Abstract
In addition to its well-defined role as an antagonist in apoptosis, we propose that BCL2 may act as an intracellular suppressor of cell motility and adhesion under certain conditions. Our evidence shows that, when over-expressed in both cancer and non-cancer cells, BCL2 can form a complex with actin and gelsolin that functions to decrease gelsolin-severing activity to increase actin polymerization, and, thus, suppress cell adhesive processes. The linkage between increased BCL2 and increased actin polymerization on the one hand, and suppression of cell adhesion, spreading, and motility on the other hand, is a novel observation that may provide a plausible explanation for why BCL2 over-expression in some tumors is correlated with improved patient survival. In addition, we have identified conditions in vitro in which F-actin polymerization can be increased while cell motility is reduced. These findings underscore the possibility that BCL2 may be involved in modulating cytoskeleton reorganization, and may provide an opportunity to explore signal transduction pathways important for cell adhesion and migration and to develop small molecule therapies for suppression of cancer metastasis.
Introduction
BCL2 (B cell lymphoma-2) is best known as an anti-apoptotic protein and this function has been the focus of most studies of this protein in cancer cells.Citation1–Citation3 However, the functional role(s) of BCL2 in tumor development and progression are quite unclear and often contradictory. For example, BCL2 has been shown not to transform cells or inhibit cell cycle progression.Citation4–Citation8 Data from previously published studies also indicate that BCL2 can inhibit cell differentiation and enhance the tumor progression in some types of cancer.Citation3,Citation9,Citation10 Also, clinical observations reporting that BCL2 expression in breast cancer can be associated with a favorable prognosis suggests a possible beneficial role for BCL2 in suppressing tumor progression and metastasis.Citation11 In our investigation, BCL2 overexpression inhibited cell adhesion, spreading and motility in NIH3T3 and MCF7 cells, which was associated with increased actin polymerization.Citation12 Taken together, these multiple and complex functions suggest that BCL2 can act as a “cell defender” to protect against signaling, which results in cell migration, division and death. Our research findings not only present a new function for BCL2, but also suggest that actin polymerization may be an important process that integrates multiple signaling pathways to govern cancer cell motility. Thus, actin polymerization represents a target for drug development for prevention of cancer metastasis.
BCL2 Inhibits Cell Adhesion, Spreading and Motility
Dynamic remodeling of the cytoskeleton is required for cell adhesion, spreading and motility. Our initial discovery came from a surprising observation that MCF7 cells engineered to overexpress BCL2 (MCF7-BCL2 cells) spread more slowly than MCF7-control cells after low density seeding. Similar results were obtained by using several different BCL2 transfected clones of MCF7 cells. These observations were confirmed in NIH3T3 cells that overexpressed BCL2 compared to vector only control NIH3T3 cells. These results suggested that overexpression of BCL2 may have affected the integrity of the cytoskeleton. To further investigate the possible effect of BCL2 on cell motility, doxycycline inducible NIH3T3 cells overexpressing mouse BCL2 and MCF7 cells overexpressing human BCL2 were established. The experiments with these cells consistently showed that BCL2 expression decreased cell motility by both in vitro “wound healing” and transwell cell migration/invasion assay using fetal bovine serum as a chemoattractant. These results raised the possibility that BCL2 may be a critical regulator of cytoskeleton reorganization.
Interestingly, we observed that Bcl2−/− mouse embryonic fibroblasts (MEFs) showed higher cell motility when compared to Bcl2+/+ MEFs and exhibited lower levels of F-actin polymerization. One earlier study showed that, compared to the wild type, there is no increase in apoptotic cells in Bcl2−/− mouse intestinal crypts (where intestinal epithelial stem cells are located), while there is an increase of apoptotic cells in the colonic crypt.Citation13 These results suggest that loss of BCL2 may not only cause apoptosis in epithelial stem cells but may also result in abnormal stem cell migration depending upon the tissue location. Another example illustrates that grey hair observed in Bcl2−/− mice may be due to the loss of melanocyte stem cells.Citation14,Citation15 The critical question remains whether the grey hair results because of a abnormal niche-to-niche migration of these cells, a niche that may not support these stem cells, thus resulting in the loss of melanocyte stem cells. These observations may provide another approach to study the role of BCL2 expression in stem cells that change their stem cell niche and exhibit reduced apoptosis potential.
