Abstract
Leukocyte chemotaxis plays an essential role in generating and delivering immune responses and is a critical component of inflammation. In order to identify novel genes and pathways important for regulating chemotaxis, we performed an RNAi-based screen and identified several genes involved with vesicle movement and fusion as mediators of chemotaxis. Our recently published data1 show that during chemotaxis vesicle trafficking proteins are required for lysosome fusion, uropod release and efficient directed cell migration.
Leukocyte chemotaxis is an essential process that directs the immune system and drives inflammatory responses.Citation2 The processes whereby an extracellular chemical signal induces a program that results in morphological changes to the cell and induces chemotaxis remain incompletely understood. Since the earliest days of the study of chemotaxis, a number of investigators have found clues suggesting that membrane flow and vesicle trafficking play roles in the movement of cells toward a chemotactic stimulus. As early as 1962 it was suggested that cell migration involves a membrane cycle.Citation3 Data emerged suggesting that the plasma membrane flowed toward the rear of the migrating cell.Citation4–Citation6 More recently, it was suggested the membrane actually flowed toward the front of the migrating cell.Citation7 This later hypothesis was supported by the finding that recycling transferrin receptors were routed to the plasma membrane of the leading edge lammellopodia.Citation8 Finally, a potential role for lysosome fusion with the uropod has been suggested.Citation9–Citation12 Despite these observations, a functional correlation of vesicle trafficking in chemotaxis had not been established.
We performed an RNAi-based genetic screen to identify novel regulators of CXCR4-mediated lymphocyte chemotaxis. In the screen, we found two genes in the synaptotagmin family of calcium sensing vesicle fusion proteins. Synaptotagmin-2 (Syt2) was identified as a negative regulator of lymphocyte chemotaxis and Synaptotagmin-like protein-5 (Sytl5) was identified as a positive regulator of chemotaxis. Lymphocytes in which SYT2 expression was reduced using RNAi demonstrated enhanced migration in a CXCL12 gradient. In contrast, cells in which SYTL5 mRNA expression was reduced demonstrated decreased migration in a CXCL12 gradient. The knock-down of these genes had similar effects in the migration of a monocyte cell line to a different chemokine, CCL2.
In order to determine the extent of the role of synaptotagmin family member proteins in chemotaxis, we extended the screen to the remaining synaptotagmin and synaptotagmin-like family member genes and identified Synaptotagmin-7 (Syt7) as encoding a positive regulator of chemotaxis. The identification of SYT7 provided potential insight into the mechanistic role of these proteins in chemotaxis as it is expressed on the lysosomal membrane of many different cell types and has been shown to be important in lysosome-cell membrane fusion as well as in membrane repair. Additionally, an SYT7-deficient mouse line was available to facilitate further experiments to analyze the function of SYT7 in chemotaxis. Lymphocytes and neutrophils obtained from SYT7-deficient mice demonstrated decreased chemotaxis in response to chemokine gradients. By analyzing cell migration tracks of cells migrating in a Zigmond chemotaxis chamber, we found that the cells lacking SYT7 had both a defect in migration velocity, as well as in migration direction. In an in vivo model of monosodium urate (MSU) crystal induced gout-like inflammation, we found that SYT7-deficient female mice demonstrated decreased neutrophil recruitment into an experimentally produced airpouch containing MSU crystals. The decreased neutrophil recruitment was not due to decreased IL-1 or neutrophil-active chemokine release as the airpouches contained similar amounts of these cytokines. These results confirmed and extended the hypothesis that synaptotagmin family member proteins have a role in chemotaxis.
In addition to synaptotagmin family members, the process of vesicle trafficking within the cell also requires SNARE proteins. Indeed, a role for the SYT7 interacting SNARE protein, VAMP7, has been shown in epithelial cell migration.Citation13 As the interaction of SYT7 and VAMP7 has also been shown to be important for lysosome fusion with the cell membrane and SYT2 has been shown to be a negative regulator of lysosomal exocytosis,Citation14,Citation15 we investigated whether lysosome fusion was important for chemotaxis. Using FACS analysis, we found that chemoattractant stimulation of lymphocytes and neutrophils resulted in the expression of the lysosomal membrane protein LAMP-1 (CD107a) on the cell surface. Expression of LAMP-1 was dependent on the expression of SYT7 as well as the release of intracellular calcium. Further, confocal micrographs of podocytes, large cells of renal origin that express CXCR3, show that podocytes express the lysosomal marker LAMP-2 on their surface following stimulation with the CXCR3 ligand CXCL11. These data support the hypothesis that chemoattractant-mediated calcium influx induces the fusion of lysosomes with the cell membrane in an SYT7-dependent manner. To further investigate the role of lysosomes in chemotaxis, we analyzed the migration of lysotracker-stained wild-type and Syt7-/- lympohocytes in a CXCL10 gradient in a Dunn chamber. We found that lysotracker accumulated in the uropods of Syt7-/- cells. Additionally, these cells were adherent to the fibronectin-coated substrate at their uropods. Taken together, these data suggested that Syt7-mediated fusion of lysosomes is required for efficient uropod release during chemotaxis.
