Abstract
Adhesion molecules are known to be important components of an active T cell-mediated immune response. Signals generated at a site of inflammation cause circulating T-cells to respond by rolling, arrest, and then transmigration through the endothelium, all of which are mediated by adhesion molecules. Consequently, strategies have been developed to treat immune disorders with specific antibodies that block the interaction of adhesion molecules. However, the therapeutic effects of such remedies are not always achieved. Our recent investigations have revealed that intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) work together with chemokines to induce immunosuppression mediated by mesenchymal stem cells (MSCs), thus demonstrating the dual role of adhesion molecules in immune responses. Since MSCs represent an important component of the stromal cells in an inflammatory microenvironment, our findings provide novel information for understanding the regulation of immune responses and for designing new strategies to treat immune disorders.
Adhesion molecules are cell surface proteins that mediate the interaction between cells, or between cells and the extracellular matrix (ECM). There are four families of adhesion molecules: immunoglobulin-like adhesion molecules, integrins, cadherins and selectins. Most of them are typical transmembrane proteins that have cytoplasmic, transmembrane and extracellular domains. In the immune system, cell adhesion plays a critical role in initiating and sustaining an effective immune response against foreign pathogens.Citation1 Based on our recent data, we discuss herein the role of immunoglobulin superfamily cell adhesion molecules, ICAM1 and VCAM-1, in the immunosuppression mediated by Mesenchymal stem cells.
Adhesion Molecules in T-Cell Mediated Immunity
In a typical T-cell mediated immune response, T cells are first activated by antigen-presenting cells in lymph nodes and then migrate through the endothelium at the site of inflammation where they help eliminate foreign pathogens. Adhesion molecules are critical mediators of this process. When T cells respond to chemokines, adhesion molecules trigger their rolling, activation, stable arrest and transmigration. Under conditions of shear flow, the rolling of T cells on the endothelial surface is conducted through the interaction of the T-cell surface adhesion molecules, L-selectin,Citation2 α4 integrinsCitation3 and lymphocyte function-associated antigen-1 (LFA-1),Citation4 with their respective endothelial ligands, glycosylation-dependent cell adhesion molecule-1 (GLYCAM1), vascular cell adhesion molecule-1 (VCAM-1) and inter-cellular adhesion molecule 1 (ICAM-1). Moreover, endothelial cells express E-selectin and P-selectin, which are also involved in leukocyte rolling.Citation5 After the transient rolling along the endothelium, stimulation of leukocytes by chemokines and other chemoattractants leads to conformational changes and clustering of their surface adhesion molecules, especially integrins. Such changes result in increased adhesive ligand-receptor binding affinity, which promotes the firm arrest of leukocytes on endothelium.Citation1 The key interaction in this process is the binding of leukocyte integrins to immuno-globulin-like adhesion molecules, such as ICAM-1 and VCAM-1 on endothelial cells. The final step, with the participation of platelet endothelial cell adhesion molecule (PECAM), is leukocyte transmigration through the endothelium to the site of injury.Citation1 Recent insights into leukocyte adhesion have demonstrated that cell-cell adhesion is a complex and finely-tuned process.Citation6
Anti-Adhesion Therapies
The fact that adhesion molecules positively participate in immune responses has led to efforts to develop anti-adhesion strategies for the treatment of inflammatory disorders such as asthma,Citation7 psoriasis,Citation8 Crohn disease,Citation9 multiple sclerosis,Citation10 inflammatory bowel diseaseCitation11 and cancer.Citation12 Most therapies target the interaction of integrins and immunoglobulin-like adhesion molecules with their targets. Several drugs in preclinical and clinical trials show future promise, such as natalizumab, a humanized monoclonal antibody targeting very late antigen-4 (VLA-4) for the treatment of Crohn diseaseCitation13 and relapsing-remitting multiple sclerosis.Citation10 In addition to having some adverse effects, such as increased viral infection,Citation14 immunotherapies that target adhesion molecules have been found to be ineffective for other inflammation-related diseases, including myocardial infarction and hemorrhagic shock,Citation15 although the targeted adhesion molecules are key players in the pathogenesis of these diseases. In animal studies, anti-ICAM-1 did not significantly affect experimental melanin-induced uveitisCitation16 or experimental auto-immune encephalomyelitis (EAE) in rats.Citation17 Likewise, anti-VCAM-1 did not protect against ischemic damage either in rats or in mice.Citation18 Several factors may account for these apparently contradictory results. Firstly, the overlapping effects of different adhesion molecules may make a single treatment insufficient to block the disease. Secondly, it is difficult to precisely target the molecules that are critical for a disease with a complicated pathogenesis. Our recent studies have revealed that under some special circumstances, adhesion molecules can lead to immunosuppression,Citation19 making it necessary to re-evaluate the function of adhesion molecules in an immune response.
