoangiogenesis. In accordance with this, decorin downregulates the IGF-IR in vitro,Citation18 indicating that signalling through, and control of IGF-IR levels by decorin could be an important factor in regulating angiogenesis. Additionally, immobilised decorin supports platelet adhesion through interactions with the collagen I-binding integrin, α2β1.Citation19 We have shown that decorin—α2β1 integrin interaction may play a part in modulating endothelial cell—collagen I interactions, and further, have demonstrated that decorin promotes motility in this context through activation of IGF-IR and the small Rho GTPase, Rac.Citation17 Similarly, decorin stimulates fibroblast motility through activation of small Rho GTPases,Citation20 supporting a direct mechanism by which decorin influences cell-matrix interactions and motility, via activation of key regulators of cytoskeleton and focal adhesion dynamics. It should also be noted that signalling by decorin directly through ErbB receptors has also been extensively demonstrated in cancer cell systems where these receptors are frequently overexpressed.Citation21 This interaction was not relevant to human umbilical vein endothelial cellsCitation18 although a recent study found that decorin activated the epidermal growth factor receptor in mouse cerebral endothelial cells.Citation8 These differences presumably depend on cell-specific factors such as receptor availability as well as relative receptor affinities. In a complex system such as angiogenesis, multiple mechanisms doubtlessly are involved. However, it is clear that modulation of cell-matrix interactions by decorin could certainly be expected to play a key role in contributing to regulation of postnatal angiogenesis.
Signals from the extracellular matrix via integrins and from growth factors to their receptors are co-ordinately integrated into the complex angiogenic cascade. Evidence exists to suggest that decorin could regulate cell-matrix interactions during early tube formation, i.e., endothelial cell sprouting and cell alignment, through both influencing integrin activity and signalling through IGF-IR.Citation17 Later stages of angiogenesis, such as lumen formation and maturation are also potentially regulated by decorin through activation of Rac and α2β1 integrin,Citation17 since activity of both these molecules is integral to this phase of angiogenesis.Citation22 Additionally, Rac activity is implicated in regulating endothelium permeability and integrity,Citation23 providing further possibilities in control of endothelium function by decorin. Further investigations would be required however, to establish whether decorin exerts its effects on tubulogenesis through these molecular mechanisms.
Of relevance to α2β1 integrin-dependent endothelial cell interaction with collagen I, sprouting endothelial cells would encounter interstitial ECM, of which collagen I is a major component. Further, a ‘provisional’ matrix containing collagen I is secreted by sprouting endothelial cells and may be required for motility,Citation24 and tube formation.Citation25 Theoretically, various interactions could exist between decorin, collagen type I and α2β1 integrin in this context, which may be differentially supported through various stages of angiogenesis. Up to eleven interaction sites of α2β1 integrin have been postulated to exist within collagen I, albeit with different affinities towards this receptor. Some of these binding sites may only be recognized by the integrin in its highly active conformation.Citation26 By influencing the collagen I binding activity of α2β1Citation17 decorin could thus alter the number of endothelial cell—collagen I contacts, thereby modulating adhesion and motility. Additionally, some decorin and α2β1 integrin binding sites may overlap, or are in close proximity.Citation27 By virtue of this location, decorin would be ideally placed to locally modulate collagen I—binding activity of the integrin. Interestingly, modulation of activity of both α2β1 integrin and the small Rho GTPase Rac by decorin also could have implications for collagen I fibrillogenesis, which in turn, would indirectly influence cell-matrix interactions. Both the related Rho GTPase RhoA, and α2β1 integrin are involved in cellular control of pericellular collagen I fibrillogenesis.Citation28 Thus in addition to regulating cell independent fibrillogenesisCitation1 decorin could potentially influence cell-mediated aspects of this process. Pertinent questions remain therefore, as to under which biological situations is the interaction between α2β1 integrin and decorin relevant, and does decorin influence α2β1 integrin activity on the cell-surface through direct interactions, and/or by inside-out signalling through the IGF-I receptor (or alternative receptors)? Further, how do differential decorin/α2β1 integrin/collagen I interactions mediate fibrillogenesis and cell-matrix interactions?
