Abstract
Investigating the ability of films of pristine (purified, without any functionalization) multiwalled carbon nanotubes (MWCNTs) to influence human bone marrow mesenchymal stem cells’ (hBMSCs) proliferation, morphology, and differentiation into osteoblasts, we concluded to the following: A. MWCNTs delay the proliferation of hBMSCs but increase their differentiation. The enhancement of the differentiation markers could be a result of decreased proliferation and maturation of the extracellular matrix B. Cell spread on MWCNTs toward a polygonal shape with many thin filopodia to attach to the surfaces. Spreading may be critical in supporting osteogenic differentiation in pre-osteoblastic progenitors, being related with cytoskeletal tension. C. hBMSCs prefer MWCNTs than tissue plastic to attach and grow, being non-toxic to these cells. MWCNTs can be regarded as osteoinductive biomaterial topographies for bone regenerative engineering.
Cellular interaction with substrate and neighboring cells plays a critical role in osteoblast survival, proliferation, differentiation as well as bone remodeling. Regulated biophysical cues, such as nanotopography, have been shown to be integral for tissue regeneration in the stem cell niche. Multiwalled carbon nanotubes (MWCNTs) represent a nanomaterial that has won enormous popularity in nanotechnology, exhibiting extraordinary physicochemical properties and supporting the growth of different kinds of cells.Citation1-3
Simultaneous enhancement of osteoblast cells’ proliferation and differentiation,Citation4,5 decrease of proliferation rates along with decreased differentiationCitation6 or increased differentiation accompanied with decreased proliferationCitation7 have been reported. Contradictory results concerning osteoblast cell adhesion, and morphology have also been reported. Osteoblast cell lines on CNTs have been found to elongate but not widen or displayed a spindle-shaped morphology.Citation8,9 Spreading and surface area covered were reduced.Citation8-10 On the contrary, Tutak et al.Citation7 reported robust spreading on medium roughness CNTs networks.
This variable behavior on CNTs is probably due to the various cell types used in these works. It is reported that primary human marrow stromal cells and cell lines use substantially different mechanisms to regulate adhesion and spreading on the substrate.Citation11
In a recent work of ours, published in Annals of Biomedical Engineering,Citation12 it was found that MWCNTs can create an osteogenic environment for human bone marrow mesenchymal stem cells (hBMSCs), even without addition of exogenous factors, representing a suitable reinforcement for bone tissue engineering scaffolds.
In the following, we will highlight and discuss some aspects of this work's results, in the context of literature findings, and provide additional material in order to elucidate issues on the influence of MWCNTs on hBMSCs’ proliferation, morphology, and differentiation into osteoblasts.
MWCNTs Delay the Proliferation of hBMSC Cells but Increase their Differentiation
Previous studies have shown that nano- or micro-rough Ti surfaces reduce osteoblast cells proliferation but enhance differentiation and local factor production, supporting a mature secretory osteoblast-like phenotype. On tissue culture plastic (TCP) and smooth Ti surfaces cells preserved a rather immature, dividing osteogenic phenotype (high proliferation rates, low integrin levels, and low specific osteogenic cell differentiation).Citation13-14 This enhancement is additive, if not synergistic, with the introduction of surface nanoscale structures (PLLA nanofiber scaffolds, electrospun poly(ϵ-caprolactone scaffolds).Citation15-16 Similar behavior of human mesenchymal stem cells has been observed on PLLA nanofiber scaffoldsCitation17 or on electrospun poly(ϵ-caprolactone) scaffolds.Citation18
Cells, not only respond to the presence of topographical features, but also to the dimensions of these features. A number of studies try to answer the question what is the optimum micro-nanodimension for enhanced response. Oh et al.Citation19 reported that small (30 nm diameter) nanotubes promoted hBMSC adhesion without noticeable differentiation, whereas larger (70 to 100 nm diameter) nanotubes elicited a selective differentiation into osteoblast-like cells.
In all above works, various types of osteoblast cells, stages of osteoblast maturation and chemistries have been used and the comparison was in respect to different substrates, making direct comparisons difficult. The general conclusion from all these results is that cell differentiation on micro- or nanostructured surfaces takes place at the expense of proliferation.
Few contrary results have been reported. Knabe et al.,Citation20 comparing the effect of various bioactive glass ceramics on the expression of bone-related genes and proteins found that all novel glass ceramics supported cellular proliferation together with expression of bone-related genes. However, these results did not show consistent tendency of lower cell numbers along with expression of the osteoblastic phenotype to a higher degree.