BCL2 Enhances Actin Polymerization
One of the common processes involved in cell spreading, adhesion and motility is actin polymerization and depolymerization.Citation16–Citation19 We showed that F-actin is increased in cells that overexpress BCL2 compared to cells with native levels of expression of BCL2. Moreover, we found that lysates from cells that overexpress BCL2 have little effect on actin polymerization, while control lysates significantly inhibited actin polymerization. This result confirmed previous findings that BCL2 rescued latrunculin B induced MCF710A apoptosis by enhancing actin polymerization.Citation20 Moreover, lysates of MCF7 cells that overexpressed BCL2 decreased gelsolin-severing activity. Altogether, these data suggest a substantial and consistent change in actin polymerization machinery in response to increased BCL2 expression.
Both cell motility and cancer cell metastasis involve cycles of adhesion and de-adhesion that are dependent on the strength of the adhesiveness to the substrate.Citation19 BCL2 causes loss of E-cadherin and α2 integrin subunit in human mammary epithelial cells, suggesting that it may inhibit cell adhesion on certain substrates.Citation6 However, others report that BCL2 may enhance cell adhesion.Citation21 We found that BCL2 expression caused a decrease in cell-cell adhesion in a 12G10 activating anti-β1 monoclonal antibody induced cell-cell adhesion assay,Citation22 a process that requires both the α2 integrin and F-actin polymerization. BCL2 affects actin polymerization and thus, can modulate cell attachment, spreading and migration. Jasplakinolide induces actin polymerization in A2058 melanoma cells, which causes inhibition of cell migration. Furthermore, its concentration is inversely correlated with the extent of cell motility ( and B). Moreover, jasplakinolide greatly inhibits transwell motility of A2058 cells (). This result supports our previous finding that higher levels of actin polymerization result in lower cell motility.
In conclusion, we propose that the enhanced actin polymerization may have a central role in BCL2 mediated inhibition of cell adhesion, spreading and motility and the association with reduced cancer metastasis.
Proposed Mechanism
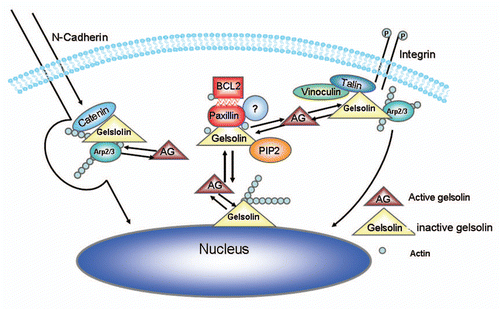
Although increased by α5β1-integrin expression in CHO cells,Citation23 BCL2 has been previously shown to reduce α2, but not β1 integrin expression.Citation6 Rac-GTP was reported to be critical in regulating F-actin turnover, nucleation, elongation, barbed end capping and decapping.Citation24,Citation25 When compared the level of Rac-GTP by control cells and cells overexpressing BCL2, we found no difference in the levels of Rac-GTP, suggesting that this pathway was not involved (). In contrast, a clear downregulation of gelsolin severing activity was detected suggesting that the activity of F-actin severing activity by gelsolin (but not the level of gelsolin expression) may be regulated by BCL2 expression.Citation12 We also found that BCL2 formed a protein complex that included gelsolin and actin. Although these findings suggest that BCL2 may affect actin polymerization by inhibiting the F-actin severing activity of gelsolin, it is uncertain whether the role of BCL2 on actin polymerization is the result of BCL2 direct binding with actin, since recombinant BCL2 appears to have little effect on increasing actin polymerization. However, we hypothesize that a protein complex is likely responsible for the BCL2 downregulation of gelsolin severing activity since gelsolin normally (in cell at rest) localized in the cytoplasm to keep its potential severing activityCitation26 (). Direct observation of enhanced actin polymerization in BCL2 expressed cells using live imaging techniques should be performed to characterize the detailed mechanism. Previously, BCL2 was reported to bind paxillin directly.Citation27 It is important to determine if paxillin is part of the BCL2-gelsolin complex. Additional questions, such as to which domains of the BCL2 protein are involved in the formation of a complex, and how this complex functions in the regulation of actin polymerization is critical to further development.
