Abstract
Embryonic stem (ES) cells have a broad potential application in regenerative medicine and can be differentiated into cells of all three germ layers. Adhesion of ES cells to extracellular matrix (ECM) proteins is essential for the differentiation pathway; Cell-ECM adhesion is mediated by integrins that have the ability to activate many intracellular signaling pathways. Therefore, we hypothesize that the expression and function of integrin receptors is a critical step in ES differentiation. Using functional cell adhesion assays, our study demonstrates that α5β1 is a major functional integrin receptor expressed on the cell surface of undifferentiated mouse ES-D3 cells, which showed significantly higher binding to fibronectin as compared to collagens. This adhesion was specific mediated by integrin α5β1 as evident from the inhibition with a disintegrin selective for this particular integrin. Differentiation of ES-D3 cells on fibronectin or on a collagen type1/fibronectin matrix, caused further selective up-regulation of the α5β1 integrin. Differentiation of the cells, as evaluated by immunofluorescence, FACS analysis and quantitative RT-PCR, was accompanied by the upregulation of mesenchymal (Flk1, isolectin B4, α-SMA, vimentin) and endodermal markers (FoxA2, SOX 17, cytokeratin) in parallel to increased expression of α5β1 integrin. Taken together, the data indicate that fibronectin-mediated, upregulation of α5β1 integrin and adhesion of ES-D3 cells to specific ECM molecules are linked to early stages of mouse embryonic stem cells commitment to meso-endodermal differentiation.
Introduction
Mouse ES cells are pluripotent cell lines derived from the inner cell mass of mouse blastocysts. These cells have unlimited proliferation potential in culture while retaining their pluripotent phenotype.Citation1,Citation2 Thus, mouse ES cells can thus serve as a convenient model of embryonic development as well as an important biological source for studies in tissue engineering.Citation3
One of the first mouse stem cell clones to be developed were the ES-D3 cellsCitation4 that have been widely used as cellular models to study stem cell differentiation in vitro, as well as for analysis of the early organization of the cells within embryoid bodies (EBs).Citation5,Citation6 ES-D3 cells have successfully been differentiated into derivatives of all three germ layers,Citation5,Citation7 such as mesodermal-derived myocardial,Citation4 endothelial,Citation8 chondrogenic cells,Citation9 ectodermal-derived neurons,Citation10 endodermal-derived hepatocytes,Citation11 and pancreatic β cells.Citation12 In the field of tissue engineering, ES-D3 cells have been used to generate 3D tissue-like structures in vitro, such as beating cardiomyocytes in a scaffold of collagen I and matrigel,Citation13 and to generate endodermal cells on fibronectin-coated collagen I gels.Citation14 While ES-D3 cells in general resemble other mouse embryonic stem cell lines (e.g., in terms of expression of stemness markers such as Oct 3/4, alkaline phosphatase, SSEA-1), certain inherent differences have been identified. For example, ES-D3 cells constitutivley express markers of endothelial differentiation Flk-1 (VEGFR-2) and PECAM even in undifferentiated state.Citation8 Given our interest in lung specific differentiation of embryonic stem cells, we hypothesized that ES-D3 cells provide a valuable source for generating specific epithelial and endothelial linages.Citation15
Integrins are transmembrane glycoproteins that form functional αβ-heterodimeric receptors for either extracellular matrix (ECM) ligands or cell membrane-anchored adhesion melocules.Citation16 Their activation initiates intracellular signaling cascades that regulate numerous cellular functions, including migration, cell proliferation, survival and apoptosis.Citation17 Integrin α5β1 is the primary receptor for the ECM protein fibronectin, which is found on most cells.Citation18 Integrin α5β1 and its fibronectin ligand play critical roles in blood vessel development in mouse embryos and within embryoid bodies (EBs) differentiated from ES cells.Citation19 Therefore studying α5β1 integrin expression in ES-D3 cells may contribute to a mechanistic understanding of the role of ECM interactions in stem cell differentiation.