Our resultsCitation12 showed that MWCNTs substrates decreased cell numbers but displayed an accelerated progression of osteoblast phenotype development, indicated by early and enhanced expression of alkaline phosphatase activity (ALP) and osteocalcin (OC) and osteopontin (OP) levels. In the absence of additional biochemical inducing agents, ALP on MWCNTs increased about fold4- as that on the control.
The above results, taken together, show that the ordered expression of genes during development of the osteoblast phenotype can be altered (osteoblastic maturation prematurely upregulated) because of micro-nanotopography. Nanotopography is probably the additional cellular signaling necessary for developmental expression of genes to pass the restriction points during osteoblast differentiation.Citation21 Differentiation markers could increase as a result of decreased proliferation and maturation of the extracellular matrix. Boyan et al.Citation14 concluded that microrough Ti surfaces can alter the maturation state of the cell, creating a microenvironment conducive to new bone formation on. This mechanism can possibly be extended to nano-topographies on different chemistries. To understand the relationship between the nano-dimensional cues and hMSC cell response, further research is required with excessive care not to misinterpret the value of various surface features to cell response in vitro and in vivo if one examines only cell attachment and proliferation without considering the ability of those cells to differentiate into competent osteoblasts in a timely manner.
Cell Spread on MWCNTs Toward a Polygonal Shape with Many Thin Filopodia to Attach to the Surfaces
Cell shape is suggested to be a key regulator of MSC commitment.Citation22 The cell morphology correlates with the physiological behavior of the cells. It is admitted that cell growth better occurs when cell adhesion is decreased. On mirror-polished samples, the lower frequency of adhering pseudopodia and focal adhesions was correlated to an increase in cell proliferation.Citation23 On microrough surfaces, the cell bodies become more cuboidal and anchor themselves to the surface through long dendritic filopodia.Citation24 In contrast, on smoother surfaces, the cells flatten and spread, resulting in a fibroblastic appearance. Zhao et al.Citation25 reported that on smooth and low energy surfaces, the cells were elongated and formed spindle like shape; on rough and high energy surfaces, the cells were polygonal in shape with many thin filopodia to attach to the surfaces. This morphology was accompanied by lower cell numbers.
Additionally, cells grown in expansion media appeared spindle-shaped whereas cells cultured under osteogenic conditions showed a more flattened and polygonal morphology. Distinct changes found in cell architecture upon osteogenic differentiation, obtained by transfection of HBCs with an OC promoter gene, provided evidence for the connection between cell shape and functional state. The fibroblast-like phenotype of pre-osteoblasts changed to the flattened and polygonal shape of differentiated osteoblasts.Citation26
The clear correlation between cell shape and differentiation leads to the assumption that changes in the assembly and disassembly of the actin cytoskeleton may be critical in supporting osteogenic differentiation.Citation27 It seems that cell spreading increases osteoblast differentiation in pre-osteoblastic progenitors. It is not yet clear if the change in morphology precedes the expression of a more mature physiology or if the differentiation of the cell is activated by another yet unidentified factor and the cell now responds differently to the surface microarchitecture.
Few contradictory results, showing that an increase in the hMSCs cellular elongation induced cells’ differentiation into osteoblast-like cells, have been reported.Citation19
shows cell spreading on MWCNTs and TCP by fluorescent staining of the cytoskeleton f-actin (green) and DNA (blue). From data presented in Kroustalli et al.,Citation12 areas of lower cell density were selected to facilitate observation of individual cell shapes. The images of the cells shown in the selected micrographs are typical of cells throughout the culture. The confocal images of hMSCs on carbon nanotubes showed the formation of more filopodia, lamellipodia, and cellular extensions compared with those on flat TCP. They also show that, on TCP, cells tend to have a more elongated shape and long, thin actin stress fibers are running in parallel to the longitudinal cell axis. Cytoplasmic processes and filopodia are slightly concentrated at the narrow cell endings. On the contrary, cells cultured on MWCNTs spread to a larger area and displayed a more flattened and polygonal morphology. They also show marked interactions through extending cytoplasmic processes and filopodia, which enabled the anchorage of the cells (). The above findings on cell morphology conform with the results on proliferation delay accompanied by differentiation acceleration.