Involvement in Cancer Metastasis
Our observation that increased BCL2 expression is correlated with increased F-actin polymerization and decreased cell motility suggests that BCL2 may participate, in general, in cellular processes requiring cell adhesion and migration (such as cancer cell metastasis and stem cell homing). The correlation of cell motility with cancer metastasis may explain why BCL2 expression in cancer cells may be associated with a lower metastatic potential, a better clinical outcome and a more favorable prognosis.Citation11 Therefore, these results suggest that BCL2 expression within a tumor may function to confine the tumor cells to the tumor mass and inhibit metastasis.
BCL2 effects on actin polymerization, cell adhesion, cell motility, and thus potentially on cancer cell metastasis appears to be cell type specific. Our observation that BCL2 enhances actin polymerization is consistent with the results with MCF10A mammary epithelial cells.Citation20 Overexpression of BCL2 in estrogen receptor-positive MCF-7 mammary carcinoma cells results in decreased cell surface E-cadherin and the disruption of junction complexes between cells.Citation28 However, transformation of human mammary epithelial cells involves a high level of BCL2 expression.Citation29 BCL2 also promotes the growth of human breast epithelial cells independent of cell anchorage.Citation30 Furthermore, BCL2-mediated cell survival promotes metastasis of EpH4 βMEKDD mammary epithelial cells.Citation31 These results indicate that cancer metastasis involves multiple complex processes and that cancer cell adhesion and motility may contribute only a part. BCL2 expression enhances cell survival under unfavorable conditions encountered in the metastatic process, resulting in the enhanced metastatic potential of bladder cancer.Citation32 Loss of BCL2 was reported to inhibit migration of retinal endothelial cells, while enhancing cell adhesion and the motility of ureteric bud cells.Citation33,Citation34 The cell type and context specific basis as to why different type of cells display different effects or even paradoxical opposite effects are not clear. In addition, distinct BCL2 expression level profiles of different cell type may dampen the BCL2 effect or even reverse its functions. Different in vitro experimental conditions may also contribute the experimental differences observed and discrete conclusions reached.
Conclusion
Our recent findings not only reveal a potential new function for BCL2 on cell adhesion and cell motility, but also suggest a target for cancer therapy. Current evidence shows that BCL2 functions include the regulation of apoptosis, cell proliferation, cell differentiation and cell motility. These functions are intrinsically inter-correlated.Citation7,Citation8 Since increased actin polymerization usually correlates with decreased cell motility, we hypothesize that actin polymerization may be a critical target for development of anti-metastatic therapeutics. Current cancer drug development strategies are confronted with many challenges; e.g., drugs targeting only one molecule may not work because of the redundant functions of other similar functional molecules. The ultimate fate of a cell must be dependent on a balance between “cell defenders” and “cell destroyers.” The evolutionary significance of the BCL2 function is still largely unknown and requires further development. We hope that our research findings can be a starting point for future research.
Figures and Tables
Figure 1 Actin polymerization agent inhibits cell motility in a concentration dependent manner. (A and B) The actin stabilizing agent, jasplakinolide, inhibits A2058 human melanoma cell migration in the wound healing migration assay in a concentration-dependent manner. Cells were cultured to confluence in 35 mm dishes. The cell layers were then wounded with a tip and cultured in the presence of the indicated concentrations of jasplakinolide for 16 h, then fixed and photographed. The cell motility is expressed by the distance from the edge of wounding to the site of front moving cells in the computer image. Dotted lines indicate the original extent of the “wound.” (C) Jasplakinolide (100 nM) inhibits A2058 cell invasion assay through a Matrigel reconstituted basement membrane after 24 h. The extent of inhibition is approximately 80%.
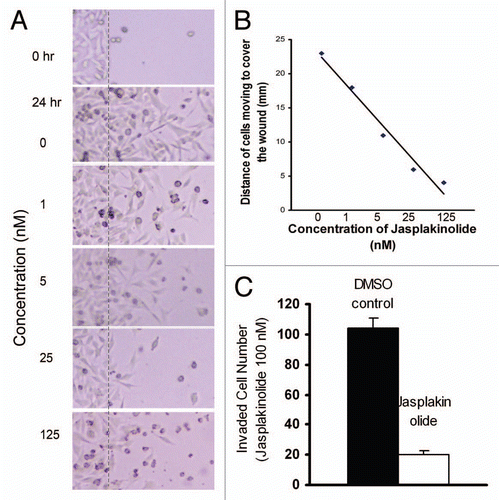
Figure 2 The level of Rac-GTP is not changed by BCL2 expression. Wild-type MCF-7 cells and NIH3T3 or cells that overexpress BCL2 were starved for 24 h before stimulation with serum containing medium. Cells were harvested 5 min after stimulation. Equal amount of cell lysate were used for Rac activation assay. Whole cell lysate was used as input to show a total Rac. The levels of PAK-1 PBD binding Rac, termed Rac-GTP, were shown in the same blot. The membrane was blotted with antibody against Rac followed with HRP-conjugated anti-mouse antibody. The results showed the same level of Rac-GTP in the vector control and the cells that overexpress BCL2 in both MCF-7 cells and NIH3T3 cells.
