Abstract
Integrin receptors cluster on the cell surface and bind to extra cellular matrix (ECM) proteins triggering the formation of focal contacts and the activation of various signal transduction pathways that affect the morphology, motility, gene expression and survival of adherent cells. Polyamine depletion prevents the increase in autophosphorylation of focal adhesion kinase (FAK) and Src during attachment. Rac activity also shows a steady decline, and its upstream guanine nucleotide exchange factor (GEF), Tiam1 also shows a reduction in total protein level when cells are depleted of polyamines. When Tiam1 and Rac1 interaction was inhibited by NSC-23766, there was not only a decrease in Rac1 activity as expected but also a decrease in FAK auto-phosphorylation. Inhibition of Src activity by PP2 also reduced FAK auto-phosphorylation, which implies that Src modulates FAK autophosphorylation. From the data obtained in this study we conclude that FAK and Src are rapidly activated upon fibronectin mediated signaling leading to Tiam1-mediated Rac1 activation and that intracellular polyamines influence the signaling strength by modulating interaction of Src with Tiam1 using focal adhesion kinase as a scaffolding site.
Introduction
The phenomenon of restitution is critical to the intestinal mucosa, which is the crucial barrier to a wide range of toxic and immunogenic substances within the lumen. Even under normal physiological conditions, proteases, resident flora, certain kinds of food additives and supplements cause damage to the intestinal epithelia. Compromised intestinal epithelial integrity is manifested in various disorders including inflammatory bowel disease, celiac disease and intestinal infections.Citation1–Citation3 Restitution of the intestinal mucosa is a highly regulated phenomenon, which follows various pathways depending on the type and extent of damage. Epithelial sheets respond to injury by reorganizing their actin cytoskeletons to migrate. Lamellae formation is a key feature of restitution observed both in vitro and in vivo. Lamellae are large flat cytoplasmic protrusions that are extended by the surrounding cells into the denuded area. The traction to pull the cell forward is provided by adhesion between the integrins and their specific extracellular matrix ligands. Eventually, the lamellae contract thus detaching the rear part of the cell and allowing it to translocate.Citation4
We have demonstrated that duodenal mucosal damage induced by stress is repaired rapidly and this process is accompanied by significant increases in ornithine decarboxylase (ODC). The hypertonic NaCl model for mucosal injury in rats showed that polyamines are essential for healing of intestinal lesions.Citation5–Citation7 In animals that were administered a-difluoromethylornithine (DFMO), mucosal repair was almost completely prevented following stress related damage. ODC activity was also reported to increase after partial resection, during lactation and after obstruction of the lumen: all instances associated with mucosal growth.Citation8–Citation10 DFMO prevented the accumulation of polyamines and growth of mucosa in each of these models. These studies established the significance of polyamines in mucosal restitution.
Intracellular polyamine levels are highly regulated and dependent on the activity of ODC, which catalyzes the first rate-limiting step in polyamine biosynthesis. In IEC-6 cells, DFMO an inhibitor of ODC, depleted putrescine within 6 h, spermidine after 24 h, and spermine (60%) after 4 days. We have extensively studied the role of polyamines in the regulation of cell proliferation, apoptosis and wound healing in IEC-6 cells. Polyamine depletion decreased the activation of integrin, FAK and Rho GTPases.Citation11–Citation13 The activation of Rac1 is sufficient to restore migration of polyamine-depleted cells indicating that polyamines might regulate a step upstream of Rac1 activation.
Studies have demonstrated that the integrin β cytoplasmic domain regulates signaling through the Src-family kinases. Src-family kinases activated downstream of integrins, directly interact with the β3 integrin cytoplasmic domain and are involved in outside-in signaling.Citation14,Citation15 Thus, it appears that polyamines modulate a macromolecular signaling complex involving extracellular matrix proteins, Src and FAK. Therefore we hypothesized that polyamines might be necessary to bring Src and FAK together to enable the formation of focal adhesions, which triggers Rac1 activation via Tiam1.
Results
Fibronectin accelerates attachment, spreading and migration.
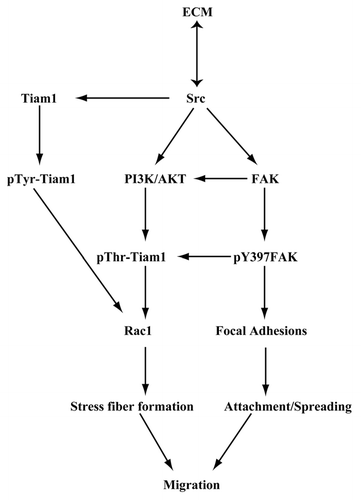
Confluent IEC-6 cells grown in control medium were trypsinized and allowed to attach on plastic and fibronectin-coated (FN) plates in serum free medium. Four hours later, the cells were observed under the microscope and photographed (, upper). A large number of cells on the plastic plate were still rounded and highly reflective, indicating that these cells though attached were not able to start the process of spreading. However, the cells on fibronectin-coated plates at this time were flattened and lamellipodial protrusions were beginning to appear. Staining for F-actin showed a well-defined labeling along the cell edges in cells seeded on FN plates. In plastic plates, the F-actin was condensed (, lower). Attached cells were trypsinized and counted at this point, and it was observed that significantly more cells attached per unit area when seeded on fibronectincoated plates (). Focal adhesions play an important role during cell attachment, spreading and migration by activating FAK (pY397). A dramatic increase in the autophosphorylation of FAK (pY397) occurred in response to FN. A concomitant increase in Src autophosphorylation was also observed. Actin served as a loading control for the western blots (). Migration of cells during wound healing was enhanced on the fibronectin-coated compared to those on plastic plates (). When the cells were plated on plastic and FN-coated plates in medium without serum, attachment and spreading were slow (). In the presence of serum, attachment and spreading were more rapid, and the difference between plastic and FN was enhanced. Total and active (GTP-Rac1) Rac1 protein significantly increased in cells plated on FN, which was reflected in GTP-Rac1 levels and accelerated wound healing (). RGDS, a peptide that blocks the interaction between integrin receptors and fibronectin, significantly inhibited the cell migration of normal and polyamine depleted cells as evident from a decrease in their ability to migrate compared to control cells 7 h after wounding. The addition of putrescine to the polyamine-depleted cells returned migration rates to normal in cells not treated with RGDS ( and B).