Figure 1. Fluorescent staining of the f-actin cytoskeleton (green) and DNA (blue), showing cell spreading. (A) 6 h on MWCNTs. (B) 3rd day on MWCNTs. (C) 6 h on TCP. (B) 3rd day on TCP.
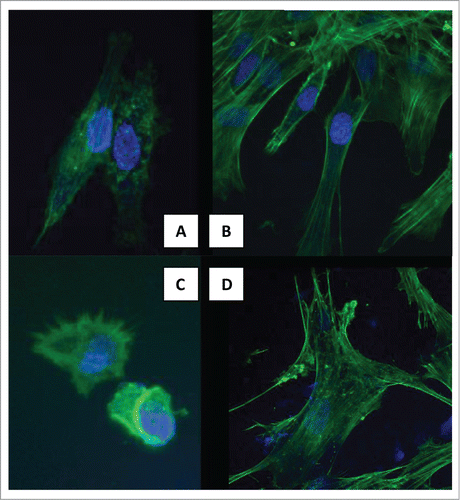
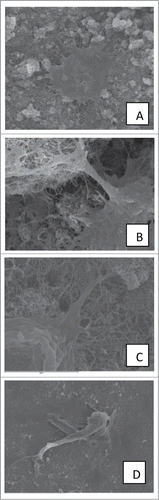
Figure 2. SEM images showing cytoplasmic processes and filopodia. (A–C) 3rd day on MWCNTs. (D) 3rd day on TCP.
Adhesion blocking assays have demonstrated that integrins are mainly involved in osteoblast adhesion to MWCNTs. One of the hypotheses that BoettingerCitation28 makes for integrin-mediated signaling on how the signals get across the plasma membrane is that integrin mediated attachment to a solid surface allows cytoskeletal tensioning. Cell is tensioned using the integrin ‘anchor’ to pull against is required, along with specific linkage to the surface and integrin clustering. As a mechanism for the enhanced osteogenic differentiation connected with cell spreading, we can propose that when the stem cells are stressed, they tend to differentiate into a specific lineage to accommodate the stress. This hypothesis has been presented in the literature. It has been suggested that hMSC sense and transduce nanotopographical signals through focal adhesions and actomyosin cytoskeleton contractility to induce differential gene expression.Citation29
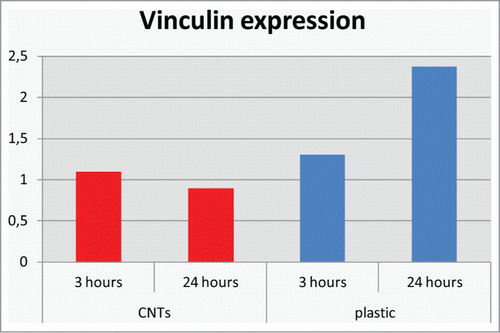
Our results on vinculin gene expression on MWCNTs -TCP contribute to this direction. FAK and vinculin are major players in the focal adhesion processes activated by integrin-fibronectin interactions. In particular, vinculin transduces integrin-mediated intracellular signaling molecules that promote cell migration.Citation30
We found that the vinculin gene expression for cells grown on MWCNTs was lower when compared with those attached on the flat TCP plate and it decreased after 24 hours (). It has been reported that decreases in the levels of vinculin benefited cell migration by increasing the cell mobility.Citation31 In contrast, decreased migration is seen in cells overexpressing a-actinin and vinculin. Cells attached on MWCNTs reorganized to spread and create long extended filopodia and this rearrangement might result in lower vinculin expression.
The differentiation ability of MSCs could be influenced by cytoskeletal rearrangement.Citation32 Live cell analysis of human bone marrow mesenchymal stem cells on transparent titanium demonstrated rapid cytoskeletal re-organization on the nanoscale surface features, which ultimately induced higher expression of osteoblast phenotype genes.Citation15 Born et al.Citation26 found that during osteogenic differentiation the actin cytoskeleton was reorganized, resulting in thick non-aligned actin stress fibers. It is likely that the reorganization of the intracellular link is responsible for the transformation of the mechanical force into a biochemical signal, which in turn triggers cytoskeleton assembly.