During the 1970s, chemoattractant stimulation of neutrophils was shown to induce lysosomal enzyme releaseCitation10,Citation11 and a role for lysosomal fusion was speculated to play a role in neutrophil adhesion.Citation9 However, it was difficult to identify a direct role of lysosomal enzyme release or to separate lysosome membrane fusion with that of enzyme release in chemotaxis.
We speculate that the migration and fusion of lysosomes is required to deliver cargo during chemotaxis that allows uropod release. It is possible that the fusion of lysosomes is required to deliver a specific protein required for uropod release. Alternatively, the cargo may be lipids required for membrane cycling during chemotaxis or for the delivery of a specific lipid or protein needed in the chemotaxis signaling pathway. Finally, it is possible that the contents of the lysosome, perhaps enzymes, are required on the outside of the cell to facilitate the release of uropods from the extracellular matrix. The identification of these factors is of great interest.
While our data specifically implicate a role for lysosomal trafficking and fusion in chemotaxis, it is likely that the flow of other vesicles and membrane is important in directed cell migration. Sytl5 was originally identified in a yeast two-hybrid screen as a binding partner for Rab27a.Citation16 Using this information, we used RNAi-mediated knockdowns of Rab27a and Rab3a mRNA and found similar to the synaptotagmins, these proteins had a role in lymphocyte chemotaxis. We have recently extended this genetic screen and have identified additional Rab family members that regulate chemotaxis (). The identified Rabs have broad functions with regard to vesicle trafficking, suggesting a more general requirement for vesicle trafficking during chemotaxis. These data support a role for the trafficking of other types of vesicles in addition to lysosomes during chemotaxis.
Future studies are needed to determine the roles of vesicle trafficking in regulating chemotaxis. We have presented experiments specifically demonstrating a role for lysosome or lysosome-like vesicle fusion in uropod release during chemotaxis (). However, it is likely that there will be many more roles for vesicle trafficking in chemotaxis. For example, we have found that the knock-down of additional Rab family member proteins not specifically associated with lysosomal movement also reduced chemotaxis in lymphocytes. It is likely that vesicle trafficking is essential for delivering lipid membrane to the cell surface as the cell shape changes.Citation17 Additionally, chemokine receptor recycling during chemotaxis depends on vesicle trafficking and SYT3.Citation18 Specific cargo, including integrins and other receptors, are likely to be delivered to the leading and trailing edges by vesicles.Citation19 It is likely that that the movement of vesicles within the cell is essential for many aspects of cell movement beyond the cell.
Figures and Tables
Figure 1 Transwell chemotaxis of SupT1 cells infected with viruses containing shRNA targeting genes encoding the indicated members of the Rab family or EGFP as a control. The number of cells migrating to the lower chamber was determined using CyQUANT as previously described.Citation1 Reductions in the chemokine dose response were significant using ANOVA (p < 0.01 for each of the knock-down cell lines).
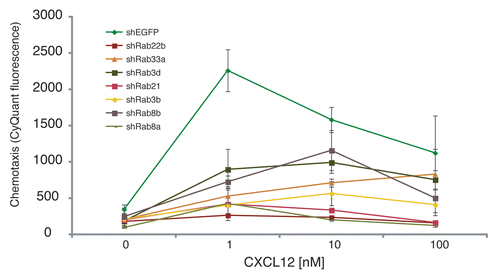
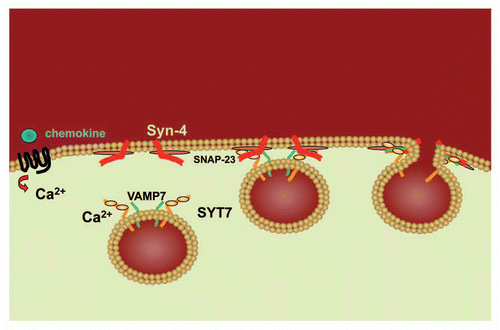
Figure 2 Model of SYT7 activity in chemotaxis. Following the binding of a chemoattractant (eg. chemokine) to its G protein-coupled seven transmembrane spanning receptor, calcium is released. Binding of calcium to Syt7 on the lysosome surface induces Vamp7 on the lysosome surface to interact with Syn-4 and SNAP-23 at the cell membrane thereby facilitating the fusion of the lysosome with the cell membrane.