Disintegrins, a family of polypeptides derived from venoms of a variety of snakes, are valuable tools for investigating the function of integrin receptors. These cysteine-rich molecules contain approximately 40–100 amino acids and are powerful competitive inhibitors of particular integrins.Citation20,Citation21 Depending on the presence of active motifs responsible for integrin binding, disintegrins can be primarily divided into three functional groups: RGD-, MLD- and KTS-containing proteins. RGD-containing disintegrins such as VLO4,Citation20 and Bitisgabonin-1,Citation22 are excellent antagonists of the integrins α5β1, αvβ3 and αIIBβ3 integrins, all of which contain RGD in their functional sequence. Similarly, MLD-containing disintegrins such as EC3,Citation23 are highly specific inhibitors of α4β1, α4β7 and α9β1 integrins and partial inhibitory of α5β1 integrins, while KTS-containing disintegrins such as VP12 and viperistatin block α2β1 and α1β1 integrins, respectively.Citation24 Disintegrins have been used in cell adhesion assay to identify the functional expression of a particular type of integrin on the cell plasma membrane surface,Citation20 but they have not yet been explored in stem cell research.
The aim of the present study was to identify by functional adhesion assays using monoclonal antibodies and disintegrins, the expression of specific integrins on the surfaces of ES-D3 cells that might be in the process of fate decision during ECM-driven differentiation. Our results suggest that α5β1 is the major integrin expressed in undifferentiated mES cells and that its expression is further upregulated by its ligand fibronectin in parallel to increased expression of mesenchymal and endodermal markers.
Results
LIF deprivation increased adhesion of ES-D3 cells grown on fibronectin to anti-α5 selective disintegrin and antibody.
In this first functional assay, we cultured undifferentiated and LIF-deprived mouse ES-D3 cells on gelatin, collagen IV or fibronectin and measured their adhesion to disintegrins known for their relative specificities towards particular α integrin subunits: VLO4 (α5β1), Bitisgabonin-1 (α5β1), EC3 (α4β1, α4β7 and lower affinity for α5β1) and VP12 (α2β1). As seen in , the adhesion of ES-D3 cells grown on fibronectin, but not on gelatin or collagen IV, was increased by 80, 40 and 70%, when plated onto the α5β1-specific disintegrins, VLO4, Bitisgabonin-1 and EC3, respectively, as compared to control (undifferentiated cells grown in presence of LIF). The increased adhesion was not observed when the cells grown on fibronectin were plated on the α2β1-specific disintegrin VP12. These findings suggest that the cells grown on fibronectin express specific a subunits, in particular α5, as recognized by some of these disintegrins.
In order to further characterize these a subunits we measured differential adhesion of undifferentiated (with LIF) and differentiated (without LIF) ES-D3 cells cultured on fibronectin to various anti-a subunits recognizing monoclonal antibodies. Since the fibronectin receptor is composed of α5 and β1 subunits we also investigated the effect of LIF deprivation on the expression of α5 and β1 subunit on ES-D3 cells grown on fibronectin. For comparison we used the adhesion to specific monoclonal antibodies to determine the functional expression of α1, α2, α3, α4, αv and β3 subunits. The data shown in clearly identify α5 and β1 as the major integrin subunits expressed in ES-D3 cells grown on fibronectin. The levels of α1, α2, α3, α4 and αv and β3 subunits were very low and not affected by LIF deprivation of the ES-D3 cells. By contrast, the expression of α5 and β1 in LIF-deprived, differentiating ES-D3 cells is significantly upregulated by almost 90%, as is also suggested from the experiments described in . These data obtained by differential adhesion of the cells, indicate that LIF deprivation specifically upregulates the expression of the α5 integrin subunit.
ES-D3 cells growth on fibronectin selectively upregulates α5 subunit expression.
To further investigate the relationship between ES-D3 cells growth on different integrin ligands as well as the expression of α5, we cultured ES-D3 cells for three days on gelatin, collagen I, IV, VCAM-1 or fibronectin in the presence or absence of LIF and assessed the adhesion to the α5 monoclonal antibody immobilized to the bottom of a 96-well plate. As seen in , the adhesion, and hence by inference the expression of α5, is significantly higher for ES-D3 cells grown on fibronectin (∼12%) than for the cells grown on the other ECM proteins, for which adhesion was less than 5%. By comparing the ES-D3 cells grown on fibronectin in the absence of LIF, to those grown in the presence of LIF, a 90% significant increase (upregulation) in the α5 expression, is evident.
