Abstract
The dioxin receptor (AhR) is possibly the best characterized xenobiotic receptor because of its essential role in mediating the harmful effects of highly toxic environmental pollutants. Despite the fact that AhR-dependent toxicity is a major environmental concern, compelling evidence is been lately produced unveiling novel and remarkable endogenous functions of AhR in cell physiology and tissue homeostasis. Adding to its role in cell proliferation and differentiation, AhR is also involved in the control of cell adhesion and migration, both highly relevant tasks in development and in disease states such as cancer. Interestingly, the effect of AhR on cell migration is cell-type specific because it can sustain or slow down cell motility. Here, I will comment on our recent report showing that AhR is a positive regulator of fibroblast cells migration. Besides characterizing the phenotype of such mesenchymal cells, the most important single finding of our study is that AhR uses the cytoskeleton regulator and oncogen Vav3 to signal through small Rho GTPases, ultimately leading to the physiological control of cell adhesion and migration. These data reveal that AhR activity is required to maintain signaling pathways governing normal cell function and open the question of whether AhR plays a role in cell migration during development and in pathological conditions such as tumor metastasis.
For several decades, the aryl hydrocarbon (dioxin) receptor (AhR) has been studied as a paradigm of xenobiotic-activated intracellular receptor that mediates many of the harmful effects of environmental toxins and carcinogens. These studies attract a great deal of interest because many xenobiotics represent a serious concern to the Biosphere, in general, and to human health, in particular. One of the most important discoveries resulting from all this work is that AhR is a transcription factor that regulates the expression of xenobiotic metabolizing enzymes and, in fact, a quite accurate signaling pathway has been proposed that explains how AhR could mediate toxic responses.Citation1–Citation3 Yet, several important observations led to the hypothesis that AhR is not sorely intended for xenobiotic metabolism. Its high degree of conservation during evolution and its constitutive expression,Citation4 its early appearance in metazoans and the physiological roles of closely related proteins are clues about the implication of AhR in physiology. Nevertheless, it has been the production of genetically modified miceCitation5–Citation7 and the study of invertebrate modelsCitation1 that has provided strong evidence for the involvement of AhR in tissue and organ homeostasis and established the bases to characterize its cellular functions. It should be noted, however, that both activities of AhR (e.g., xenobiotic-related vs. xenobiotic-unrelated) could follow similar molecular pathways since xenobiotics seem to act by exacerbating/deregulating endogenous cellular processes.Citation1,Citation8,Citation9
AhR shares important functional motifs with proteins that regulate myogenesis, circadian rhythms, organ development and neurophysiology; all members of the basic-helix-loop-helix (bHLH) family of transcriptional regulators.Citation10 Specifically, AhR belongs to the Class VII of bHLH proteins, which have the unique property of containing a PAS (PER-ARNT-SIM) domain for ligand binding. To be functional, AhR needs to dimerize with the Aryl Hydrocarbon Receptor Nuclear Translocator (ARNT), a molecular interaction that allows the heterodimer to recognize consensus regulatory binding elements (xenobiotic responsive element, XRE) located in the promoter of target genes; classically cytochromes P450 and phase II enzymes.Citation11 The interest to decipher the endogenous activity of AhR has encouraged the identification of novel AhR target genes through either microarray-based studiesCitation12 or by functional analyses of individual candidates (reviewed in refs. Citation1 and Citation13). Such ability to regulate the expression of xenobiotic-unrelated genes gives additional support for the role of AhR in normal cell function.