FAK autophosphorylation increased in cells plated on FN, which was inhibited in the presence of RGDS. FN-induced Rac1 activation was prevented by RGDS, though it did not affect the level of Rac1 protein (). RGDS also prevented FN-induced tyrosine phosphorylation of Tiam1 (). These results suggest that ECM-mediated signaling plays an important role in the migration of IEC-6 cells, and that polyamines are required for the effective signal transduction.
Polyamine-depletion delays signaling events during cell attachment, spreading and migration.
Confluent IEC-6 cells grown in control and DFMO containing media were trypsinized, plated on FN-coated plates and allowed to attach for up to 8 h. Cells were photographed at various time points. Control cells attached rapidly and began spreading within 2 h and completed spreading within 4 h. However, polyamine-depleted cells required a much longer time to attach and spread compared to control cells (). FAK and Src were not activated in nonadherent cells (0 h), but a robust increase in autophosphorylation of FAK (pY397) and Src (pY418) occurred within 2 h, when most cells were still in the process of attachment and spreading (). Although, a similar increase in Src and FAK autophosphorylation occurred in polyamine-depleted cells, the magnitude of increase was less compared to control cells ( and D). The amounts of FAK and Src were similar in cells grown under control and polyamine-depleted conditions. Tiam1 protein increased during attachment and spreading of control cells on FN, which was not evident in polyamine depleted cells ( and B). The phosphorylation of Tiam1 (pThr-Tiam1) observed in the control cells was not evident in the polyaminedepleted cells ( and D). The level of GTP-Rac1 in control cells was double that found in polyamine depleted cells at 0 time point. As time progressed Rac1 activity decreased in both groups (). Upon plating, cells attach, spread and then begin to proliferate until contact inhibited. Once the cells have covered most of the surface area (contact inhibited), lamellipodia are no longer required, and hence, there is a decrease in Rac1 activity over time. Although, polyamine-depleted cells were not contact inhibited, the decrease in Rac1 activity was similar to that in control cells indicating that lamellipodial formation was inhibited in the absence of polyamines. Although, the patterns of Rac1 activation observed in every experiment were similar, the times at which it occurred varied; hence we have shown data from a representative experiment as a bar graph.
Cells grown in control, DFMO and DFMO plus putrescine containing medium were allowed to attach on fibronectin-coated plates for 4 h and were analyzed to ascertain that the observed effects were due to polyamine depletion. Results in , show that the addition of putrescine along with DFMO prevented the decrease in FAK, Src and Tiam1 phosphorylation as compared to that shown in and . Tiam1 levels were also restored to control levels.
Effect of Rac1 inhibition during cell attachment and spreading.
NSC-23766 is a Rac1 inhibitor that fits into the surface groove of Rac1 known to be critical for GEF specification.Citation16 120 µM concentration of this molecule inhibited Rac1 activity during the lamellipodial formation phase of cell adhesion, about 4–8 h after seeding cells under our experimental conditions (). Total as well as threonine phosphorylated Tiam1 (pThr-Tiam1) also exhibited a similar profile (). As shown earlier, we observed a rapid increase in pY397FAK when control cells were plated on fibronectin. However in the presence of NSC-23766, the increased activation of FAK at 6 and 8 h was largely prevented (). Cell migration after wounding was also reduced by about 50% in the presence of NSC23766 ().
Inhibition of Src kinase attenuates cell attachment and spreading.
PP2 is a potent and selective inhibitor of the Src family tyrosine kinases. Confluent IEC-6 cells were trypsinized and plated on fibronectin-coated plates with or without PP2 (5 µM and 10 µM). It was evident from observing the cells under the microscope that Src kinase is critical for the early events that dictate cell attachment and spreading (). There was a dose dependent reduction in the number of cells that attached and spread in the presence of PP2 (). Autophosphorylation of Src and FAK were significantly inhibited by PP2 (). AKT phosphorylation was also inhibited by PP2 indicating that Src-mediated PI3kinase was effectively inhibited and could not exert its effect on downstream substrate (). Total Tiam1 and GTP-Rac1 were also decreased, as seen in the same figure. We have shown previously that integrin activation induces EGFR-mediated MAPK signaling and Src-mediated PI3K activation.Citation17 Cell migration post wounding was monitored in the presence or absence of PP2 (10 µM), and we observed that inhibition of Src kinase activity prevented cell migration by more than 50% (). Furthermore, PP2 also inhibited FN-induced tyrosine phosphorylation of paxillin, a FAK binding partner, without altering the level of vinculin, a structural component of adhesion complexes (). FAK14, a specific inhibitor of FAK autophosphorylation, completely prevented the attachment of cells to FN (). It inhibited integrin-mediated FAK, AKT and Rac1 activation without modulating Src autophosphorylation ( and C). Interestingly, FAK14 decreased the levels of phosphorylated as well as total Tiam1 protein (). These results indicate the crucial role of FAK in integration and relay of signals downstream to activate Rho-GTPases.
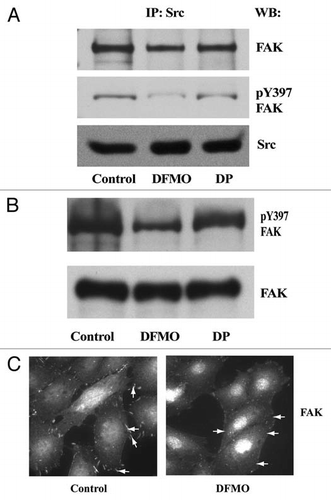
Polyamines modulate association of FAK and Src.
