Abstract
During vertebrate brain development, migration of neurons from the germinal zones to their final laminar positions is essential to establish functional neural circuits1-3. Whereas key insights into neuronal migration initially came from landmark studies identifying the genes mutated in human cortical malformations4, cell biology has recently greatly advanced our understanding of how cytoskeletal proteins and molecular motors drive the morphogenic cell movements that build the developing brain. This Commentary & View reviews recent studies examining the role of the molecular motors during neuronal migration and critically examines current models of acto-myosin function in the two-step neuronal migration cycle. Given the apparent emerging diversity of neuronal sub-type cytoskeletal organizations, we propose that two approaches must be taken to resolve differences between the current migration models: the mechanisms of radial and tangential migration must be compared and the loci of tension generation, migration substrates, and sites of adhesion dynamics must be precisely examined in an integrated manner.
Basic Mechanisms of Neuronal Migration: A Multistep Process
Since the first time-lapse studies documented cerebellar granule neuron (CGN) migration along Bergmann glial fibers,Citation5 in vitro and ex vivo imaging has revealed many features of migrating neurons that are conserved throughout the nervous system.Citation6–Citation10 Most migrating neurons extend a dynamic leading process from the cell body to sample the surrounding microenvironment while the soma and nucleus remain largely stationary. Leading processes differ in morphology across classes of neurons, but all leading processes require microtubule stability and efficient membrane trafficking for their extension and function. After the leading process commits to a single direction, the cell body and nucleus translocate forward in a two-step process termed nucleokinesis. During the first step, a distal swelling appears in the proximal leading process and the centrosome and Golgi apparatus move toward it.Citation10–Citation13 These two events are thought to be strong predicators of the final step of migration, in which the nucleus and cell body translocate, occasionally leaving behind a trailing process.
Our understanding of the mechanisms of migration has advanced greatly during the past five years, with improved imaging techniques and reagents. Neuronal migration is now known to be a highly orchestrated event involving intricate interplay between microtubules, actin and their associated motor proteins. To better understand how neurons move, it is important to know where cytoskeletal elements are located and how they interact during the migratory cycle, which is perhaps best investigated using drugs that alter the natural state of the cytoskeleton and motor proteins. In cytoskeletal perturbation experiments, 50 µM blebbistatin, which inhibits ATPase activity of the A and B isoforms of non-muscle myosin ii, halted somal translocation without affecting leading process advancement, whereas 100 µM blebbistatin completely arrested migration of anterior subventricular zone (SVZa) cells.Citation9 Conversely, depolymerization of microtubules via nocodazole treatment did not affect cell body movement but halted the advance of the leading process. Whereas such studies demonstrate that the acto-myosin and the microtubule cytoskeletal components have different functional requirements during discrete migration stages, the spatio-temporal sites of action of these components remains a topic of much debate.
Nucleokinesis
Centrosome movement into the dilated region is commonly used as a predictor of subsequent nuclear movement. In recent slice experiments, dynein was shown to be responsible for centrosome and nuclear movement into the proximal leading process, as knockdown of LIS1 (a dynein regulator) or dynein itself inhibited centrosomal displacement, whereas blebbistatin did not affect centrosome movement.Citation10 However, we suspect that the concentration of blebbistatin in these experiments may have been insufficient to affect centrosome movement; subsequent studies demonstrated that addition of blebbistatin to purified CGNs did in fact arrest centrosomal movement, as did jasplakinolide, an actin-anchoring drug.Citation14
Many previously believed that the nucleus is tethered to the centrosome and that the centrosomes' movement into the proximal region of the leading process induces forward nuclear movement.Citation12 Umeshima et al. confirmed that the nuclei of CGNs migrating in ex vivo slice preparations are surrounded by a cage of microtubules,Citation13 as had been observed in previous in vitro studies.Citation15 Interestingly, the cage is composed of different populations of microtubules, most of which are tyrosinated and are thought to be dynamically unstable. However, the microtubules around the anterior surface of the nucleus were found to be acetylated, a post-translational modification associated with stability. The acetylated microtubules extended from the nucleus toward the proximal leading process and were not associated with the centrosomes as previously thought. In live imaging experiments ex vivo and in vitro, they observed that the nucleus, powered by cytoplasmic dynein, frequently moved ahead of the centrosome. In fact, the microtubules emanating from the anterior portion of the nucleus tended to bypass the centrosome and extend into the leading process. This population of microtubules was shown to be essential for migration of the nucleus, possibly because they provide a direct bridge between the microtubules surrounding the nucleus and the tension in the leading process provided by acto-myosin.