During the last few years, our laboratory has been interested in analyzing the endogenous roles of AhR in vitro and in vivo and, in particular, we have focused on its role in cell proliferation and tumor development. A question that is considered of paramount importance in cancer is how the stroma interacts with tumors and by doing so how it modulates their growth and dissemination. In early studies,Citation14 we produced and characterized immortalized fibroblast cell lines from mammary gland stroma of AhR-expressing (AhR+/+) and AhR-null (AhR−/−) mice. Subcutaneous xenografts of these cell lines in immunocompromised mice revealed that AhR−/− fibroblasts had a very limited ability to induce tumors in vivo and, importantly, that they had decreased migration efficiency in a collagen I matrix.Citation14 The fact that this same migratory phenotype was observed in primary fibroblasts isolated from AhR−/− embryos,Citation13 and since mutation of the Caenorhabditis elegans heterolog AHR-1 also impairs cell and axon migration,Citation15 we decided to further analyze the role of AhR in cell adhesion and migration. A first clue about the potential mechanisms involved come from the differences in cell area and polarization observed between both cell lines. AhR−/− fibroblasts had larger cell area and were less polarized than AhR+/+ fibroblasts. Considering that cell morphology is closely related to F-actin and cytoskeleton dynamics and to the recycling of cell-cell and cell-substratum interactions,Citation16 these results prompted us to investigate actin stress fibers and focal adhesions (FAs) in cells of both genotypes. In agreement, AhR-null cells had a major increase in actin stress fibers and FAs, suggesting the existence of a mechanism connecting AhR to the cytoskeleton.Citation17 Moreover, when we tested the functional significance of these alterations, we found that AhR−/− fibroblasts had a marked increase in cell adhesion and spreading associated to their impaired migration. A potentially relevant candidate to help explain such effects is the RhoA/Rac1 small GTPase family of proteins. Rho/Rac GTPases are essential regulators of cell adhesion, spreading and migration.Citation18,Citation19 Accordingly, RhoA regulates the formation of F-actin stress fibers and focal adhesionsCitation20,Citation21 while Rac1 promotes the formation of lamellipodia and membrane ruffles.Citation19,Citation22 Interestingly enough, in certain cell types, high levels of Rac1 activity are able to inhibit RhoA and to decrease their actin stress fibers content and number of focal adhesions.Citation23,Citation24 In agreement to these observations, highly adherent and less motile AhR−/− fibroblasts had decreased Rac1 and increased RhoA activities that were relevant for the AhR-null morphology as determined by the use of specific pharmacological inhibitors.
Biochemically, Rho/Rac proteins cycle between inactive (GDP-bound) and active (GTP-bound) states. The GDP to GTP transition is controlled by GDP/GTP exchange factors (GEFs) that favor the rapid transition to the activated state during cell signaling. Remarkably, the proto-oncogen Vav3, a GEF for Rho GTPases with a relevant role in cytoskeleton dynamics, was severely downregulated at the mRNA and protein levels in AhR−/− fibroblasts. It is particularly relevant that the constitutive expression of Vav3 significantly contributes to the reorganization of the cytoskeleton and to the formation of lamellipodia and membrane ruffles.Citation25,Citation26 Considering these data altogether, we hypothesized that decreased Vav3 expression in AhR-null fibroblasts could alter Rac1 and RhoA activities, cytoskeleton stability and the morphology of the cell, ultimately leading to increased adhesion and reduced VIEW migration. In addition to the interest of these observations, these results also identified Vav3 as a novel AhR target gene that expands the number of cellular functions requiring AhR activity. Promoter analyses, chromatin immunoprecipitation (ChIP) assays and site directed mutagenesis led to the observation that Vav3 expression is not inducible by exogenous AhR ligands in neither immortalized nor embryonic fibroblasts. This means that AhR is devoted to the regulation of the constitutive transcription of Vav3. These results add even further strength to our hypothesis stating that AhR signals in a major pathway that governs cell morphology, adhesion and migration. The fact that AhR, a ligand-activated transcription factor, regulates the constitutive expression of Vav3 could appear intriguing. However, Vav3 is not the only gene to be constitutively regulated by AhR, albeit only few have been characterized to date. Former studies on AhR−/− mouse liver revealed that lack of receptor produced a large reduction in constitutive cytochrome P450 1A2 (CYP1A2)5 mRNA while AhR represses the constitutive expression of the c-Myc proto-oncogeneCitation27 and the latent TGFβ binding protein LTBP1.Citation28,Citation29 Taken together, these data support the attractive possibility that AhR regulates a battery of genes controlling cellular homeostasis.