Interaction of decorin with multiple binding partners makes it challenging to fully understand the role of decorin in angiogenesis (). A consideration of the relative accessibility and affinity of binding sites on both decorin and its' binding partners would facilitate further understanding. It is still an open question whether collagen I—bound decorin can simultaneously interact with other ligands. In the case of the IGF-IR, the binding site on the concave surface of decorin overlaps with that of collagen I, thus mutually exclusive interactions seem more likely. That decorin clearly influences both collagen I matrix integrity and IGF-IR activity in vivo, would suggest that decorin is not exclusively associated with collagen I. Perhaps decorin occurs in a more ‘soluble’ form when locally secreted by endothelial cells undergoing angiogenic morphogenesis. Does collagen-bound decorin interact simultaneously with α2β1 integrin? This could be a possibility, since decorin core protein interacts with collagen I, allowing the possibility of GAG—integrin interaction. In this scenario however, interaction of α2β1 integrin with the GAG moiety of decorin in preference to collagen I might sound improbable. Nevertheless, during remodelling, interactions such as these could occur in a transient manner, and be crucial in controlling cell-matrix interactions in a rapidly changing environment. Interestingly, decorin interacts with IGF-IR via the core protein,Citation18 and with α2β1 integrin via the GAG moietyCitation17 raising yet another possibility of simultaneous decorin interaction with multiple binding partners. Additionally, while it is a matter of some debate whether decorin exists predominantly as a monomer or as a dimer in a physiologically relevant environment, it has been proposed that collagen-bound decorin could support simultaneous interactions of decorin with additional binding partners, and that dimer-monomer transitions also could facilitate differential interactions.Citation29 Perhaps supporting multiple simultaneous interactions of decorin, the phenotype of patients with a progeroid variant of Ehlers-Danlos Syndrome indicates an essential role for properly glycosylated decorin (and the related SLRP biglycan). These patients exhibit skeletal and craniofacial abnormalities, loose skin and deficiencies in wound healing as a direct result of abnormal decorin and biglycan glycosylation, such that approximately half the population of decorin is secreted as the core protein only.Citation30 Notably, the defect in loose skin and in wound healing is similar to the phenotype of the decorin knockout mouse.Citation2,Citation4 Evidently, the core protein alone cannot maintain normal function in vivo, despite being responsible for several important interactions of decorin, in particular, binding to collagen I and the IGF-IR. These studies may therefore support a requirement for simultaneous interactions of the core protein and GAG moieties for proper function of decorin.
Modulation of cell-matrix interactions by decorin plays a key role in modulating endothelial cell motility and angiogenesis in vivo, and some of the mechanisms responsible have been elucidated in conjunction with in vitro studies. The large number of potential interactions of decorin with multiple matrix components and cell-surface receptors makes a clear understanding difficult. However, direct activation of signalling pathways by decorin has been highlighted recently as likely to play an important role. In conclusion, a better understanding of the mechanisms by which decorin regulates vessel formation and persistence would contribute to understanding how angiogenesis is dysregulated in a clinical setting, and how rational therapeutic strategies can be developed to restore tissue function and homeostasis.
Abbreviations
| IGF-IR | = | insulin-like growth factor I receptor |
| ECM | = | extracellular matrix |
| GAG | = | glycosaminoglycan |
| SLRP | = | small leucine-rich repeat proteoglycan |
| HUVEC | = | human umbilical vein endothelial cells |
| HDMEC | = | human dermal microvascular endothelial cells |
| EGFR | = | epidermal growth factor receptor |
| BAE | = | bovine aortic endothelial cells |
Figures and Tables
Figure 1 Decorin influences cell-matrix interactions through multiple mechanisms. Decorin signals through the IGF-IR via the core protein moiety (grey diamond), and may simultaneously interact with the α2 subunit (cross-hatched subunit) of α2β1 integrin via the GAG moiety (wavy black line) (A). Activation of Rac through IGF-IR enhances motility by modulating cytoskeleton dynamics and may influence α2β1 integrin activity for collagen I through inside-out signalling (B). Decorin induces large, peripheral vinculin (grey oval)-positive focal adhesions by signalling through IGF-IR and/or α2β1 integrin (C and D). Decorin could also directly influence α2β1 integrin activity through binding to the α2 subunit and/or simultaneous interactions with collagen I (thick wavy black line) through the core protein. Collagen I interacts with the A-domain (white circle) of the α2 subunit at a site distinct to that of decorin (D). In summary, activation of IGF-IR, Rac and modulation of α2β1 integrin affinity for collagen I by decorin modulates cell-matrix interactions and contributes to enhanced motility and tubulogenesis in a collagen I environment.