Conflicting Acto-Myosin Models: Engine in the Leading Process or Cell Rear?
While a central role for acto-myosin dynamics in driving cell migrations has been established for nearly every cell type examined,Citation16 there is considerable diversity in cytoskeletal architecture or location of motor components for many models of cell motility. For example, blood cells possess prominent accumulation of acto-myosin at the cell rearCitation17 while epithelial cells or migrating fibroblasts possess significant myosin ii accumulation in the lamellum forward of the nucleus.Citation18
Given the apparent cytoskeletal diversity observed in non-neuronal cells it is no surprise that differences in acto-myosin function have been observed in migrating neurons (). Initial studies suggested that acto-myosin contractility is localized at the rear of migrating neurons.Citation9,Citation10,Citation12,Citation19 This theory, based largely on Medial Ganglionic Eminence (MGE) interneurons migrating in cortical slice preparations or in vitro culturesCitation12 and SVZa precursors migrating in 3D matrigel matrix,Citation9 surmises that myosin ii-based contraction at the rear of migrating neurons functions to squeeze the contents of the neuron forward during somal translocation and nucleokinesis ().
More recently, our laboratory and the laboratory of Xiaobing Yuan, have shown the leading process of radially migrating CGNs contains a high concentration of F-actin and myosin ii motors, with very little acto-myosin located in the cell body rear or trailing process ().Citation14,Citation20 Fluorescence recovery after photobleaching (FRAP) analysis also showed the leading process is the region of highest acto-myosin turnover while pulse chase experiments further consolidated these findings, showing that F-actin flows from the cell soma toward the direction of migration.Citation14,Citation20 The co-localization of F-actin and myosin ii strongly suggests that acto-myosin contractility events play an important role in the cytoplasmic dilation and distal leading process of CGNs. Further, we have observed that leading process acto-myosin contractility is required for translocation of the centrosomeCitation14 and Golgi (unpublished data) into the leading process during CGN migration. Thus, acto-myosin tension anterior to the cell body may be required for organelle positioning events prior to the final stage of movement and may “prime” the cell for somal/nuclear translocation.
While the observed differences in migration mode between MGE interneurons, SVZa cells and CGNs may represent bona fide cell type-specific differences in acto-myosin organization, we'd like to note there were significant differences in experimental conditions and how acto-myosin organization was assessed. For example, migration substrates significantly differed: MGE migration was assayed in both ex vivo slices or cortical mixed cultures, SVZa migration was assayed in matrigel matrix and CGN migration was assayed in neuron-glial co-cultures that recapitulate radial migration. Moreover, most of the studies demonstrating the localization of myosin ii at the cell rear lack correction for changes in cytoplasmic volume and have not directly probed cytoskeletal dynamics using techniques like FRAP or photo-activation. Thus, it is still unclear if the cell rear is a site for specific acto-myosin accumulation (i.e., specifically concentrated relative to cytoplasmic volume) or if actin undergoes rapid myosin ii-dependent turnover in the cell rear in MGE interneurons or SVZa cells.
Local inactivation of myosin ii motor activity in the leading process and at the cell rear would shed much needed light on this controversy and would be a significant advance compared to the bath application of myosin ii inhibitors used in previous studies.Citation9,Citation12,Citation14 Recently, He and colleagues have re-examined acto-myosin localization in migrating CGNs and performed local inactivation of myosin ii and f-actin dynamics.Citation20 These studies confirmed the high-leading process and low cell rear distribution of myosin ii. Moreover, focal inactivation of acto-myosin activity in the leading process but not cell rear slowed somal translocation. Similar studies will be needed in MGE interneurons or SVZa neurons to confirm the proposed differences in subcellular acto-myosin function or if leading process acto-myosin function has been underestimated in these cells.