The functional relevance of Vav3/Rac1/RhoA signaling in mediating the AhR-dependent fibroblastic phenotype was demonstrated by down-modulating Vav3 expression through RNA interference (RNAi) in AhR+/+ fibroblasts. Specific Vav3 small interfering RNAs (siRNA) switched the patterns of actin stress fibers and FAs, as well as the attachment and adhesion efficiencies of AhR+/+ cells to values typical of AhR−/− fibroblasts. Furthermore, the analysis of embryonic fibroblasts from Vav3 knock-out miceCitation30 showed increased cell area and higher actin stress fibers content, a phenotype that resembles that of AhR−/− fibroblasts. Although the AhR-Vav3 pathway appears functionally relevant for the control of cell adhesion and migration in mesenchymal fibroblast cells, other interesting mechanisms could be also involved. For instance, since AhR has cullin 4B ubiquitin ligase activity,Citation31 the ligand-bound receptor could use the proteasome to adjust the levels of migration-associated proteins by a mechanism similar to that previously reported for steroid receptors.Citation31 On the other hand, additional signaling pathways could collaborate and/or converge with Vav3 to finally determine the AhR-dependent cell phenotype. One interesting possibility relies on the dynamics of the cell-substratum interactions because suspension of adherent primary mouse keratinocytesCitation32 and mouse hepatoma Hepa-1c1 cellsCitation33 induced the nuclear translocation and transcriptional activation of AhR, thus supporting AhR as an intermediate molecule in cell adhesion. Current studies are underway to address those questions and to more precisely define, within the context of AhR expression, the importance of Rac1/RhoA activities in pathologies concerning an altered control of cell migration, as for instance in tumor metastasis.
As discussed above, the physiological activities of AhR could be closer to its xenobiotic-related functions than previously thought. Based on this assumption, it is worthy to consider that AhR could also mediate the effects of toxic xenobiotics on cell adhesion and migration. Interestingly, an initial study revealed that suspension of C3H10T1/2 fibroblasts increased the transcriptional activity of AhR to levels similar to those obtained after treatment with dioxin,Citation34 suggesting the existence of common mechanisms of regulation. More recently, several relevant studies have defined additional signaling pathways that could mediate the effects of xenobiotics on cell adhesion and migration through AhR. Using human breast cancer MCF-7 cells, Diry and collaborators first reported that dioxin induced a reorganization of the cytoskeleton and an increase in the number of lamellipodia that were associated to higher migration rates of these cells. Mechanistically, such effects on morphology and migration involved reduced expression of the cell-cell interaction protein E-Cadherin and activation of the c-Jun N-terminal kinase (JNK) pathway.Citation35 In following studies, the same laboratory has further defined the signaling involved in the modulation of adhesion and migration by dioxin. They found that dioxin induces the expression of a member of the Cas family of proteins, Nedd9/Hef1/CasL,Citation36 which has been postulated as a pro-metastatic gene in the dissemination of several cancers including melanomaCitation37 and glioblastoma.Citation38 Moreover, Nedd9/Hef1/CasL appears to have a relevant role in the mechanisms controlling epithelial cell adhesion and migration because it is required for dioxin to modulate E-Cadherin expression and JNK activation through AhR. Thus, altogether, these studies provide strong experimental evidence for a role of AhR in cell adhesion and migration and further support the hypothesis that dioxin acts by mimicking/exacerbating physiological signaling pathways requiring endogenous AhR activity.
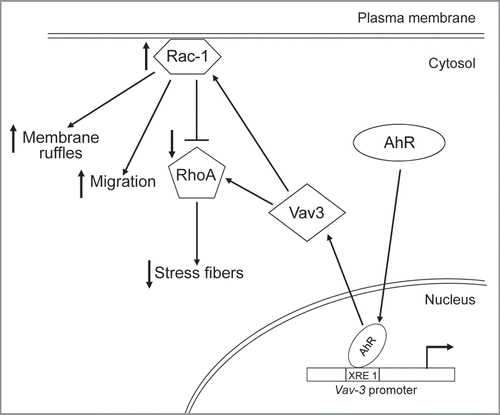
The proposed mechanism that could integrate AhR in the Vav3-dependent signaling pathway that controls fibroblast cell adhesion and migration is depicted in . We suggest that under physio-logical cell conditions, endogenous AhR activity maintains constitutive Vav3 expression. A cell status favoring migration will increase Vav3-dependent Rac1 activity which, in turn, will promote the formation of membrane ruffles and stimulate cell migration. In addition, the net balance between the positive modulation through Vav3 and the inhibitory effect exerted by Rac1 will determine RhoA activity and, as a result, the amount of actin stress fibers observed in AhR+/+ fibroblasts.