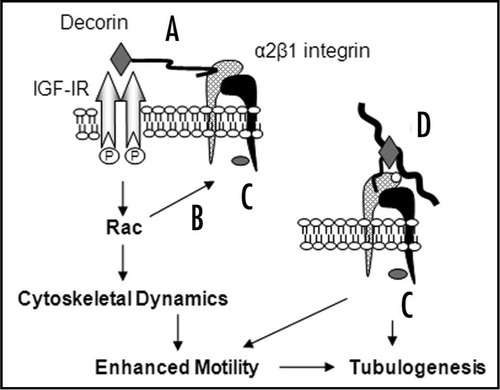
Table 1 Summary of the key functions of decorin in controlling cell behaviour
Acknowledgements
This manuscript is dedicated to the memory of Dr. Elke Schönherr, without whom this work would not have been possible. This work was supported by a studentship to L.F. from Cardiff University, Deutsche Forschungsgemeinschaft Grants SFB 293 and 492, SPP1086 and Eb177/5-1.
References
- Vogel KG, Paulsson M, Heinegard D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J 1984; 223:587 - 597
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol 1997; 136:729 - 743
- Schönherr E, Sunderkotter C, Schaefer L, Thanos S, Grassel S, Oldberg A, et al. Decorin deficiency leads to impaired angiogenesis in injured mouse cornea. J Vasc Res 2004; 41:499 - 508
- Jarvelainen H, Puolakkainen P, Pakkanen S, Brown EL, Hook M, Iozzo RV, et al. A role for decorin in cutaneous wound healing and angiogenesis. Wound Rep Regen 2006; 14:443 - 452
- Nelimarkka L, Salminen H, Kuopio T, Nikkari S, Ekfors T, Laine J, et al. Decorin is produced by capillary endothelial cells in inflammation-associated angiogenesis. Am J Pathol 2001; 158:345 - 353
- Schönherr E, O'Connell BC, Schittny J, Robenek H, Fastermann D, Fisher LW, et al. Paracrine or virus-mediated induction of decorin expression by endothelial cells contributes to tube formation and prevention of apoptosis in collagen lattices. Eur J Cell Biol 1999; 78:44 - 55
- Davies Cde L, Melder RJ, Munn LL, Mouta-Carreira C, Jain RK, Boucher Y. Decorin inhibits endothelial migration and tube-like structure formation: role of thrombospondin-1. Microvasc Res 2001; 62:26 - 42
- Santra M, Santra S, Zhang J, Chopp M. Ectopic decorin expression upregulates VEGF expression in mouse cerebral endothelial cells via activation of the transcription factors Sp1, HIF1alpha and Stat3. J Neurochem 2008; 105:324 - 337
- Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, Iozzo RV. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene 2002; 21:4765 - 4777
- Sulochana KN, Fan H, Jois S, Subramanian V, Sun F, Kini RM, et al. Peptides derived from human decorin leucine-rich repeat 5 inhibit angiogenesis. J Biol Chem 2005; 280:27935 - 27948
- Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1995; 1:1024 - 1028
- Kinsella MG, Fischer JW, Mason DP, Wight TN. Retrovirally mediated expression of decorin by macrovascular endothelial cells; effects on cellular migration and fibronectin fibrillogenesis in vitro. J Biol Chem 2000; 275:13924 - 13932
- Schönherr E, Hausser HJ. Extracellular matrix and cytokines: a functional unit. Dev Immunol 2000; 7:89 - 101
- Ehnis T, Dieterich W, Bauer M, Schuppan D. Localization of a Cell Adhesion Site on Collagen XIV (Undulin). Exp Cell Res 1998; 239:477 - 480
- Merle B, Malaval L, Lawler J, Delmas P, Clezardin P. Decorin inhibits cell attachment to thrombospondin-1 by binding to a KKTR-dependent cell adhesive site present within the N-terminal domain of thrombospondin-1. J Cell Biochem 1997; 67:75 - 83