In a complementary approach, we verified the effect of LIF deprivation on integrin expression in cells grown on either fibronectin or gelatin and thereafter tested for adhesion to the respective ECM ligand (). In this approach, the nature of integrin subunit expressed by bound cells is inferred from the selectivity of binding to the respective ECM proteins, collagens I and IV and fibronectin, which were immobilized to the plate (). The adhesion of ES-D3 cells grown in the absence of LIF is expressed as percentage of control (cells grown in the presence of LIF). It is clear that ES-D3 cells grown on fibronectin in the absence of LIF display an increase of 60% in adhesion to immobilized fibronectin. This increased binding was found to be selective to fibronectin and did not occur on collagen I or IV. Cumulatively these findings indicate ligand (fibronectin)-dependent upregulation of α5 in ES-D3 cells upon LIF deprivation induced differentiation.
Since the physiological ECM is more abundant in collagens than fibronectin and due to the fact that type I collagen is most commonly used to generate three-dimensional scaffolds for tissue engineering purposes, we used, unless stated otherwise, a mixture of 10:1 collagen I/fibronectin in the subsequent studies. Under these conditions upregulation of α5 expression is preserved (data not shown).
Fibronectin-induced ES-D3 cell spreading.
When plated on gelatin, in the presence of LIF, ES-D3 cells show a round morphology () and which at low density was maintained for 3 days (); thereafter the cells assembled into large dome shaped colonies adopting the well-established morphology of undifferentiated mES cells. After LIF deprivation, the majority of the cells on gelatin remained round for about 24 h; after 2–3 days however they began to extend filipodia (data not shown). In contrast, when plated on fibronectin, in the presence of LIF, the ES-D3 cells tended to rapidly flatten out and spread within 6 h, producing long processes (data not shown). Plating ES-D3 cells in the absence of LIF on fibronectin coated surfaces increased the proportion of spread cells in the first 6 h (), suggesting that in the wake of LIF deprivation, differentiation-induced spreading is increased by fibronectin as compared to gelatin. After 3 days of growth in the absence of LIF, the cells plated on fibronectin are characterized by a monolayer configuration with many cells exhibiting an elongated shape (). Indeed, when comparing the cell spreading on fibronectin and gelatin in the absence of LIF (), we observed significantly more spreading on fibronectin at 6, 18 and 24 h than on gelatin, suggesting α5 involvement in this process. In support of this notion, cell spreading on fibronectin was significantly inhibited by the continuous presence of 2 µg VLO4 in the culture medium (data not shown).
The effects of fibronectin on the expression of stemness and differentiation markers.
Analysis by qPCR suggests that LIF deprivation of ES-D3 cells grown on fibronectin for three days induced a change in the phenotype of the ES-D3 cells as inferred from the disappearance of the stem cell pluripotency markers Oct 3/4 ( and insert) and SSEA-1 (data not shown). In the absence of LIF the differentiated cells exhibited increased expression of diverse meso-endodermal markers such as FoxA2 (endoderm), cytokeratin (epithelial), α-SMA (smooth muscle) as well as of endothelial markers vimentin and isolectin B4 as measured by immunostaining (). The expression of these differentiation markers is lacking in the undifferentiated ES-D3 cells ( and inserts). The expression levels of Flk-1 (VEGFR-2) an endothelial marker, measured by immunoprecipitation followed by western blotting, was similar in undifferentiated and differentiated cells (). FACS analysis further confirmed its expression in the differentiated ES-D3 cells (). These findings support the concept that VEGFR-2 in ES-D3 cells is constitutivley expressed, independent of the differentiation status of the cells.Citation8
The time course of gene expression levels of the pluripotency marker Oct 3/4 and of the meso-endodermal lineage markers FoxA2 and Sox17 in LIF-dperived ES-D3 cells grown on fibronectin was assessed by qRT-PCR and immunostaining. As seen in , during the eight-day culture period, mRNA expression of FoxA2 and Sox17 significantly increased over time by up to 20- and 45-fold, respectively. Concomitantly and as expected, the pluripotency marker Oct 3/4 significantly decreased, during this differentiation process ( and insert). shows the co-expression of FoxA2 and Sox17 transcription factors in differentiated ES-D3 cells, which acquired a more endodermal morphology (, phase contrast). Taken together our data cumulatively indicate that following LIF deprivation, culture on fibronectin enhances ES-D3 cell differentiation, presumably though upregulation of integrin α5 expression.