In order to determine whether the dependence of FAK autophosphorylation on polyamines affected its binding to Src, we immunoprecipitated Src from control, DFMO treated and DFMO plus putrescine treated cells. Polyamine depletion by DFMO prevented FAK phosphorylation and hence, its association with Src (). The addition of putrescine to the DFMO containing medium restored phosphorylation and binding to Src. Polyamine depletion decreased the phosphorylation of FAK at tyrosine residue 397 without altering the level of FAK protein (). FAK localized at distinct focal adhesion plaques in control cells, whereas in DFMO treated cells it was concentrated within the nucleus and prominent focal adhesion plaques were absent (). These results suggest that polyamines modulate the assembly of active signaling complexes leading to formation of focal adhesions, lamellipodia, and the activation of Rho-GTPases during migration.
Discussion
Polyamine metabolism is dysregulated in cancers and other hyperproliferative diseases. This along with the observation that cells synthesize more polyamines when induced to grow and that polyamines are essential for eukaryotic cell growth generated an interest in these molecules being viewed as potential candidates for the development of therapeutics.Citation18 Since ODC activity is linked to various cellular functions including cell division, transformation and cytoskeletal reorganization, inhibitors of polyamine synthesis have traditionally been developed as potential antitumor and/or antiparasitic agents.Citation19,Citation20 Recently, deHart et al. have shown that local changes in polyamine concentrations caused by association of spermidine/spermine acetyltransferase (SSAT) with α9β1 integrin increased potassium efflux and enhanced migration.Citation21 Furthermore, a report by Makitie et al. showed that transglutaminase-catalyzed polyamination of RhoA regulates its activity and that ODC influenced RhoA distribution in the cells.Citation22 These studies suggested a role for polyamines in global as well localized effects on migration. Therefore, we predicted that the polyamines might influence the membrane proximal signaling events during attachment and spreading leading to migration.
In this study, we employed fibronectin to delineate the polyamine-dependent processes in the ECM-mediated signaling leading to activation of Rho GTPases and migration. FN-induced activation increased attachment, spreading and migration with concomitant activation of FAK, Src and Rac1 (). The tetra-peptide (RGDS) prevented FN-induced autophosphorylation of FAK and Src and Rac1 activity ( and D). Furthermore, RGDS significantly inhibited the migration of control and polyamine depleted cells ( and B). Src and FAK were rapidly phosphorylated during fibronectin-induced cell adhesion indicating that these events are crucial to transmitting the signal down the pathway for other proteins involved in cell adhesion, spreading and migration. In polyamine-depleted cells plated on FN, autophosphorylation of FAK and Src was significantly lower at 2 and 4 h compared to control cells () and polyamine-depletion also decreased migration ( and B, ). The decreased tyrosine phosphorylation of FAK and Src in polyamine-depleted cells appears to be due to a decrease in the respective kinase or increase in tyrosine phosphatase activity. Oetken et al. showed that DFMO treatment decreased the tyrosine phosphorylation of various cellular proteins during proliferation of the Moloney murine leukemia virus-transformed T lymphoma cell line (LSTRA). Since addition of exogenous putrescine with DFMO restored tyrosine phosphorylation, they suggested that polyamines regulate the rate of dephosphorylation of specific target proteins.Citation23
Inhibition of Src by PP2 significantly decreased attachment, spreading and migration by preventing the phosphorylation of FAK and Rac1 activation. These results indicate that ECM-mediated Src activation is required for the assembly of focal adhesions and Rac1 activation and that polyamines are probably essential for the docking of Src and FAK at focal adhesions to further the signaling resulting in cell migration.
It is now well established that the Rho family of GTPases are crucial regulators of integrin-mediated signaling that controls the cytoskeletal structure involved in changes in cell morphology and migration.Citation24 Rac1 is critical for migration of most cell types.Citation25 When bound to GTP, the GTPases undergo a conformational change, which allows them to bind to their respective downstream effector molecules and transmit the signal.Citation26 Formation of active Rac-GTP is stimulated by GEFs, such as Tiam1, and once activated they stimulate signaling pathways that regulate actin organization, membrane ruffling and other events leading to cell migration.Citation27,Citation28 It has been shown that membrane translocation of Tiam1 is crucial for its capacity to induce Rac-mediated membrane ruffles.Citation26 Servitja et al. have shown that Vav2 and Tiam1 are phosphorylated on tyrosine residues in cells expressing active Src or oncogenic Src.Citation29 Furthermore, they showed that active Src increased Tiam1-induced Rac1 activation in NIH3T3 cells. Lysophosphatidic acid (LPA)-induced threonine phosphorylation of Tiam1 by protein kinase C increases Rac1 activity in Swiss 3T3 fibroblasts.Citation30 Thus, the activity of Rac1 specific GEFs appears to be regulated by extracellular as well as intracellular stimuli. Our results also show that Tiam1 is phosphorylated in response to FN in serum-starved cells without change in total protein (). However, Tiam1 protein level increased in control cells during attachment and spreading in the presence of dialyzed serum. This was not observed in polyamine-depleted cells (). These results suggest that polyamines might regulate the turnover of Tiam1.