Toward Resolving Model Differences: Tangential vs. Radial Migration
Whereas at first it appears difficult to resolve the apparent cytoskeletal diversity of migrating neurons, closer inspection reveals that these neurons differ in their migration pathways and morphology. Acto-myosin organization has been closely examined in two tangentially migrating neuronal types to date: MGE interneurons and SVZa cellsCitation9,Citation12,Citation19 and radially migrating CGNs (by time-lapse imaging).Citation14,Citation20 SVZa cells migrate as coherent chains of neuroblastsCitation21 that require laminin recognition by β1 integrin receptors to reach the olfactory bulb.Citation22 While tangentially migrating MGE cells also require an integrin receptor to migrate,Citation23 these neurons do not use laminin substrates or migrate as chains; rather, they interact with multiple migratory pathways including axons, dendrites and eventually radial glia.Citation24 In contrast, radially migrating neurons are β1 integrin-independentCitation25 and use a different set of molecules to adhere to glial fibers as they migrate to a final laminar position: CGNs use astrotactinCitation26 and cortical pyramidal neurons use connexin 43.Citation27
Diversity in migration paths or substrates can have important implications for cytoskeletal organization. For example, in recent studies variation in the migratory environment's geometry (one-, two- or three-dimensional) dramatically altered the position of actin or myosin ii motors in fibroblasts. Fibroblasts normally harbor myosin ii at the cell rear, but they localized it forward of the nucleus when forced to migrate on thin collagen fibers.Citation28 Because radial migration of neurons along glial fibers is similarly one-dimensional, acto-myosin may be more prominently localized in the leading process in radially migrating neurons, while the multifocal adhesive environment experienced by tangentially migrating neurons may favor an alternative acto-myosin organization.
Another important difference between the radial and tangential migration mode is seen in the morphology of neuronal cell types. Mature CGNs possess a characteristic T-shaped morphology in which the “T” is an axonal stalk connected to parallel fiber axons that extend hundreds of microns into the molecular layer (ML) of the cerebellar cortex. As these neurons extend their axons well before they leave the external granule layer (EGL), they must trail an extensive axonal structure from the rear of the cell body as the soma migrates forward. Remarkably, very little is known about how granule neurons elaborate this axonal process as they migrate along Bergmann glial fibers. Because parallel fibers are anchored in the ML, complete de-adhesion of the trailing process, as seen at the rear of migrating fibroblasts or as proposed for tangentially migrating neurons, would likely result in misplacement or loss of contact between the granule neuron soma and parallel fiber axons. In contrast, both MGE interneurons and SVZa neurons possess a less extensive trailing process and do not trail an axon.Citation9,Citation21,Citation24,Citation29 Thus, extensive retraction of the trailing process could create the appearance of “squeezing at the rear” of these cells. It will be of interest to examine whether other types of neurons that undergo radial migration and possess an axonal trailing process, like cortical pyramidal neurons, possess an acto-myosin organization like that of CGNs. If tangentially migrating cells generally show prominent “squeezing at the rear” whereas radially migrating neurons do not, the controversy surrounding the two opposing models will be somewhat resolved. In that case, the cerebellar granule neuron would be an invaluable tool to discover the molecular mechanisms controlling the switch in cytoskeletal organization, as these cells undergo two migration phases: tangential migration near the cerebellar surface followed by radial migration away from the EGL after they cross the ML and enter the internal granule layer.
Adhesion Dynamics and Myosin II Motors
Most neuronal migration studies to date have indirectly examined sites of myosin ii function by using movement of the cytoplasmic organelles, nucleus or soma as surrogate indicators. We believe that the site of action of acto-myosin-based contraction can be firmly established only by local determination of myosin ii function. Historically, myosin ii activity has long been believed to power migration by squeezing cytoplasm toward the front of the cell, shaping the cell rear and breaking adhesions in the trailing portion of migrating cells.Citation30–Citation34 Recent advances in general cell motility suggest that myosin ii motors not only act in the cell rear, as older studies suggested, but also play a direct role in the leading portion of the cell where myosin ii-based contractions generated forward of the nucleus is required for efficient adhesion and cell motility.Citation35,Citation36 (reviewed in refs. Citation16 and Citation18, for addressing myosin ii and general cell motility: Ridley et al. highlight the cell rear and sides as the primary sites for myosin ii action, while Vicente-Manzanares et al. review recent advances that suggest a significant subset of myosin ii functions is anterior to the nucleus in the lamellum). While the migratory mechanisms of non-neuronal cells may differ significantly from those of neurons and may not be directly applicable to the observed differences in tangential or radial migration, the oscillations in F-actin and myosin ii dynamics in CGNs suggests the leading process of radially migrating neurons may share similarities to the acto-myosin rich lamellum of epithelial cells and fibroblasts. If leading process myosin ii function is similar to adhesion supporting lamellar acto-myosin, two predictions can be made regarding myosin ii and adhesion dynamics in migrating neurons: (1) multiple actin-based adhesions should reside near regions of high acto-myosin concentration in migrating neurons and (2) inhibition of myosin ii motor activity should prevent adhesion dynamics at these sites. When myosin ii is inhibited, the “reach and pull” model would predict reduced adhesion dynamics in the neuronal leading and soma process due to its high acto-myosin concentration, whereas the “squeezing at the rear” model would predict reduced adhesion disassembly only in the trailing aspect of the neuronal soma. Previous electron microscopy studies observed adhesion disengagement within the soma and not the trailing process of migrating CGNs, suggesting the first model may be the active mechanism for radially migrating cells.Citation37 Our laboratory is currently generating pHluorin-based probes to measure functional neuronal adhesion sites and directly test these hypotheses as they relate to acto-myosin dynamics in the CGN system.