Discussion
In this study we describe an increased adhesion process to the ECM protein fibronectin, mediated by α5 integrin, for differentiated murine ES cells. It is known that depriving ES-D3 stem cells of LIF, an essential cytokine that preserves the pluripotent phenotype of the stem cells,Citation2 will initiate ES cell differentiation.Citation32 In our study incipient ES cell differentiation was inferred from a strong reduction in the expression of the stemness markers Oct 3/4 with concomitant enhanced expression of the endothelial/mesenchymal markers, vimentin and isolectin B4, as previously reported.Citation33,Citation34 However, in our hands, the cells also initiated differentiation towards the endodermal lineage suggesting either the coexistence of diverse populations of cells of different germ layersCitation35 or co-expression of markers of more than one lineage in the same cells. The novel finding in this research is that integrin α5 is a crucial component of this differentiative process involves, as inferred from the fact that the initial culture of LIF-deprived ES-D3 cells on fibronectin increases the expression of α5 integrin in these cells concomitant with an increased adhesion to α5 ligands, such as fibronectin. This functional upregulation was not observed when the cells were cultured on other extracellular matrix proteins ( and ). Our notion of a positive feedback mechanism is strongly supported by the three different experimental approaches to measure α5 subunit expression, using: (1) a monoclonal antibody selective for α5, (2) fibronectin, as a preferred ligand of α5β1 integrin and (3) VLO4, and Bitisgabonin-1, two relative selective disintegrins as antagonistic tools towards α5β1 integrin. VLO4 is a homodimeric disintegrin that inhibits α5β1 integrin-mediated adhesion of cells to fibronectin with an IC50 of 3.5 nM.Citation21 This indicates high specific and potent inhibition of the α5β1 integrin. The disintegrin adhesion assays clearly showed that the RGD-disintegrins used were the only ones to inhibit ES-D3 adhesion. Since we were unable to detect neither αvβ3 nor the collagen receptors α1β1 or α2β1 we conclude that that the cells were mainly adhering to matrices via α5β1 integrin. Also, the cells did not bind to immobilized VCAM-1, which is the ligand of α4β1. Interestingly, upregulation of integrin α5 could not be reproduced by treating the cells grown on gelatin in the absence or presence of LIF with 10 µg/ml soluble fibronectin (data not shown), indicating the requirement of its immobilization in order to induce this effect. These findings suggest involvement of other recognition motifs present in the fibronectin central cell-binding domain.Citation36 However, we cannot completely exclude the expression of some other fibronectin- or laminin-binding integrin subunits in undifferentiated cells,Citation37 which subsequently may disappear with mES differentiation on fibronectin. Garcia et al. reported that adsorption of fibronectin on collagen results in conformational changes of fibronectin that lead to differences in binding to α5β1 integrin, switching between cell proliferation and differentiation.Citation38 Their study demonstrates that the conformation of the ECM ligand, like the conformation of the integrin receptor, can be modified to regulate the integrin-ligand interaction and integrin-mediated signaling. This notion may be particularly important in the case of fibronectin because of the large variety of processes that this matricellular protein controls, its widespread expression in different tissues, and its ability to associate with a variety of other extracellular molecules.
In this study we also established a causal relationship between the fibronectin-induced increased in ES-D3 adhesion and cell spreading as previously reported for bonafide endothelial cellsCitation39 and fibroblasts.Citation40 The process of spreading on fibronectin occurred in the first 24 h after plating of the cells ( and ) in correlation with the increased integrin α5 expression. In the case of stem cell differentiation, control of α5β1-fibronectin expression levels and signaling through substrate-dependent conformational changes in the ECM represents a versatile approach to manipulate cellular responses for tissue engineering applications.