Adhesion Molecules ICAM-1 and VCAM-1 Play a Key Role in Mesenchymal Stem Cell-Mediated Immunosuppression
Mesenchymal stem cells (MSCs) are a population of tissue stromal/stem cells that have been successfully isolated from bone marrow, adipose tissue, placenta, umbilical cord, skin, liver and intestine. Investigations by various laboratories have shown that these cells have enormous potential for the treatment of a number of immune disorders, owing to their exquisite ability to suppress immune responses. In animal models, preclinical and clinical trials, MSC-based cell therapy has become a promising strategy for the prevention and treatment of graft-versus-host disease,Citation20 liver fibrosis,Citation21 sepsis,Citation22 multiple sclerosisCitation23 and rheumatoid arthritis.Citation24 To achieve better clinical application of these cells, we have examined the cellular and molecular mechanisms through which MSCs suppress immune responses. We found that these cells are not innately immunosuppressive, rather they become immunosuppressive upon interaction with the inflammatory environment. The specific immunosuppressive effector varies among species: the effector molecule in mouse MSCs is nitric oxide (NO), while indoleamine 2,3 dioxygenase (IDO) serves the same purpose in human and non-human primate MSCs.Citation25,Citation26
NO is a highly-labile small molecule and many biochemical compounds affect its perfusion rate. Mathematical models have predicted that NO can be active only at a very limited distance from its source of production.Citation27 To achieve immunomodulation NO occurs at a high concentration, which results in the nitration of some vital proteins or formation of toxic reactive nitrogen species.Citation28 In the human system, IDO catalyzes an essential amino acid, tryptophan, leading to the production of many immunomodulatory tryptophan metabolites along the kynurenine pathway, as well as creating a local tryptophan-deficient microenvironment.Citation29 Previous studies have shown that separating MSCs from immune cells in a transwell system inhibits immunomodulation by MSCs.Citation25,Citation26 In addition, direct interaction between MSCs and T cells favors the formation of regulatory T cells.Citation30 Therefore, effective T-cell inhibition by MSCs depends on their proximity to MSCs.
Our recent findings have demonstrated that, in an inflammatory environment, MSCs secrete high concentrations of various chemokines and express high levels of surface adhesion molecules such as ICAM-1 and VCAM-1.Citation19,Citation26 These traditionally immune-promoting chemokines and adhesion molecules mediate the close interaction between MSCs and T cells.Citation19,Citation26 Interestingly, inflammatory cytokines induce the production of high concentrations of NO or IDO by MSCs and the resulting metabolites are immunosuppressive only in close proximity with T cells.Citation25,Citation26 Normally, MSCs express very low levels of chemokines and adhesion molecules. In the presence of an active immune response, however, the inflammatory cytokines, interferonγ (IFNγ), tumor necrosis factorα (TNFα) and interleukin-1 (IL-1) stimulate the upregulation of several T-cell-specific chemokines by MSCs, especially ligands of CXCR3 and CCR5 and immunoglobulin-like adhesion molecules ICAM-1 and VCAM-1.Citation19,Citation25,Citation26 Chemokines and adhesion molecules attract and anchor T cells to MSCs, where high concentrations of immunosuppressive effector molecules can act on the T cells and inhibit their proliferation. Blockade of chemokine receptors CXCR3 and CCR5 or ICAM-1/VCAM-1, significantly reversed such MSC-mediated immunosuppression in vitro and in vivo,Citation25,Citation26 suggesting that chemokines and adhesion molecules indeed cooperate with the effector molecues (NO or IDO) to exert the immunosuppressive effect by inflammatory cytokine-stimulated MSCs.
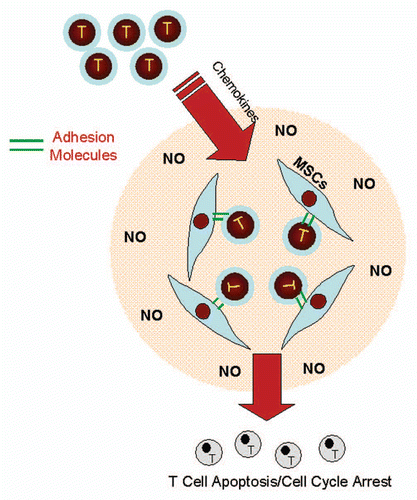
Therefore, MSCs and possibly other stromal cells recruit leukocytes in a similar fashion as endothelium, but they have set a trap with a high concentration of NO or IDO-catalyzed metabolites. Once the leukocytes are in proximity with and adhere to MSCs, these immunosuppressive molecules lead the T cells to undergo apoptosis, cell cycle arrest or become regulatory T cells ().Citation25,Citation26,Citation30 Further elucidation of the dual role of adhesion molecules in an immune response will improve our understanding of immunoregulation and help to develop better adhesion molecule-targeting therapies.