Does Myosin II or Dynein Power the First Step?
The largest remaining discrepancy between the “reach and pull” and “squeezing at the rear” models is the identity of the motor(s) that power the first step of the two-stroke movement cycle. Whereas it is well established that cytoplasmic dynein powers nuclear translocation in migrating neurons and regulates the positioning of microtubule arrays,Citation38 recent studies suggest that acto-myosin also plays an important role in centrosome positioning. Piel et al. have shown that the actin cytoskeleton is needed to attract daughter centrioles to the future site of mid-body abscission during mitosis.Citation39 Rosenblatt et al. have shown that cortical myosin ii motors are essential to control mitotic spindle rotation.Citation40 Our studies using blebbistatin and the F-actin stabilizer jasplakinolide strongly suggest that myosin ii provides the force needed to move centrosomes and thus coordinate the first step of the migration cycle.Citation14 We therefore propose that myosin ii in the leading process plays a significant role in positioning organelles within the actin-rich cytoplasmic dilation before nuclear/somal translocation.
As both dynein and myosin ii are required for centrosome positioning events, further studies are required to dissect the functions of these motor components. It is possible that dynein and myosin ii cooperate to power organelle positioning events before somal/nuclear translocation. For example, an intact actin cytoskeleton is required for dynein transport of microtubule fragments into axons during slow axonal transport.Citation41 How could acto-myosin and dynein cooperate during the first step of the migration cycle? Dynein interacts with the plasma membrane of a variety of cell types in an actin-dependent manner via a linkage between dynactin and scaffolding molecules like β-cateninCitation38 and PLAC-24.Citation42 Interestingly, β-catenin and PLAC-24 recruitment to the cell cortex depend on an intact actin cytoskeleton and myosin ii motors.Citation42,Citation43 Therefore, interaction of dynein with the actin-rich leading process cortex via molecules like β-catenin or PLAC-24 could link acto-myosin flow to microtubule movement associated with centrosome positioning. Studies under way in our laboratory are examining whether inhibition of myosin ii motor activity or actin cytoskeletal dynamics in live CGNs alters the localization of dynein or dynactin in the leading process or alters their turnover to begin to directly test this hypothesis.
Concluding Remarks
Examination of the genes and signaling pathways regulating neuronal migration has led to the discovery of a host of cytoskeletal components required for neuronal migration. The remarkable successes of the past decade have created a new challenge—that of weaving the ever-increasing array of diverse cytoskeletal regulators required for neuronal migration into an unbiased and integrated model of neuronal migration. Meeting this challenge in a manner that allows a better understanding of the pathology of neuronal positioning disorders and perhaps eventually repair defective migration will require careful consideration of the differences in neuronal migration model systems and an integrated approach to the function of the neuronal migration machinery, as opposed to the study of cytoskeletal components in isolation.
Future Directions
Whereas cell type-specific variations in migration substrate and morphology may explain some differences in migration mode and cytoskeletal organization, more important factors, such as the true site of action of acto-myosin-based tension and the functional cooperativity of actin- and microtubule-based motors in generating the two-stroke migration cycle, remain to be fully elucidated.