In a broad perspective, the involvement of AhR in cell migration adds an important function to its well known implication in the control of cell proliferation, differentiation and apoptosis. Future work will surely provide novel and exciting proofs for the importance of this receptor in developmental processes and in disease states. Particularly relevant are the epithelial-to-mesenchymal transition (EMT) and its reversal, the mesenchymal-to-epithelial transition (MET) since they have major roles in both normal development and in the acquisition of a metastatic phenotype by many tumor cells. The characterization of these new activities will luckily fulfill the mechanistic and functional requirements needed to propose AhR as a marker/target molecule susceptible to be modulated in such health threatening ilness as cancer.
Figures and Tables
Figure 1 Proposed mechanism of AhR-dependent control of cell migration through interaction with Vav3 signaling. Under physiological cell conditions, AhR binds to the Vav3 promoter and regulates its constitute expression. Vav3 protein then serves as GDP/GTP exchange factor (GEF) for the small GTPases Rac1 and RhoA. Once in their GTP-bound form, Rac1 and RhoA modulate cell migration, plasma membrane protrusions and cytoskeleton reorganization. The inhibitory effect of high levels of Rac1 activity on RhoA-dependent control of stress fibers is considered (blunt arrow).
Acknowledgements
Research at P.M.F.-S. laboratory is supported by grants from the Spanish Ministry of Education and Sciences (SAF2008-0462), the Junta de Extremadura (2PR04A060) and the Red Temática de Investigación Cooperativa en Cáncer (RTICC) (RD06/0020/1016, Fondo de Investigaciones Sanitarias (FIS), Carlos III Institute, Spanish Ministry of Health). All Spanish funding is co-sponsored by the European Union FEDER program. I am grateful to Dr. Jaime M. Merino for critical review of this manuscript.
References
- Barouki R, Coumoul X, Fernandez-Salguero PM. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett 2007; 581:3608 - 3615
- Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer 2006; 6:947 - 960
- Whitlock JP Jr. Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol 1999; 39:103 - 125
- Hahn ME. Aryl hydrocarbon receptors: diversity and evolution. Chem Biol Interact 2002; 141:131 - 160
- Fernandez-Salguero PM, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 1995; 268:722 - 726
- Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K, et al. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells 1997; 2:645 - 654
- Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: Involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci USA 1996; 93:6731 - 6736
- Bock KW, Kohle C. Ah receptor: dioxin-mediated toxic responses as hints to deregulated physiologic functions. Biochem Pharmacol 2006; 72:393 - 404
- Furness SG, Lees MJ, Whitelaw ML. The dioxin (aryl hydrocarbon) receptor as a model for adaptive responses of bHLH/PAS transcription factors. FEBS Lett 2007; 581:3616 - 3625
- Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol 2000; 20:429 - 440
- Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control and apoptosis. Biochem Pharmacol 2000; 59:65 - 85
- Tijet N, Boutros PC, Moffat ID, Okey AB, Tuomisto J, Pohjanvirta R. Aryl hydrocarbon receptor regulates distinct dioxin-dependent and dioxin-independent gene batteries. Mol Pharmacol 2006; 69:140 - 153
- Gomez-Duran A, Carvajal-Gonzalez JM, Mulero-Navarro S, Santiago-Josefat B, Puga A, Fernandez-Salguero PM. Fitting a xenobiotic receptor into cell homeostasis: How the dioxin receptor interacts with TGFbeta signaling. Biochem Pharmacol 2009; 77:700 - 712
- Mulero-Navarro S, Pozo-Guisado E, Perez-Mancera PA, Alvarez-Barrientos A, Catalina-Fernandez I, Hernandez-Nieto E, et al. Immortalized mouse mammary fibroblasts lacking dioxin receptor have impaired tumorigenicity in a subcutaneous mouse xenograft model. J Biol Chem 2005; 280:28731 - 28741