Cells Prefer MWCNTs Than TCP to Attach and Grow
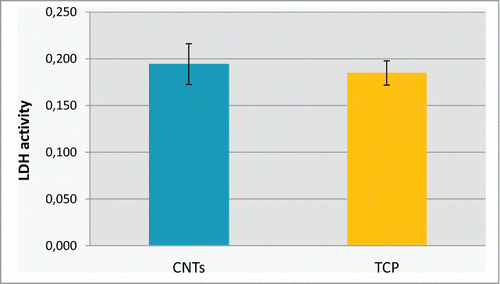
Several studies have described contrasting results regarding cytotoxicity of CNTs.Citation33-35 Such different results are probably caused by variations both in the specific characteristics of the CNTs tested (single versus multi; length and diameter; concentration; and impurities) and the type of cells used. Regarding our substrate, after very careful washing, the lactate dehydrogenase (LDH) activity was measured in cell supernatant after 24 h of culture, which was not significantly different from a positive control TCP ().
Figure 4. Lactate dehydrogenase (LDH) activity measured in cell supernatant after 24 h of culture on MWCTs and on TCP.
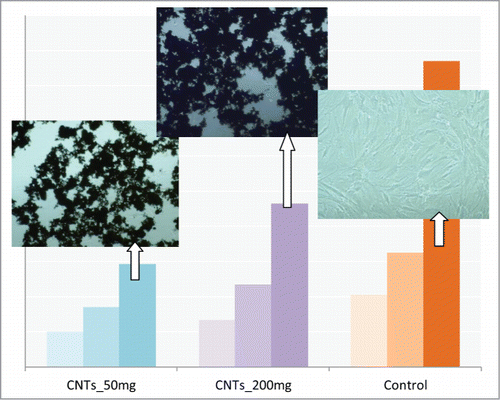
Moreover, total protein synthesis experiments with various concentrations of MWCNTs (80, 160 and 320 μg/mL) for 1, 3 and 7 d at cell seeding densities of 3000 cells/cm2 have showed that the total protein increased with concentration at each culture time point. Images of cultures displayed a very low presence of cells on TCP substrate among CNTs (). These results lead to the conclusion that cells prefer CNTs than TCP to attach. The BMSCs, even at very low seeding densities, grow and tend to seek CNTs in order to adhere and spread, and not TCP. Thus, increasing concentration of CNTs resulted in increased adhered cells and, consequently, more total protein.
Figure 5. Total-protein of the hMSCs cells after 1, 3 and 7 d of culture on 2 different concentrations of MWCNTs. CNTs_50: 50 μg/mL, CNTs_200: 200 μg/mL and on TCP. Images of cultured cells on the substrates are also shown.
Perspectives
MWCNTs represent a structure that provides the sustained effects on the organization of the extracellular matrix to modify the progression of differentiation of proliferating cells of the osteoblast lineage.
Despite literature evidence supporting the nanostructures’ ability to be both osteoconductive and osteoinductive, there is still disparity regarding how nanostructures regulate the progression toward an osteoblastic phenotype. It is necessary to explore unique micro- and nano-architectures, to understand how they initiate osteoinductive signals through pathways similar to BMPs, and how these unique geometries can be translated to the clinic. More fundamental questions are related to defining the specific mechanisms operative in proliferating cells that allow for increased phenotypic alterations and the signals that promote progressive differentiation of the hBMSCs.