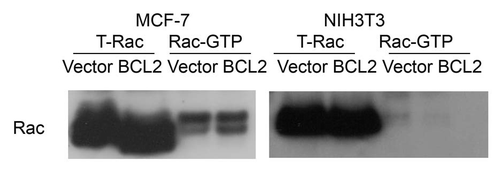
Figure 3 Model illustrating a proposed mechanism for regulation of cell adhesion and motility by BCL2. The cells normally have a balance between active (free) gelsolin and bound gelsolin within the cytoplasm. Outside-in signaling results in the recruitment of actin regulating proteins, including gelsolin, resulting in cytoskeleton reorganization. Under certain conditions, such as increased BCL2 expression, the BCL2 binds actin and forms a complex containing gelsolin resulting in reduction of free gelsolin. The increase of F-actin thus results in inhibition of cell motility.
Acknowledgements
This manuscript was supported by the Division of Intramural Research, NIEHS, NIH and by NIAMSD, NIH grant K01AR051470 awarded to Dr. Jennifer Y. Zhang. Thanks to Dr. Douglas S. Tyler, Duke University, Durham, NC for providing A2058 melanoma cell lines and to Dr. Christine Sorenson, University of Wisconsin, Madison, WI, for the generous gift of the mouse Bcl2 deficient cells. We thank Ms. Vineela Gandham for her editing of this manuscript.
References
- Coultas L, Strasser A. The role of the Bcl-2 protein family in cancer. Semin Cancer Biol 2003; 13:1 - 3
- Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 1988; 335:440 - 442
- Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 1990; 348:331 - 333
- Murphy KL, Kittrell FS, Gay JP, Jager R, Medina D, Rosen JM. Bcl-2 expression delays mammary tumor development in dimethylbenz(a)anthracene-treated transgenic mice. Oncogene 1999; 18:6597 - 6604
- de la Coste A, Fabre M, McDonell N, Porteu A, Gilgenkrantz H, Perret C, et al. Differential protective effects of Bcl-xL and Bcl-2 on apoptotic liver injury in transgenic mice. Am J Physiol 1999; 277:702 - 708
- Lu PJ, Lu QL, Rughetti A, Taylor-Papadimitriou J. bcl-2 overexpression inhibits cell death and promotes the morphogenesis, but not tumorigenesis of human mammary epithelial cells. J Cell Biol 1995; 129:1363 - 1378
- Gil-Gomez G, Berns A, Brady HJ. A link between cell cycle and cell death: Bax and Bcl-2 modulate Cdk2 activation during thymocyte apoptosis. EMBO J 1998; 17:7209 - 7218
- Quinn L, Coombe M, Mills K, Daish T, Colussi P, Kumar S, et al. Buffy, a Drosophila Bcl-2 protein, has anti-apoptotic and cell cycle inhibitory functions. EMBO J 2003; 22:3568 - 3579
- Trisciuoglio D, Desideri M, Ciuffreda L, Mottolese M, Ribatti D, Vacca A, et al. Bcl-2 overexpression in melanoma cells increases tumor progression-associated properties and in vivo tumor growth. J Cell Physiol 2005; 205:414 - 421
- Von Wangenheim KH, Peterson HP. A mechanism of intracellular timing and its cooperation with extracellular signals in controlling cell proliferation and differentiation, an amended hypothesis. J Theor Biol 2001; 211:239 - 251
- Callagy GM, Webber MJ, Pharoah PD, Caldas C. Meta-analysis confirms BCL2 is an independent prognostic marker in breast cancer. BMC Cancer 2008; 8:153
- Ke H, Parron VI, Reece J, Zhang JY, Akiyama SK, French JE. BCL2 inhibits cell adhesion, spreading and motility by enhancing actin polymerization. Cell Res 2010; 20:458 - 469
- Merritt AJ, Potten CS, Watson AJ, Loh DY, Nakayama K, Hickman JA. Differential expression of bcl-2 in intestinal epithelia. Correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J Cell Sci 1995; 108:2261 - 2271