Src kinase activity is also important during the initial events of cell adhesion. There was an instantaneous arrest of spreading immediately after the addition of PP2 ( and B). The inhibition of Src kinase significantly decreased Rac1 activation, and migration ( and E). When Src kinase was inhibited by PP2, PI3K-mediated AKT phosphorylation was also inhibited in a dose dependent manner. Therefore, it is possible that the inhibition of Src kinase prevented Src- or PI3K-mediated Tiam1 activation leading to decreased Rac1 activation. NSC23766, a chemical compound that specifically binds to Rac1 at the Tiam1 binding pocket prevented Rac1 activation. Decreased attachment and migration by NSC23766 correlated with FAK autophosphorylation and Rac1 activity implying that Tiam1 mediated Rac1 activation occurs via FAK during migration (). Inhibition of FAK phosphorylation at tyrosine 397 by FAK14 significantly decreased FN-induced Tiam1 levels and Rac1 activity suggesting the involvement of FAK in Tiam1-mediated Rac1 activation ().
In polyamine-depleted cells FAK predominantly localized in the nucleus, as opposed to the focal adhesions in control cells (). The association between Src and FAK has been described by other workers.Citation31,Citation32 Polyamine depletion decreased the association of Src with FAK, which was restored by putrescine when added with DFMO (). Integrin activation has been shown to increase the activity of Src and its localization to focal adhesions in fibroblasts.Citation32 The major autophosphorylation site of FAK, tyr397, serves as a binding site for the SH2 domain of Src family kinases.Citation33,Citation34 Our results clearly demonstrate that the ECM-mediated Src activation increases Tiam1 levels and activity in a FAK dependent manner, which in turn increases Rac1 activity.
From our present observations, we propose that, ECM-mediated signaling involves the autophosphorylation of FAK and Src, which are essential components of the focal adhesion complex in IEC-6 cells. The activation of these two key players modulated by polyamines leads to Rac1 activation via Tiam1. Our model () suggests that activation of integrin receptors in response to fibronectin triggers a signaling cascade, leading to activation of Src, which binds to FAK and increases its autophosphorylation at the tyrosine-397 and probably recruits Tiam1 to the focal adhesions. Src activation also modulates Tiam1 protein levels, which is upstream of Rac1. Activated FAK modulates attachment while activated Rac1 regulates lamellipodia formation and actin cytoskeletal reorganization during migration. Although, basal levels of activated Src increased in confluent polyamine depleted IEC-6 cells, pY397-FAK and migration were decreased.Citation12 This led us to propose that polyamines are required for the association of Src, FAK, Tiam1, and other adhesion molecules to form the dynamic signaling complex, which activates Tiam1 leading to Rac1 activation and enhanced attachment and migration.
Materials and Methods
Antibodies and reagents.
Polyclonal rabbit anti-Tiam1 antibody was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA), mouse monoclonal anti-Src clone GD11, mouse monoclonal anti-Rac1, rabbit polyclonal anti-FAK, rabbit polyclonal anti-phospho-Src (Tyr418), mouse monoclonal anti-phospho tyrosine and mouse monoclonal anti-actin antibodies were obtained from Millipore Corporation (Temecula, CA, USA). Affinity BioReagent's rabbit polyclonal antiphospho-FAK (Tyr397) was purchased from Thermo Fisher Scientific Inc. (Rockford, IL, USA). Rabbit polyclonal antiphospho AKT and rabbit polyclonal anti-phospho threonine antibodies were purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). Bicinchoninic acid (BCA™) and mammalian protein extraction (M-PER) reagents were purchased from Thermo Fisher Scientific Inc. (Rockford, IL, USA). Cell culture medium and fetal bovine serum (FBS) were obtained from Mediatech Inc., (Herndon, VA, USA). Dialyzed FBS and glutathione agarose beads were purchased from Sigma (St. Louis, MO, USA), and trypsin-EDTA, antibiotics and insulin from GIBCO-BRL (Grand Island, NY, USA). The enhanced chemiluminescence substrate, Western Lightning™ was purchased from Perkin Elmer Life and Analytical Sciences (Shelton, CT, USA). DFMO was a gift from ILEX Oncology, (San Antonio, TX, USA). NSC23766 and PP2 were purchased from EMD Biosciences (La Jolla, CA, USA). FAK inhibitor 14 was purchased from Tocris Biosciences, (Ellisville, MO, USA). All the other reagents used were of the highest analytical grade commercially available.
Cell culture.
IEC-6 cell line (CRL-1592) was obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in T-150 flasks in Dulbecco's Minimal Essential Medium (DMEM) supplemented with 10% (FBS), 10 µg/ml insulin and 50 µg/ml gentamycin sulfate at 37°C and 10% CO2. Stock cells were passaged once a week and medium was changed three times a week. Prior to an experiment cells were trypsinized, counted using a Beckman Coulter counter and grown for 3 days in dialyzed FBS in control, DFMO (5 mM) or DFMO plus 10 µM putrescine containing media and were serum starved for 24 h prior to an experiment. Cell culture plates pre-coated with fibronectin were obtained from BD Bioscience, (Bedford, MA, USA) were used throughout the study.
Attachment assay.
The attachment assay was performed as previously described.Citation34 Confluent serum starved cells were trypsinized, counted, resuspended and conditioned in DMEM containing dialyzed FBS for 30 min at room temperature. 3 × 106 cells were plated in a final volume of 2.0 ml on fibronectin-coated six-well plates. 30 and 60 min later, cells were washed two times with Hank's buffered salt solution (HBSS) and photographed using a Nikon Diaphot inverted microscope. The cells were trypsinized at this time, and the number of attached cells was estimated by counting using the Coulter counter.
Cell migration assay.
This assay was carried out as described previously.Citation35,Citation36 Cells grown in control, DFMO, or DFMO plus putrescine containing media were serum starved for 24 h before the experiment. Six-well plates containing a confluent monolayer of cells were marked in the center (along the diameter) with a marker. A wound was created perpendicular to the mark by scraping the monolayer with a gel loading micro-tip. Plates were washed, and the wound area was captured with a charged coupled device (CCD) camera system using NIH Image (Version1.62) at the intersection of the marked line at 0 h and at desired time point (7 h). The wound area covered during migration was measured using NIH Image J program. Cell migration was calculated as wound area or wound width covered. Each experiment was carried out in triplicate and two observations were recorded from each well (n = 6).