The α5 integrin is particularly important for mesodermal development, and plays a major role in vascular formation.Citation41 Both α5β1 integrin and its ligand fibronectin are critical for vascular development emphasizing the importance of studying this integrin in embryos as well as embryonic stem cells.Citation19 Integrin α5β1 is upregulated in migrating endothelial cells as well as in cells under shear stress and in tumors secreting fibronectin.Citation42 It is tempting to suggest that the upregulation of the α5 integrin subunit represents a very early signal in the commitment of progenitor cells towards meso-endodermal,Citation43 specifically endothelial lineage and that this effect facilitates the migration/spreading abilities of the cells required for organization into capillaries. In mouse embryos lacking α5 integrin, greatly distended blood vessels are seen in the vitelline yolk sac and in the embryo itself.Citation44 Additionally, overall blood vessel pattern complexity is reduced in α5-null tissues.Citation45 This defective vascular phenotype is correlated with a decrease in the ligand for α5β1 integrin, fibronectin, in the endothelial basement membranes. Normal vascular phenotype could be partially restored to fibronectin-null mice by the addition of whole fibronectin to the culture system. These findings establish a clear role for α5β1 and fibronectin in early blood vessel development.Citation19 Our finding of an increased adhesion and α5 upregulation in ES-D3 cells deprived of LIF and grown on fibronectin may mimic an important early event in the physiology of the differentiating endothelial progenitors, which requires further investigation.
Mouse embryonic stem cells, such as ES-D3, provide a useful tool for developing regenerative cardiovascular tissue engineering strategies.Citation13 In addition to creating cardiac grafts, an as yet largely elusive goal of regenerative engineering is to generate vascular grafts in which the luminal lining is comprised of stem cells derived endothelial monolayer. Matrix enhanced directed ES differentiation not only tries to emulate the embryonic development but also allows accelerated ES differentiation on ECM proteins such as collagen/fibronectin that are frequently critical components of tissue engineered grafts. To that end, our results that show fibronectin induced α5β1 integrin upregulation in ES-D3 stem cells may propose the design of vascular graft scaffolds containing fibronectin and adherent ES-D3 cells. Currently experiments are underway to further enhance this process while driving a more homogeneous differentiation of ES-D3 cells towards endothelial phenotype.
Materials and Methods
Materials.
Antibodies against markers of ES stemness and differentiation, anti-Oct-4, anti-Sox17 and anti-Flk-1 were from Santa Cruz Biotechnology, Santa Cruz, CA. The fluorescein-conjugated Griffonia simplicifolia lectin I/isolectin B4 (murine endothelial cell marker)Citation25 was purchased from Vectors laboratories, Burlingham, CA. The integrin antibodies anti-α1 (CD49a), anti-α2 (CD49b), anti-α4 (CD49d), anti-α5 (CD49e), anti-αv (CD51), and anti-β3 (CD61) were purchased from BD Pharmingen (San Diego, CA). The anti-β1 integrin (CD29) antibody was obtained from Chemicon International (Temecula, CA). The following cell culture reagents were all purchased from Mediatech, Inc., (Herndon, VA): Dulbecco's modified Eagle's medium (DMEM), antibacterial solution (10,000 I.U/ml penicillin, 10,000 µg/ml streptomycin), L-glutamine (200 mM-29.20 mg/ml), Trypsin/EDTA solution (0.25%/2.21 mM), Dulbecco's Phosphate Buffered Salt Solution (10x) and tissue-culture grade distilled water were all purchased from Mediatech, Inc. (Herndon, VA). Fetal bovine serum (FBS)Citation26 and Hank's Balanced Salt Solution (HBSS) with calcium and magnesium was obtained from Hyclone (Logan, UT). β-mercaptoethanol and ESGRO-murine Leukemia Inhibitory Factor (LIF) were purchased from Chemicon International (Temecula, CA). ES cells were cultured in standard tissue culture-treated 25 cm2 polystyrene Falcon flasks (BD, Franklin Lakes, NJ). For adhesion assays, 96-well polystyrene EIA/RIA plates made of polystyrene were obtained from Corning Inc. (Corning, NY). Bovine serum albumin (BSA) fraction V was purchased from Sigma Aldrich (St. Louis, MO). CMFDA (5-choloromethylfluorescin diacetate), a cell tracker dye used to fluorescently label cells was obtained from Invitrogen (San Diego, CA).
Disintegrins.