References
- Colvin RA, Means TK, Diefenbach TJ, Moita LF, Friday RP, Sever S, et al. Synaptotagmin-mediated vesicle fusion regulates cell migration. Nat Immunol 2010; 11:495 - 502
- Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol 2008; 48:171 - 197
- Marcus PI. Dynamics of surface modification in myxovirus-infected cells. Cold Spring Harb Symp Quant Biol 1962; 27:351 - 365
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. II. “RRuffling”. Exp Cell Res 1970; 60:437 - 444
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. I. Movements of the leading edge. Exp Cell Res 1970; 59:393 - 398
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. 3. Movements of particles on the dorsal surface of the leading lamella. Exp Cell Res 1970; 62:389 - 398
- Lee J, Gustafsson M, Magnusson K, Jacobson K. The direction of membrane lipid flow in locomoting polymorphonuclear leukocytes. Science 1990; 247:1229 - 1233
- Hopkins CR, Gibson A, Shipman M, Strickland DK, Trowbridge IS. In migrating fibroblasts, recycling receptors are concentrated in narrow tubules in the pericentriolar area, and then routed to the plasma membrane of the leading lamella. J Cell Biol 1994; 125:1265 - 1274
- Fehr J, Dahinden C. Modulating influence of chemotactic factor-induced cell adhesiveness on granulocyte function. J Clin Invest 1979; 64:8 - 16
- Becker EL, Showell HJ. The ability of chemotactic factors to induce lysosomal enzyme release. II. The mechanism of release. J Immunol 1974; 112:2055 - 2062
- Becker EL, Showell HJ, Henson PM, Hsu LS. The ability of chemotactic factors to induce lysosomal enzyme release. I. The characteristics of the release, the importance of surfaces and the relation of enzyme release to chemotactic responsiveness. J Immunol 1974; 112:2047 - 2054
- Becker EL. Some interrelations of neutrophil chemotaxis, lysosomal enzyme secretion and phagocytosis as revealed by synthetic peptides. Am J Pathol 1976; 85:385 - 394
- Proux-Gillardeaux V, Raposo G, Irinopoulou T, Galli T. Expression of the Longin domain of TI-VAMP impairs lysosomal secretion and epithelial cell migration. Biol Cell 2007; 99:261 - 271
- Baram D, Adachi R, Medalia O, Tuvim M, Dickey BF, Mekori YA, et al. Synaptotagmin II negatively regulates Ca2+-triggered exocytosis of lysosomes in mast cells. J Exp Med 1999; 189:1649 - 1658
- Rao SK, Huynh C, Proux-Gillardeaux V, Galli T, Andrews NW. Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J Biol Chem 2004; 279:20471 - 20479
- Kuroda TS, Fukuda M, Ariga H, Mikoshiba K. Synaptotagmin-like protein 5: a novel Rab27A effector with C-terminal tandem C2 domains. Biochem Biophys Res Commun 2002; 293:899 - 906
- Traynor D, Kay R. Possible roles of the endocytic cycle in cell motility. J Cell Sci 2007; 120:2318 - 2327
- Masztalerz A, Zeelenberg IS, Wijnands YM, de Bruijn R, Drager AM, Janssen H, et al. Synaptotagmin 3 deficiency in T cells impairs recycling of the chemokine receptor CXCR4 and thereby inhibits CXCL12 chemokine-induced migration. J Cell Sci 2007; 120:219 - 228
- Li J, Ballif BA, Powelka AM, Dai J, Gygi SP, Hsu VW. Phosphorylation of ACAP1 by Akt regulates the stimulation-dependent recycling of integrin beta1 to control cell migration. Dev Cell 2005; 9:663 - 673