- Schmidt G, Robenek H, Harrach B, Glossl J, Nolte V, Hormann H, et al. Interaction of small dermatan sulfate proteoglycan from fibroblasts with fibronectin. J Cell Biol 1987; 194:1683 - 1691
- Fiedler LR, Schönherr E, Waddington R, Niland S, Seidler DG, Aeschlimann D, et al. Decorin regulates endothelial cell motility on collagen I through activation of insulin-like growth factor I receptor and modulation of alphα2beta1 integrin activity. J Biol Chem 2008; 283:17406 - 17415
- Schönherr E, Sunderkötter C, Iozzo RV, Schaefer L. Decorin, a novel player in the insulin-like growth factor system. J Biol Chem 2005; 280:15767 - 15772
- Guidetti G, Bertoni A, Viola M, Tira E, Balduini C, Torti M. The small proteoglycan decorin supports adhesion and activation of human platelets. Blood 2002; 100:1707 - 1714
- Tufvesson E, Westergren-Thorsson G. Biglycan and decorin induce morphological and cytoskeletal changes involving signalling by the small GTPases RhoA and Rac1 resulting in lung fibroblast migration. J Cell Sci 2003; 116:4857 - 4864
- Reed CC, Waterhouse A, Kirby S, Kay P, Owens RT, McQuillan DJ, et al. Decorin prevents metastatic spreading of breast cancer. Oncogene 2005; 24:1104 - 1110
- Davis GE, Koh W, Stratman AN. Mechanisms controlling human endothelial lumen formation and tube assembly in three-dimensional extracellular matrices. Birth Defects Res C Embryo Today 2007; 81:270 - 285
- Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol 2002; 39:187 - 199
- Madri JA, Stenn KS. Aortic endothelial cell migration I. Matrix requirements and composition. Am J Pathol 1982; 106:180 - 186
- Fouser L, Iruela-Arispe L, Bornstein P, Sage EH. Transcriptional activity of the alpha 1(I)-collagen promoter is correlated with the formation of capillary-like structures by endothelial cells in vitro. J Biol Chem 1991; 266:18345 - 18351
- Siljander PRM, Hamaia S, Peachey AR, Slatter DA, Smethurst PA, Ouwehand WH, et al. Integrin Activation State Determines Selectivity for Novel Recognition Sites in Fibrillar Collagens. J Biol Chem 2004; 279:47763 - 47772
- Sweeney SM, Orgel JP, Fertala A, McAuliffe JD, Turner KR, Di Lullo GA, et al. Candidate cell and matrix interaction domains on the collagen fibril, the predominant protein of vertebrates. J Biol Chem 2008; 283:21187 - 21197
- Li S, Van Den Diepstraten C, D'Souza SJ, Chan BM, Pickering JG. Vascular smooth muscle cells orchestrate the assembly of type I collagen via alpha2beta1 integrin, RhoA and fibronectin polymerization. Am J Pathol 2003; 163:1045 - 1056
- McEwan PA, Scott PG, Bishop PN, Bella J. Structural correlations in the family of small leucine-rich repeat proteins and proteoglycans. J Struct Biol 2006; 155:294 - 305
- Seidler D, Faiyaz-Ul-Haque M, Hansen U, Yip G, Zaidi S, Teebi A, et al. Defective glycosylation of decorin and biglycan, altered collagen structure, and abnormal phenotype of the skin fibroblasts of an Ehlers Danlos syndrome patient carrying the novel Arg270Cys substitution in galactosyltransferase I (beta4GalT-7). J Mol Med 2006; 84:583 - 594