Preparation of cell lysate.
For western blot analyses of the various proteins, plates containing cells were placed on an ice bath and washed 2 times with cold PBS and harvested in cold cell lysis buffer (M-PER containing protease inhibitor cocktail, 150 mM NaCl and the phosphatase inhibitors sodium orthovanadate, sodium fluoride and sodium β-glycerophosphate). The cells were scraped off the plate and the lysate thus obtained was centrifuged at 10,000-xg, for 15 min in the cold. The supernatant obtained was estimated for protein by the Bicinchonic acid (BCA) method as per the manufacturer's protocol. 100 µg of protein was precipitated with 2% TCA. The precipitate obtained was dissolved in 2× SDS sample buffer, boiled and used for separation by SDS-PAGE.
Preparation of GST-PAK.
E. coli BL-21DE3 containing GST-PAK (glutathione S-transferase tagged p21 activated kinase) was grown in Luria Broth. Protein expression was induced with IPTG and the bacterial pellet was resuspended in a buffer containing 50 mM Tris pH 7.4, 1% Nonidet P-40, 100 mM NaCl, 5 mM MgCl2 and 10% glycerol supplemented with protease inhibitors. The cell suspension was further sonicated and clarified by centrifugation at 10,000 × g, for 30 min. The fusion protein was then recovered by the addition of glutathione agarose beads. The quality and quantity of the GST-PAK protein was checked by gel electrophoresis. Protein was stored in the buffer containing 50% glycerol at −20°C for pull down assays.
Rac1 activation assay.
Rac1 activity was determined by pull down assay as described previously.Citation36,Citation37 GST-PAK fusion protein bound with the glutathione agarose beads was mixed with cell lysate (200 µg). The binding was allowed to proceed for 1.5 h at 4°C, the beads were washed with lysis buffer and the amount of GTP-Rac1 bound was analyzed by SDS-PAGE and western blot using Rac1 specific antibody. 10 µg of cell lysate was loaded simultaneously to determine the level of total Rac1 protein levels and western blot for actin on the same membrane served as loading control.
Western blotting.
Protein samples (20 ug) were separated by SDS-PAGE and transferred to PVDF (polyvinylidene difluoride) membrane. The membranes were then blocked with either 5% bovine serum albumin (BSA) or blocking grade non-fat dry milk made in tris-buffered saline containing 0.1% Tween 20. Appropriate primary and secondary antibodies were used to detect the proteins of interest by enhanced chemiluminescence detection reagents.
Immunocytochemistry.
Immunostaining for localization studies of proteins was done as described previously.Citation38,Citation39 Cells were grown on poly-L-lysine-coated glass coverslips placed in 24-well plates. Cells were fixed with 3.7% para-formaldehyde for 15 minutes, washed twice with DPBS, permeabilized with 0.1% Triton X-100 for 10 min, and washed again with PBS. Blocking was carried out with 3% BSA for 20 min followed by a two hour incubation with the appropriate primary antibody. The coverslips were washed with PBS, followed by incubation with an appropriate fluorescent dye-conjugated secondary antibody. The coverslips were mounted on glass slides and photographed using a Nikon Diaphot inverted fluorescence microscope with appropriate filters.
Statistics.
All data are expressed as means ± SE. Densitometric analysis of western blots from three different experiments was performed. Analysis of variance and appropriate post-hoc testing determined the significance of the differences between means. Values of p < 0.05 were considered significant.
Figures and Tables
Figure 1 Fibronectin accelerates cell attachment and spreading. IEC-6 cells were grown to confluence in DMEM containing 5% dialyzed FBS for 3 days followed by serum starvation for 24 h. The cells were trypsinized, counted, conditioned and equal numbers were seeded on plastic- and fibronectin (FN)-coated plates in DMEM without serum. (A) Upper: phase contrast pictures 4 h post plating. Lower: Cells were fixed and stained for F-actin. (B) One set of plates was washed with HBSS, trypsinized and the attached cells were counted. Cell numbers plotted with respect to the area of the plate. Values are mean ± SEM of triplicates. *Significantly different (p < 0.05). (C) Equal amounts of protein were separated using SDS-PAGE for western blot analysis. Blots were probed with specific antibodies as shown in the figure. Actin was used as loading control. Representative blots from three observations are shown. (D) Cell migration assay was carried out as described in the methods and phase contrast pictures taken at 0 and 7 h post wounding are shown. (E) IEC-6 cells grown as described above were trypsinized, counted and equal numbers were seeded on plastic and fibronectin coated plates in DMEM in the presence of 5% dialyzed FBS. Phase contrast pictures at 4 h post plating are shown. Rac-1 activity (GTP-Rac1) was determined by GST-PAK pull down assay. Representative blots for the total- and active-Rac1 levels from three observations are shown.