Abbreviations
| CGN | = | cerebellar granule neuron |
| SVZa | = | anterior subventricular zone |
| FRAP | = | fluorescence recovery after photobleaching |
| RMS | = | rostral migratory stream |
| EGL | = | external granule layer |
| ML | = | molecular layer |
| IGL | = | internal granule layer |
| MGE | = | medial ganglionic eminence |
Figures and Tables
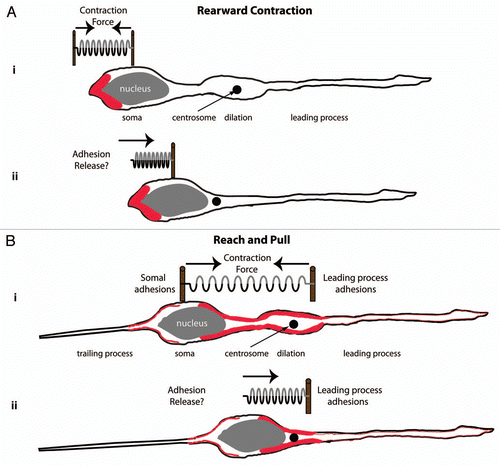
Figure 1 Acto-myosin pulling models for glial-guided neuronal migration. (A) Rearward Contraction model. (i) Prior to somal movement, acto-myosin (red) is heavily enriched at the cell rear. (ii) During somal movement myosin ii squeezing at the rear is thought to “push” the cell body forward. (B) Reach and Pull model. (i) Prior to somal movement, acto-myosin (red) is heavily enriched in the leading process from the cytoplasmic dilation to the neuronal soma. Given a muscle-like contraction of this f-actin array by myosin ii, a taut spring effectively describes the forces produced when leading process and somal acto-myosin anchoring (i.e., adhesions) are balanced before somal movement: one force vector points from the leading process back towards the soma whereas another force vector points from the soma towards the dilation (the future direction of somal movement). (ii) Once somal adhesions release, as described in Gregory et al.,Citation37 acto-myosin tension generated in the leading process primes somal movement towards the cytoplasmic dilation.
Acknowledgements
We wish to thank Sharon Naron for excellent editorial assistance. Supported by the American Lebanese Syrian Associated Charities (ALSAC, D.J.S.), a National Cancer Institute Cancer Center Support Grant (D.J.S.) and a March of Dimes Basil O'Connor Starter Scholar Research Award (D.J.S.).
References
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol 1972; 145:61 - 83
- Hatten ME. New directions in neuronal migration. Science 2002; 297:1660 - 1663
- Metin C, Vallee RB, Rakic P, Bhide PG. Modes and mishaps of neuronal migration in the mammalian brain. J Neurosci 2008; 28:11746 - 11752
- Kato M, Dobyns WB. Lissencephaly and the molecular basis of neuronal migration. Hum Mol Genet 2003; 12:89 - 96
- Edmondson JC, Hatten ME. Glial-guided granule neuron migration in vitro: a high-resolution time-lapse video microscopic study. J Neurosci 1987; 7:1928 - 1934
- O'Rourke NA, Dailey ME, Smith SJ, McConnell SK. Diverse migratory pathways in the developing cerebral cortex. Science 1992; 258:299 - 302
- Komuro H, Rakic P. Distinct modes of neuronal migration in different domains of developing cerebellar cortex. J Neurosci 1998; 18:1478 - 1490
- Solecki DJ, Model L, Gaetz J, Kapoor TM, Hatten ME. Par6alpha signaling controls glial-guided neuronal migration. Nat Neurosci 2004; 7:1195 - 1203
- Schaar BT, McConnell SK. Cytoskeletal coordination during neuronal migration. Proc Natl Acad Sci USA 2005; 102:13652 - 13657
- Tsai JW, Bremner KH, Vallee RB. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat Neurosci 2007; 10:970 - 979
- Zmuda JF, Rivas RJ. The Golgi apparatus and the centrosome are localized to the sites of newly emerging axons in cerebellar granule neurons in vitro. Cell Motil Cytoskeleton 1998; 41:18 - 38
- Bellion A, Baudoin JP, Alvarez C, Bornens M, Metin C. Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J Neurosci 2005; 25:5691 - 5699
- Umeshima H, Hirano T, Kengaku M. Microtubule-based nuclear movement occurs independently of centrosome positioning in migrating neurons. Proc Natl Acad Sci USA 2007; 104:16182 - 16187
- Solecki DJ, Trivedi N, Govek EE, Kerekes RA, Gleason SS, Hatten ME. Myosin II motors and F-actin dynamics drive the coordinated movement of the centrosome and soma during CNS glial-guided neuronal migration. Neuron 2009; 63:63 - 80
- Rivas RJ, Hatten ME. Motility and cytoskeletal organization of migrating cerebellar granule neurons. J Neurosci 1995; 15:981 - 989
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science 2003; 302:1704 - 1709