Figures and Tables
Figure 1 Adhesion molecules are involved in suppressing immune responses. Chemokines and adhesion molecules mediate leukocyte migration and adhesion to MSCs. The high concentration of NO or IDO-catalyzed metabolites produced by inflammatory cytokine-stimulated MSCs bathes the recruited leukocytes, and can induce apoptosis, cell cycle arrest or the development of immunoregulatory cells leading to downregulation of the immune response.
References
- Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol Rev 2007; 218:178 - 196
- von Andrian UH, Chambers JD, Berg EL, Michie SA, Brown DA, Karolak D, et al. L-selectin mediates neutrophil rolling in inflamed venules through sialyl LewisX-dependent and -independent recognition pathways. Blood 1993; 82:182 - 191
- Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, et al. Alpha4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell 1995; 80:413 - 422
- Sigal A, Bleijs DA, Grabovsky V, van Vliet SJ, Dwir O, Figdor CG, et al. The LFA-1 integrin supports rolling adhesions on ICAM-1 under physiological shear flow in a permissive cellular environment. J Immunol 2000; 165:442 - 452
- Kanwar S, Bullard DC, Hickey MJ, Smith CW, Beaudet AL, Wolitzky BA, et al. The association between alpha4-integrin, P-selectin and E-selectin in an allergic model of inflammation. J Exp Med 1997; 185:1077 - 1087
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007; 7:678 - 689
- Woodside DG, Vanderslice P. Cell adhesion antagonists: therapeutic potential in asthma and chronic obstructive pulmonary disease. BioDrugs 2008; 22:85 - 100
- Bauer RJ, Dedrick RL, White ML, Murray MJ, Garovoy MR. Population pharmacokinetics and pharmacodynamics of the anti-CD11a antibody hu1124 in human subjects with psoriasis. J Pharmacokinet Biopharm 1999; 27:397 - 420
- Leung Y, Panaccione R. Anti-adhesion molecule strategies for Crohn disease. BioDrugs 2008; 22:259 - 264
- Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2003; 348:15 - 23
- Stefanelli T, Malesci A, De La Rue SA, Danese S. Anti-adhesion molecule therapies in inflammatory bowel disease: touch and go. Autoimmun Rev 2008; 7:364 - 369
- Schmidmaier R, Baumann P. ANTI-ADHESION evolves to a promising therapeutic concept in oncology. Curr Med Chem 2008; 15:978 - 990
- Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, et al. Natalizumab induction and maintenance therapy for Crohn's disease. N Engl J Med 2005; 353:1912 - 1925
- Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med 2005; 353:369 - 374
- Harlan JM, Winn RK. Leukocyte-endothelial interactions: clinical trials of anti-adhesion therapy. Crit Care Med 2002; 30:214 - 219
- Smith JR, O'Rourke LM, Becker MD, Cao M, Williams KA, Planck SR, et al. Anti-rat ICAM-1 antibody does not influence the course of experimental melanin-induced uveitis. Curr Eye Res 2000; 21:906 - 912
- Willenborg DO, Simmons RD, Tamatani T, Miyasaka M. ICAM-1-dependent pathway is not critically involved in the inflammatory process of autoimmune encephalomyelitis or in cytokine-induced inflammation of the central nervous system. J Neuroimmunol 1993; 45:147 - 154
- Justicia C, Martin A, Rojas S, Gironella M, Cervera A, Panes J, et al. Anti-VCAM-1 antibodies did not protect against ischemic damage either in rats or in mice. J Cereb Blood Flow Metab 2006; 26:421 - 432
- Ren G, Zhao X, Zhang L, Zhang J, L'Huillier A, Ling W, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol 2010; 184:2321 - 2328
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008; 371:1579 - 1586
- Abdel Aziz MT, Atta HM, Mahfouz S, Fouad HH, Roshdy NK, Ahmed HH, et al. Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clin Biochem 2007; 40:893 - 899
- Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 2009; 15:42 - 49
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 2005; 106:1755 - 1761
- Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther 2008; 10:223
- Ren G, Su J, Zhang L, Zhao X, Ling W, L'Huillie A, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells 2009; 27:1954 - 1962
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008; 2:141 - 150
- Lancaster JR Jr. A tutorial on the diffusibility and reactivity of free nitric oxide. Nitric Oxide 1997; 1:18 - 30
- Radi R. Nitric oxide, oxidants and protein tyrosine nitration. Proc Natl Acad Sci USA 2004; 101:4003 - 4008
- Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 2004; 4:762 - 774
- Quaedackers ME, Baan CC, Weimar W, Hoogduijn MJ. Cell contact interaction between adipose-derived stromal cells and allo-activated T lymphocytes. Eur J Immunol 2009; 39:3436 - 3446