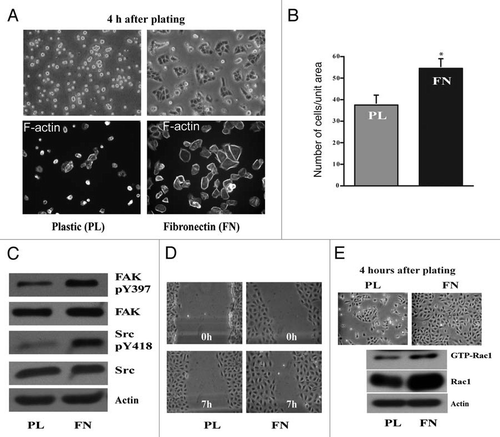
Figure 2 RGDS inhibits migration. IEC-6 cells were grown to confluence in control, DFMO and DFMO plus putrescine containing media for 3 days followed by serum starvation for 24 h. Confluent monolayers were wounded with a gel loading tip in the center of the plates, washed and left untreated or treated with 1 mM RGDS. (A) Plates were photographed immediately to record the wound width (0 h) and again at the marked wound location after 7 h. (B) Wound area covered during migration was calculated as described in the methods. Values are mean ± SEM of triplicates. *Significantly different compared to respective untreated samples. (C) IEC-6 cells were grown to confluence in control medium for 3 days and serum starved for 24 h. Cells were trypsinized and conditioned for 30 min at room temperature before plating on plastic and fibronectin-coated plates in the presence or absence of RGDS (1 mM). Cell lysates were analyzed for pY397FAK, total FAK, and active Rac1 by SDS-PAGE. Actin served as loading control. (D) IEC-6 cells were grown to confluence in control medium for 3 days and serum starved for 24 h. Cells were trypsinized and conditioned for 30 min at room temperature before plating on plastic and fibronectin-coated plates in the presence or absence of RGDS (1 µM) and allowed to attach for about 3 h in serum free medium. Equal amounts of cell lysate immunoprecipitated using Tiam1 antibody were probed with phosphotyrosine (pY99) and Tiam1 antibodies.
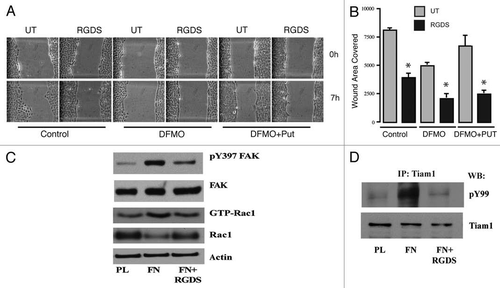
Figure 3 Polyamine depletion delays cell attachment and spreading. IEC-6 cells were grown to confluence in control and DFMO containing media for 3 days and serum starved for 24 h. Cells were trypsinized and conditioned in medium with or without DFMO for 30 min at room temperature before plating on fibronectin-coated plates. One aliquot of cells was taken at time 0. (A) Phase contrast images taken at different time points during attachment and spreading are shown (B) 20 µg protein from each sample was analyzed by western blotting to detect phosphorylated Src and FAK. Actin was used as loading control. Densitometric analysis of western blots was carried out using NIH image J software. The ratio of the phosphorylated versus total protein (specific activity) for FAK (C) and Src (D) from 3 experiments is shown. Values are mean ± SEM of triplicates. *Significantly different compared to respective control (p < 0.05).
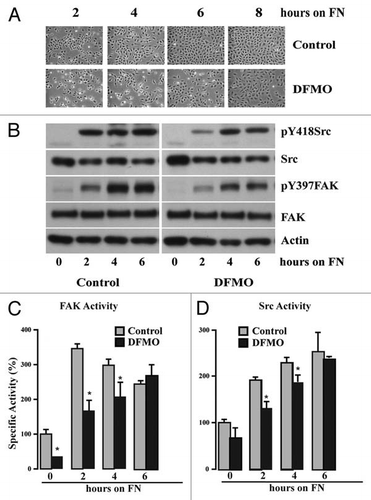
Figure 4 Polyamine depletion decreases Tiam1 protein and Rac1 activity. IEC-6 cells were grown to confluence in control and DFMO containing media for 3 days and serum starved for 24 h. Cells were trypsinized and conditioned in medium with or without DFMO for 30 minutes at room temperature before plating on fibronectin-coated plates. One aliquot of cells was taken at time 0. (A) 20 µg protein from each sample was analyzed by western blotting to detect Tiam1 and pThr-Tiam1 proteins. (B) Densitometric analysis of Tiam1 levels from 3 different experiments. Values are mean ± SEM of triplicates. *Significantly different compared to respective control (p < 0.05). (C) GTP-Rac1 separated using GST-PAK pull down as described in the methods. The western blots were scanned and the specific activity of Rac1 was calculated as a ratio of GTP-Rac1 (active) to the total Rac1. A representative experiment for the pattern of Rac1 activity is shown. (D) Cells were grown to confluence in control, DFMO and DFMO plus putrescine containing media for 3 days and serum starved for 24 h, trypsinized and plated on fibronectin-coated plates and allowed to attach for 4 h. Attached cells were lysed and 20 µg protein from each sample was analyzed by SDS-PAGE using specific antibodies. Representative blots from three observations are shown.
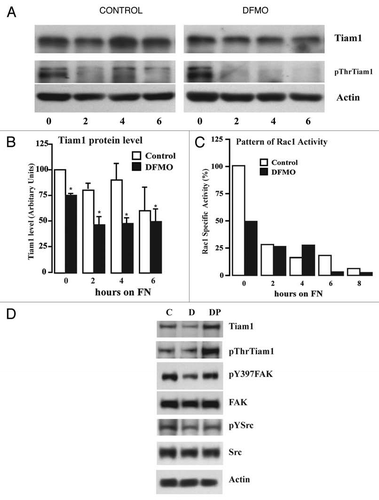
Figure 5 Inhibition of Tiam1-Rac1 binding abrogates Rac1 and FAK activity. IEC-6 cells were grown to confluence for 3 days and serum starved for 24 h. Cells were trypsinized, counted and conditioned in medium with 5% dialyzed FBS for 30 min at room temperature before plating on fibronectin-coated plates. One aliquot of cells was taken at 0 h. Cells were allowed to attach in the presence and absence of NSC23766 (120 µM) and lysed at timed intervals. (A) Western blot after GTP-Rac1 pull down (B) 20 µg protein was separated on SDS-PAGE and analyzed by western blotting using specific antibodies. (C) Migration studies were carried out as described in methods with or without NSC23766. Values are mean ± SEM of triplicates. *Significantly different compared to untreated (p < 0.05). Representative blots from three observations are shown.