References
- Khang D, Park GE, Webster TJ. Enhanced chondrocyte densities on carbon nanotube composites: the combined role of nanosurface roughness and electrical stimulation. J Biomed Mater Res A 2008; 86(1):253-60; PMID:18186050; http://dx.doi.org/10.1002/jbm.a.31803
- Mattson MP, Haddon RC, Rao AM. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J Mol Neurosci 2000; 14(3):175-82; PMID:10984193; http://dx.doi.org/10.1385/JMN:14:3:175
- Zanello LP, Zhao Bin, Hu Hui, and Haddon Robert C. Bone Cell Proliferation on Carbon Nanotubes. NanoLetters 2006; 6:562-7; PMID:16522063; http://dx.doi.org/10.1021/nl051861e
- Li X, Liu H, Niu X, Yu B, Fan Y, Feng Q, Cui FZ, Watari F. The use of carbon nanotubes to induce osteogenic differentiation of human adipose-derived MSCs in vitro and ectopic bone formation in vivo. Biomaterials 2012; 33:4818-27; PMID:22483242; http://dx.doi.org/10.1016/j.biomaterials.2012.03.045
- Aoki N, Akasaka T, Watari F, and Yokoyama A. Carbon nanotubes as scaffolds for cell culture and effect on cellular function. Dent Mater J 2007; 26(2):178-85; PMID:17621932; http://dx.doi.org/10.4012/dmj.26.178
- Zhang D, Yi C, Zhang J, Chen Y, Yao X, and Yang M. Effects of carbon nanotubes on the proliferation and differentiation of primary osteoblasts. Nanotechnology 2007; 18:475102-10; http://dx.doi.org/10.1088/0957-4484/18/47/475102
- Tutak W, Park KH, Vasilov A, Starovoytov V, Fanchini G, Cai SQ, Partridge NC, Sesti F, Chhowalla M. Toxicity induced enhanced extracellular matrix production in osteoblastic cells cultured on single-walled carbon nanotube networks. Nanotechnology 2009; 20:255101; PMID:19487801; http://dx.doi.org/10.1088/0957-4484/20/25/255101
- George JH, Shaffer MS, and Stevens MM. Investigating the cellular response to nanofibrous materials by use of a multi-walled carbon nanotube model. J Exp Nanosci 2006; 1(1):1-12; http://dx.doi.org/10.1080/17458080500463149
- Ishikawa K, Akasaka T, Yawaka Y, Watari F. High functional expression of osteoblasts on imogolite, aluminosilicate nanotubes. J Biomed Nanotechnology 2010; 6:59-65; PMID: 20499833; http://dx.doi.org/10.1166/jbn.2010.1092
- 10. Akasaka T, Yokoyama A, Matsuoka M, et al. Adhesion of human osteoblast-like cells (Saos-2) to carbon nanotube sheets. Biomed Mater Eng 2009; 19:147-53; PMID:19581708; http://dx.doi.org/10.3233/BME-2009-0574
- Kilpadi KL, Sawyer AA, Prince CW, Chang PL, and Bellis SL. Primary human marrow stromal cells and SaOs-2 osteosarcoma cells use different mechanisms to adhere to hydroxylapatite. J Biomed Mater Res A 2004; 68A:273-85 PMID:14704969
- Kroustalli AA, Kourkouli SN, Deligianni DD. Cellular function and adhesion mechanisms of human bone marrow mesenchymal stem cells on multi-walled carbon nanotubes. Ann Biomed Eng 2013; 41(12):2655-65; PMID:23820769; http://dx.doi.org/10.1007/s10439-013-0860-0
- Klein MO, Bijelic A, Ziebart T, Koch F, Kämmerer PW, Wieland M, Konerding MA, Al-Nawas B. Scale-Structured Hydrophilic Titanium Surfaces Promote Early Osteogenic Gene Response for Cell Adhesion and Cell Differentiation. Clin Implant Dent Rel Res 2011; 15(2):166-75; PMID: 21682843; http://dx.doi.org/10.1111/j.1708-8208.2011.00339.x
- Boyan BD, Lossdörfer S, Wang L, Zhao G, Lohmann CH, Cochran DL and Schwartz Z. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. European cells and Materials 2003; 6:22-7; PMID:14577052
- Khang D, Choi J, Im Y-M, Kim Y-J, Jang J-H, Kang S S, Nam T-H, Song J and Park J-W. Role of subnano-, nano- and submicron-surface features on osteoblast differentiation of bone marrow mesenchymal stem cells. Biomaterials 2012; 33:5997-6007; PMID:22632766; http://dx.doi.org/10.1016/j.biomaterials.2012.05.005
- Gittens RA, Olivares-Navarrete R, McLachlan T, Cai Y, Hyzy SL, Schneider JM, Schwartz Z, Sandhage KH, Boyan BD. Differential responses of osteoblast lineage cells to nanotopographically-modified, microroughened titanium-aluminum-vanadium alloy surfaces. Biomaterials 2012; 33:8986-94; PMID:22989383; http://dx.doi.org/10.1016/j.biomaterials.2012.08.059