- Qin H, Powell-Coffman JA. The Caenorhabditis elegans aryl hydrocarbon receptor, AHR-1, regulates neuronal development. Dev Biol 2004; 270:64 - 75
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 2005; 6:56 - 68
- Carvajal-Gonzalez JM, Mulero-Navarro S, Roman AC, Sauzeau V, Merino JM, Bustelo XR, et al. The dioxin receptor regulates the constitutive expression of the vav3 proto-oncogene and modulates cell shape and adhesion. Mol Biol Cell 2009; 20:1715 - 1727
- Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol 1998; 142:573 - 586
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion and adhesion during cell movement. J Cell Biol 1999; 144:1235 - 1244
- Chen YH, Tukey RH. Protein Kinase C Modulates Regulation of the CYP1A1 Gene by the Aryl Hydrocarbon Receptor. J Biol Chem 1996; 271:26261 - 26266
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992; 70:389 - 399
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 1992; 70:401 - 410
- Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol 1999; 147:1009 - 1022
- Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol 1999; 9:640 - 648
- Movilla N, Bustelo XR. Biological and regulatory properties of Vav-3, a new member of the Vav family of oncoproteins. Mol Cell Biol 1999; 19:7870 - 7885
- Bustelo XR. Regulatory and signaling properties of the Vav family. Mol Cell Biol 2000; 20:1461 - 1477
- Yang X, Liu D, Murray TJ, Mitchell GC, Hesterman EV, Karchner SI, et al. The aryl hydrocarbon receptor constitutively represses c-myc transcription in human mammary tumor cells. Oncogene 2005; 24:7869 - 7881
- Gomez-Duran A, Ballestar E, Carvajal-Gonzalez JM, Marlowe JL, Puga A, Esteller M, et al. Recruitment of CREB1 and histone deacetylase 2 (HDAC2) to the mouse Ltbp-1 promoter regulates its constitutive expression in a dioxin receptor-dependent manner. J Mol Biol 2008; 380:1 - 16
- Santiago-Josefat B, Mulero-Navarro S, Dallas SL, Fernandez-Salguero PM. Overexpression of latent transforming growth factor-{beta} binding protein 1 (LTBP-1) in dioxin receptor-null mouse embryo fibroblasts. J Cell Sci 2004; 117:849 - 859
- Sauzeau V, Sevilla MA, Rivas-Elena JV, de Alava E, Montero MJ, Lopez-Novoa JM, et al. Vav3 proto-oncogene deficiency leads to sympathetic hyperactivity and cardiovascular dysfunction. Nat Med 2006; 12:841 - 845
- Ohtake F, Baba A, Takada I, Okada M, Iwasaki K, Miki H, et al. Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature 2007; 446:562 - 566
- Sadek CM, Allen-Hoffmann BL. Cytochrome P450IA1 is rapidly induced in normal human keratinocytes in the absence of xenobiotics. J Biol Chem 1994; 269:16067 - 16074
- Sadek CM, Allen-Hoffmann BL. Suspension-mediated induction of Hepa 1c1c7 Cyp1a-1 expression is dependent on the Ah receptor signal transduction pathway. J Biol Chem 1994; 269:31505 - 31509
- Cho YC, Zheng W, Jefcoate CR. Disruption of cell-cell contact maximally but transiently activates AhR-mediated transcription in 10T1/2 fibroblasts. Toxicol Appl Pharmacol 2004; 199:220 - 238
- Diry M, Tomkiewicz C, Koehle C, Coumoul X, Bock KW, Barouki R, et al. Activation of the dioxin/aryl hydrocarbon receptor (AhR) modulates cell plasticity through a JNK-dependent mechanism. Oncogene 2006; 25:5570 - 5574
- Bui LC, Tomkiewicz C, Chevallier A, Pierre S, Bats AS, Mota S, et al. Nedd9/Hef1/Cas-L mediates the effects of environmental pollutants on cell migration and plasticity. Oncogene 2009; 28:3642 - 3651
- Kim M, Gans JD, Nogueira C, Wang A, Paik JH, Feng B, et al. Comparative oncogenomics identifies NEDD9 as a melanoma metastasis gene. Cell 2006; 125:1269 - 1281
- Natarajan M, Stewart JE, Golemis EA, Pugacheva EN, Alexandropoulos K, Cox BD, et al. HEF1 is a necessary and specific downstream effector of FAK that promotes the migration of glioblastoma cells. Oncogene 2006; 25:1721 - 1732