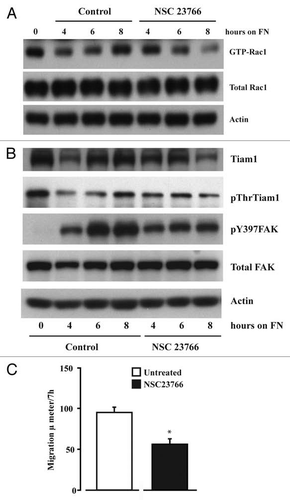
Figure 6 Src activity is required for cell adhesion and migration. IEC-6 cells were grown to confluence for 3 days and serum starved for 24 h. Cells were trypsinized, counted, and conditioned in medium with 5% dialyzed FBS for 30 min at room temperature before plating on fibronectin-coated plates. One aliquot of cells was taken at 0 h (C-0). Conditioned cells were plated in the presence of 5 or 10 µM PP 2 or DMSO (vehicle). (A) Images were captured at 0.5 and 1.0 h. (B) The cells were washed gently and attached cells were trypsinized and counted using the coulter counter. Values are mean ± SEM of triplicates. *Significantly different compared to vehicle (V) DMSO (p < 0.05). (C) Cells were lysed after 4 h and 20 µg protein was used for western blot analysis of pY418 Src, total Src, pY397FAK and total FAK. (D) Western blots for pAKT, Tiam1, GTP-Rac1, and total Rac1. Actin was used as loading control. (E) Migration studies carried out as described in methods with or without PP 2 (10 µM). Values are mean ± SEM of triplicates. *Significantly different compared to vehicle (V) DMSO (p < 0.05). (F) IEC-6 cells plated on plastic and fibronectin-coated plates with or without PP 2 (10 µM) for 4 h were lysed and 20 µg protein was analyzed by western blotting for pY397FAK, pY118Paxillin, and vinculin using specific antibodies. Representative blots from three observations are shown.
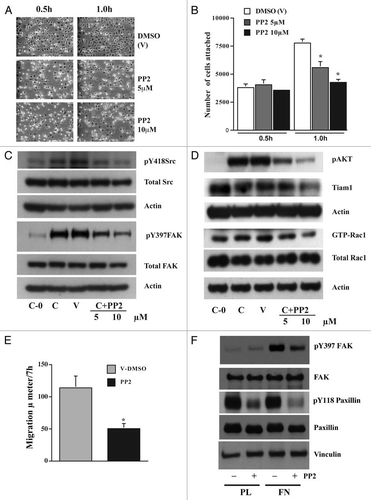
Figure 7 FAK modulates Tiam1. IEC-6 cells were grown to confluence for 3 days and serum starved for 24 h. Conditioned cells were seeded on fibronectin-coated plates with or without the focal adhesion kinase inhibitor FAK-14 (10 µM). (A) Cells were photographed after 45 min and lysed. (B and C) 20 µg protein was separated on SDS-PAGE and analyzed by western blotting using specific antibodies specific antibodies. GTP-Rac1 levels were determined by pull down assay as described in methods. Representative blots from three observations are shown.
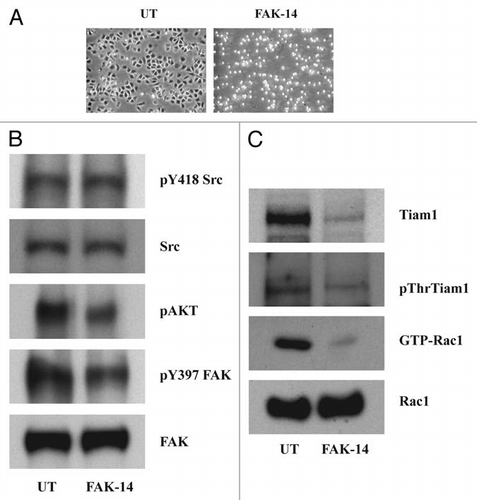
Figure 8 Src and FAK associate at the focal adhesions. IEC-6 cells grown in control, DFMO and DFMO plus putrescine containing media for 3 days, serum starved for 24 h were washed and lysed. (A) Src was immunoprecipitated from these lysates and FAK/pY397FAK bound to it was detected by immunoblotting with specific antibodies. (B) 20 µg protein from whole cell lysate separated on SDS-PAGE was subjected to western blot analysis using pY397FAK and total FAK antibodies. Representative blots from three observations are shown. (C) Cells grown in control and DFMO media were plated on glass cover slips and immuno-stained for FAK. Representative images from three experiments are shown.
Acknowledgements
This publication was made possible by Grant (DK-052784) from the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Health. We also thank Mary Jane Viar and Becky West for their technical help and advice.