- Sanchez-Madrid F, del Pozo MA. Leukocyte polarization in cell migration and immune interactions. EMBO J 1999; 18:501 - 511
- Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 2009; 10:778 - 790
- Martini FJ, Valdeolmillos M. Actomyosin contraction at the cell rear drives nuclear translocation in migrating cortical interneurons. J Neurosci 2010; 30:8660 - 8670
- He M, Zhang ZH, Guan CB, Xia D, Yuan XB. Leading tip drives soma translocation via forward F-actin flow during neuronal migration. J Neurosci 2010; 30:10885 - 10898
- Wichterle H, Garcia-Verdugo JM, Alvarez-Buylla A. Direct evidence for homotypic, glia-independent neuronal migration. Neuron 1997; 18:779 - 791
- Belvindrah R, Hankel S, Walker J, Patton BL, Muller U. Beta1 integrins control the formation of cell chains in the adult rostral migratory stream. J Neurosci 2007; 27:2704 - 2717
- Stanco A, Szekeres C, Patel N, Rao S, Campbell K, Kreidberg JA, et al. Netrin-1-alpha3beta1 integrin interactions regulate the migration of interneurons through the cortical marginal zone. Proc Natl Acad Sci USA 2009; 106:7595 - 7600
- Polleux F, Whitford KL, Dijkhuizen PA, Vitalis T, Ghosh A. Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development 2002; 129:3147 - 3160
- Belvindrah R, Graus-Porta D, Goebbels S, Nave KA, Muller U. Beta1 integrins in radial glia but not in migrating neurons are essential for the formation of cell layers in the cerebral cortex. J Neurosci 2007; 27:13854 - 13865
- Fishell G, Hatten ME. Astrotactin provides a receptor system for CNS neuronal migration. Development 1991; 113:755 - 765
- Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature 2007; 448:901 - 907
- Meshel AS, Wei Q, Adelstein RS, Sheetz MP. Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nat Cell Biol 2005; 7:157 - 164
- Bortone D, Polleux F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron 2009; 62:53 - 71
- Pasternak C, Spudich JA, Elson EL. Capping of surface receptors and concomitant cortical tension are generated by conventional myosin. Nature 1989; 341:549 - 551
- Verkhovsky AB, Svitkina TM, Borisy GG. Self-polarization and directional motility of cytoplasm. Curr Biol 1999; 9:11 - 20
- Clow PA, McNally JG. In vivo observations of myosin II dynamics support a role in rear retraction. Mol Biol Cell 1999; 10:1309 - 1323
- Chung CY, Potikyan G, Firtel RA. Control of cell polarity and chemotaxis by Akt/PKB and PI3 kinase through the regulation of PAKa. Mol Cell 2001; 7:937 - 947
- Kolega J. Asymmetric distribution of myosin IIB in migrating endothelial cells is regulated by a rho-dependent kinase and contributes to tail retraction. Mol Biol Cell 2003; 14:4745 - 4757
- Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell 2006; 125:1361 - 1374
- Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics and polarity by myosins IIA and IIB in migrating cells. J Cell Biol 2007; 176:573 - 580
- Gregory WA, Edmondson JC, Hatten ME, Mason CA. Cytology and neuron-glial apposition of migrating cerebellar granule cells in vitro. J Neurosci 1988; 8:1728 - 1738
- Dujardin DL, Vallee RB. Dynein at the cortex. Curr Opin Cell Biol 2002; 14:44 - 49
- Piel M, Nordberg J, Euteneuer U, Bornens M. Centrosome-dependent exit of cytokinesis in animal cells. Science 2001; 291:1550 - 1553
- Rosenblatt J, Cramer LP, Baum B, McGee KM. Myosin II-dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell 2004; 117:361 - 372
- Myers KA, He Y, Hasaka TP, Baas PW. Microtubule transport in the axon: Re-thinking a potential role for the actin cytoskeleton. Neuroscientist 2006; 12:107 - 118
- Karki S, Ligon LA, DeSantis J, Tokito M, Holzbaur EL. PLAC-24 is a cytoplasmic dynein-binding protein that is recruited to sites of cell-cell contact. Mol Biol Cell 2002; 13:1722 - 1734
- Ma X, Bao J, Adelstein RS. Loss of cell adhesion causes hydrocephalus in nonmuscle myosin II-B-ablated and mutated mice. Mol Biol Cell 2007; 18:2305 - 2312