- Schofer MD, Veltum A, Theisen C, Chen F, Agarwal S, Fuchs-Winkelmann S, Paletta JRJ. Functionalisation of PLLA nanofiber scaffolds using a possible cooperative effect between collagen type I and BMP-2: impact on growth and osteogenic differentiation of human mesenchymal stem cells. J Mater Sci Mater Med 2011; 22:1753-62; PMID:21604139; http://dx.doi.org/10.1007/s10856-011-4341-4
- Chang J-C, Fujita S, Tonami H, Kato K, Iwata H and Hsu S. Cell orientation and regulation of cell–cell communication in human mesenchymal stem cells on different patterns of electrospun fibers. Biomed Mater 2013; 8:055002; PMID:24002690; http://dx.doi.org/10.1088/1748-6041/8/5/055002
- Oh S, Brammer KS, Julie Li YS, Teng D, Engler AJ, Chien S, and Jin S. Stem cell fate dictated solely by altered nanotube dimension. PNAS 2009; 106: 2130-35; PMID:19179282; http://dx.doi.org/10.1073/pnas.0813200106
- Knabe C, Stiller M, Berger G, Reif D, Gildenhaar R, Howlett CR, Zreiqat H. The effect of bioactive glass ceramics on the expression of bone-related genes and proteins in vitro. Clin Oral Impl Res 2005; 16:119-27; PMID:15642039; http://dx.doi.org/10.1111/j.1600-0501.2004.01066.x
- Lian JB and Stein GS. Development of the osteoblast phenotype: molecular mechanisms mediating osteoblast growth and differentiation. Iowa Orthop J 1995; 15:118-40; PMID:7634023
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and rhoA regulate stem cell lineage commitment. Dev Cell 2004; 6:483-95; PMID:15068789; http://dx.doi.org/10.1016/S1534-5807(04)00075-9
- Linez-Bataillon P, Monchau F, Bigerelle M, Hildebrand HF. In vitro MC3T3 osteoblast adhesion with respect to surface roughness of Ti6Al4V substrates. Biomol Eng 2002; 19:133-41; PMID:12202174; http://dx.doi.org/10.1016/S1389-0344(02)00024-2
- Brunette DM. The effects of implant surface topography on the behavior of cells. Int J Oral Maxillofac Implants 1988; 3:231-46; PMID:3075965
- Zhao G, Raines AL, Wieland M, Schwartz Z, and Boyan BD. Requirement for Both Micron and Submicron Scale Structure for Synergistic Responses of Osteoblasts to Substrate Surface Energy and Topography. Biomaterials 2007; 28: 2821-29; PMID:17368532; http://dx.doi.org/10.1016/j.biomaterials.2007.02.024
- Born AK, Rottmar M, Lischer S, Pleskova M, Bruinink A, and Maniura-Weber K. Correlating cell architecture with osteogenesis: first steps towards live single cell monitoring. European Cells and Materials 2009; 18:49-62; PMID:19856264
- Yourek G, Hussain MA, Mao JJ. Cytoskeletal changes of mesenchymal stem cells during differentiation. ASAIO J 2007; 53:219-28; PMID:17413564; http://dx.doi.org/10.1097/MAT.0b013e31802deb2d
- Boettiger D. Mechanical control of integrin-mediated adhesion and signaling. Current Opinion in Cell Biology 2012; 24:592-599; PMID:22857903; http://dx.doi.org/10.1016/j.ceb.2012.07.002
- Teo BK, Wong ST, Lim CK, Kung TY, Yap CH, Ramagopal Y, Romer LH, Yim EK. Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS Nano 2013; 7:4785-98; PMID:23672596; http://dx.doi.org/10.1021/nn304966z
- Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol 2007; 213:565-73; PMID:17680633; http://dx.doi.org/10.1002/jcp.21237
- Huttenlocher A, Sandborg RR and Horwitz AF. Adhesion in cell migration, Current Opinion in Cell Biology 1995; 7:697-706; PMID:8573345; http://dx.doi.org/10.1016/0955-0674(95)80112-X
- Hwang NS, Varghese S and Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev 2008; 60:199-214; PMID:18006108; http://dx.doi.org/10.1016/j.addr.2007.08.036
- Reddy AR, Reddy YN, Krishna DR, Himabindu V. Multi wall carbon nanotubes induce oxidative stress and cytotoxicity in human embryonic kidney (HEK293) cells. Toxicology 2010; 272:11-6; PMID:20371264; http://dx.doi.org/10.1016/j.tox.2010.03.017
- Firme CP 3rd, Bandaru PR. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomedicine 2010; 6:245-256; PMID:19699321; http://dx.doi.org/10.1016/j.nano.2009.07.003
- Tsukahara T, Haniu H. Cellular cytotoxic response induced by highly purified multi-wall carbon nanotube in human lung cells. Mol Cell Biochem 2011; 352:57-63; PMID:21298324; http://dx.doi.org/10.1007/s11010-011-0739-z