References
- Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol 2003; 81:1 - 44
- Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport and inflammation. Gut 2003; 52:439 - 451
- Laukoetter MG, Bruewer M, Nusrat A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr Opin Gastroenterol 2006; 22:85 - 89
- Lotz MM, Rabinovitz I, Mercurio AM. Intestinal restitution: progression of actin cytoskeleton rearrangements and integrin function in a model of epithelial wound healing. Am J Pathol 2000; 156:985 - 996
- Wang JY, Johnson LR. Role of ornithine decarboxylase in repair of gastric mucosal stress ulcers. Am J Physiol 1990; 258:78 - 85
- Wang JY, Johnson LR. Gastric and duodenal mucosal ornithine decarboxylase and damage after corticosterone. Am J Physiol 1990; 258:942 - 950
- Wang JY, Viar MJ, Johnson LR. Transglutaminase in response to hypertonic NaCl-induced gastric mucosal injury in rats. Gasroenterol 1993; 104:65 - 74
- Luk GD, Baylin SB. Polyamines and intestinal growth-increased polyamine biosynthesis after jejunectomy. Am J Physiol 1983; 245:556 - 660
- Yang P, Baylin SB, Luk GD. Polyamines and intestinal growth :absolute requirement for ODC activity in adaptation during lactation. Am J Physiol 1984; 247:553 - 557
- Seidel ER, Haddox MR, Johnson LR. Polyamines in the response to intestinal obstruction. Am J Physiol 1984; 246:649 - 653
- Bhattacharya S, Ray RM, Johnson LR. Integrin beta3-mediated Src activation regulates apoptosis in IEC-6 cells via Akt and STAT3. Biochem J 2006; 397:437 - 447
- Ray RM, Viar MJ, McCormack SA, Johnson LR. Focal adhesion kinase signaling is decreased in polyamine-depleted IEC-6 cells. Am J Physiol Cell Physiol 2001; 281:475 - 485
- Ray RM, McCormack SA, Covington C, Viar MJ, Zheng Y, Johnson LR. The requirement for polyamines for intestinal epithelial cell migration is mediated through Rac1. J Biol Chem 2003; 278:13039 - 13046
- Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci USA 2003; 100:13298 - 13302
- Shattil SJ. Integrins and Src: dynamic duo of adhesion signaling. Trends Cell Biol 2005; 15:399 - 403
- Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA 2004; 101:7618 - 7623
- Ray RM, Bhattacharya S, Johnson LR. EGFR plays a pivotal role in the regulation of polyamine-dependent apoptosis in intestinal cells. Cell Signal 2007; 19:2519 - 2527
- Casero RA, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev 2007; 6:373 - 390
- Gerner EW, Meyskens FL. Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer 2004; 4:781 - 792
- Zou Y, Wu Z, Sirisoma N, Woster PM, Casero RA, Weiss LM, et al. Novel alkylpolyamine analogues that possess both antitrypanosomal and antimicrospoidial activity. Bioorg Med Chem Lett 2001; 11:1613 - 1617
- deHart GW, Jin T, McCloskey DE, Pegg AE, Sheppard D. The α9β1 integrin enhances cell migration by polyamine-mediated modulation of an inward-rectifier potassium channel. Proc Natl Acad Sci USA 2008; 105:7188 - 7193
- Makitie LT, Kanerva K, Andersson LC. Ornithine decarboxylase regulates the activity and localization of rhoA via polyamination. Exp Cell Res 2009; 315:1008 - 1014
- Oetken C, Pessa-Morikawa T, Autero M, Anderson LC, Mustelin T. Exp Cell Res 1992; 202:370 - 375
- Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J 1999; 18:578 - 585
- Cruz-Monserrate Z, O'Connor KL. Integrin alpha6-beta4 promotes migration, invasion through Tiam1 upregulation, and subsequent Rac activation. Neoplasia 2008; 10:408 - 417
- Mertens AE, Roovers RC, Collard JG. Regulation of Tiam1-Rac signalling. FEBS Lett 2003; 546:11 - 16
- Lambert JM, Lambert QT, Reuther GW, Malliri A, Siderovski DP, Sondek J, et al. Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat Cell Biol 2002; 4:621 - 625
- Teramoto H, Salem P, Robbins KC, Bustelo XR, Gutkind JS. Tyrosine phosphorylation of the vav protooncogene product links FcepsilonRI to the Rac1-JNK pathway. J Biol Chem 1997; 272:10751 - 10755
- Servitja JM, Marinissen MJ, Sodhi A, Bustelo XR, Gutkind JS. Rac1 function is required for Src-induced transformation. Evidence of a role for Tiam1 and Vav2 in Rac activation by Src. J Biol Chem 2003; 278:34339 - 34346
- Fleming IN, Elliott CM, Collard JG, Exton JH. Lysophosphatidic acid induces threonine phosphorylation of Tiam1 in Swiss 3T3 fibroblasts via activation of protein kinase C. J Biol Chem 1997; 272:33105 - 33110
- Cary LA, Klinghoffer RA, Sachsenmaier C, Cooper JA. SRC catalytic but not scaffolding function is needed for integrin-regulated tyrosine phosphorylation, cell migration and cell spreading. Mol Cell Biol 2002; 22:2427 - 2440
- Chaturvedi LS, Marsh HM, Shang X, Zheng Y, Basson MD. Repetitive deformation activates focal adhesion kinase and ERK mitogenic signals in human Caco-2 intestinal epithelial cells through Src and Rac1. J Biol Chem 2007; 282:14 - 28
- Schlaepfer DD, Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol Cell Biol 1996; 16:5623 - 5633
- Brunton VG, Avizienyte E, Fincham VJ, Serrels B, Metcalf CA 3rd, Sawyer TK, Frame MC. Identification of Src-specific phosphorylation site on focal adhesion kinase: dissection of the role of Src SH2 and catalytic functions and their consequences for tumor cell behavior. Cancer Res 2005; 65:1335 - 1342
- Calalb MB, Zhang X, Polte TR, Hanks SK. Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src. Biochem Biophys Res Commun 1996; 228:662 - 668
- Ray RM, Guo H, Patel M, Jin S, Bhattacharya S, Johnson LR. Role of myosin regulatory light chain and Rac1 in the migration of polyamine-depleted intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 2007; 292:983 - 995
- Ray RM, Vaidya RJ, Johnson LR. MEK/ERK regulates adherens junctions and migration through Rac1. Cell Motil Cytoskeleton 2007; 64:143 - 156
- Vaidya RJ, Ray RM, Johnson LR. Akt-mediated GSK-3beta inhibition prevents migration of polyaminedepleted intestinal epithelial cells via Rac1. Cell Mol Life Sci 2006; 63:2871 - 2879
- Jin S, Ray RM, Johnson LR. Rac1 mediates intestinal epithelial cell apoptosis via JNK. Am J Physiol Gastrointest Liver Physiol 2006; 291:1137 - 1147