- Nakayama K, Negishi I, Kuida K, Sawa H, Loh DY. Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease and lymphocytopenia. Proc Natl Acad Sci USA 1994; 91:3700 - 3704
- Mak SS, Moriyama M, Nishioka E, Osawa M, Nishikawa S. Indispensable role of Bcl2 in the development of the melanocyte stem cell. Dev Biol 2006; 291:144 - 153
- Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science 2009; 326:1208 - 1212
- Reinhart-King CA, Dembo M, Hammer DA. The dynamics and mechanics of endothelial cell spreading. Biophys J 2005; 89:676 - 689
- Koestler SA, Rottner K, Lai F, Block J, Vinzenz M, Small JV. F- and G-actin concentrations in lamellipodia of moving cells. PLoS One 2009; 4:e4810
- Moissoglu K, Schwartz MA. Integrin signalling in directed cell migration. Biol Cell 2006; 98:547 - 555
- Martin SS, Leder P. Human MCF10A mammary epithelial cells undergo apoptosis following actin depolymerization that is independent of attachment and rescued by Bcl-2. Mol Cell Biol 2001; 21:6529 - 6536
- Zhang W, Pantschenko AG, McCarthy MB, Gronowicz G. Bone-targeted overexpression of Bcl-2 increases osteoblast adhesion and differentiation and inhibits mineralization in vitro. Calcif Tissue Int 2007; 80:111 - 122
- Whittard JD, Akiyama SK. Activation of beta1 integrins induces cell-cell adhesion. Exp Cell Res 2001; 263:65 - 76
- Lee BH, Ruoslahti E. alpha5beta1 integrin stimulates Bcl-2 expression and cell survival through Akt, focal adhesion kinase and Ca2+/calmodulin-dependent protein kinase IV. J Cell Biochem 2005; 95:1214 - 1223
- Nobes CD, Hall A. Rho, rac and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell 1995; 81:53 - 62
- Yamazaki D, Oikawa T, Takenawa T. Rac-WAVE-mediated actin reorganization is required for organization and maintenance of cell-cell adhesion. J Cell Sci 2007; 120:86 - 100
- Hartwig JH, Chambers KA, Stossel TP. Association of gelsolin with actin filaments and cell membranes of macrophages and platelets. J Cell Biol 1989; 108:467 - 479
- Sorenson CM. Interaction of bcl-2 with Paxillin through its BH4 domain is important during ureteric bud branching. J Biol Chem 2004; 279:11368 - 11374
- Li L, Backer J, Wong AS, Schwanke EL, Stewart BG, Pasdar M. Bcl-2 expression decreases cadherin-mediated cell-cell adhesion. J Cell Sci 2003; 116:3687 - 3700
- Zhu T, Starling-Emerald B, Zhang X, Lee KO, Gluckman PD, Mertani HC, et al. Oncogenic transformation of human mammary epithelial cells by autocrine human growth hormone. Cancer Res 2005; 65:317 - 324
- Lin HM, Lee YJ, Li G, Pestell RG, Kim HR. Bcl-2 induces cyclin D1 promoter activity in human breast epithelial cells independent of cell anchorage. Cell Death Differ 2001; 8:44 - 50
- Pinkas J, Martin SS, Leder P. Bcl-2-mediated cell survival promotes metastasis of EpH4 betaMEKDD mammary epithelial cells. Mol Cancer Res 2004; 2:551 - 556
- Miyake H, Hara I, Yamanaka K, Gohji K, Arakawa S, Kamidono S. Overexpression of Bcl-2 enhances metastatic potential of human bladder cancer cells. Br J Cancer 1999; 79:1651 - 1656
- Kondo S, Tang Y, Scheef EA, Sheibani N, Sorenson CM. Attenuation of retinal endothelial cell migration and capillary morphogenesis in the absence of bcl-2. Am J Physiol Cell Physiol 2008; 294:1521 - 1530
- Sheibani N, Scheef EA, Dimaio TA, Wang Y, Kondo S, Sorenson CM. Bcl-2 expression modulates cell adhesion and migration promoting branching of ureteric bud cells. J Cell Physiol 2007; 210:616 - 625