Disintegrins were isolated from different snake venoms by HPLC, as previously described.Citation23,Citation27 In brief: the crude venoms were dissolved in 0.1% trifluoroacetic acid (TFA), solid precipitate was removed after centrifugation (5,000 rpm for 5 min). The clear supernatant was separated by reverse-phase HPLC (High-Performance Liquid Chromatography) on a Vydac C-18 (250 mm × 10 mm) column (Vydac, Hesperia, CA). 10 ml fractions were collected manually, dried in a Speed-Vac system and dissolved in water. The disintegrin fraction was identified by adhesion inhibition of cells overexpressing a specific α integrin subunit.Citation23 The purity was checked by electrophoresis, amino acid analysis and mass spectroscopy.Citation27
Cell culture.
Murine embryonic stem (mES) cells (line D3, ES-D3), obtained from ATTC (Manassas, VA), were cultured in an incubator at 37°C, high humidity, 5% CO2 as previously described,Citation28 with the following modifications: the undifferentiated cells were initially maintained without a fibroblast feeder in BD Falcon T-25 tissue culture flaks coated with 0.1% gelatin (Millipore, Billerica, MA) in ES-maintenance medium (ES-MM) consisting of DMEM medium supplemented with 4.5 g/L glucose, 10% FBS, 103 U/ml LIF (ESGRO, Millipore), 0.5% penicillin/streptomycin (100 U/ml, Cellgro, Manassas, VA), 2 mM L-glutamine (Invitrogen) and 0.1 mM β-mercaptoethanol (Gibco). Medium was changed daily. Cells were split every 2–3 days at 80% confluency by gentle trypsinization with 0.025% trypsin, 2.2 mM EDTA in HBSS without calcium, magnesium and sodium bicarbonate. The cells were plated at a density of about 28,000 cells/cm2.
Differentiation was initiated by two methods. Method I: The cells at 80% confluency were detached by gentle trypsinization with 0.025% trypsin, 2.2 mM EDTA in HBSS without calcium, magnesium and sodium bicarbonate. The cells were then plated at a density of 1 million cells/ml on T-25 flasks coated with either fibronectin (10 µg/ml), type IV collagen (30–50 µg/ml) or type I collagen (30 µg/ml) and cultured without LIF in aMEM (GIBCO) containing 10% FBS, 0.5% penicillin/streptomycin (100 U/ml), and 10 µM β-mercaptoethanol (Sigma). After 3–4 days the cells were harvested non-enzymatically and used for the adhesion assays.
Method II: 24 h prior to the initiation of differentiation, ES-D3 cells at about 60% confluency in ES-MM were switched to a serum replacement medium (SR-MM) containing Knockout-DMEM (Invitrogen) supplemented with 4.5 g/L glucose, 15% knockout serum replacement, 0.5% penicillin/streptomycin (100 U/ml), 0.1 mM non-essential amino acids (Invitrogen), 10 µM β-mercaptoethanol (Sigma) and 103 U/ml LIF. To induce differentiation, the cells were first detached by 2 ml of Accutase (Invitrogen) solution for 2 min then plated at a density of 10,000 cells/well in a 12-well tissue culture plate coated with a protein mixture of 10:1 collagen I (0.1 mg/ml) and fibronectin (0.01 mg/ml) and cultured in the absence of LIF in DMEM supplemented with 4.5 g/L glucose, 0.5% penicillin/streptomycin (100 U/ml), 0.1 mM β-mercaptoethanol and 5% FBS. Differentiating ES cells were maintained for 6–8 days and medium was changed at 24–48 h.
Cell spreading assay.
ES-D3 cells grown on 0.1% gelatin were detached by trypsin and collected, as above. Aliquots of 50,000 cells were seeded on six-well dishes coated with different ECMs proteins. Microphotographs of the cultures were taken at different time points with a digital camera and images were stored. Fields containing approximately 400 cells in each image and at each time point were evaluated, and the number of round, flattened, and spread cells was counted. The percentage of spread cells is defined as the ratio of spread elongated cells to the total number of cells in the field ×100.
Adhesion assay.
Adhesion assays were carried out essentially as described previously with minor changes.Citation29 Before the day of the experiment, each well of a 96-well plate (Corning Inc., Costar) was coated with 10 µg/ml monoclonal integrin antibody or 20 µg/ml disintegrin dissolved in 1x phosphate buffered saline (PBS) and incubated overnight at 4°C. Thereafter non-specific binding was blocked by incubating the wells with 1% (w/v) bovine serum albumin (BSA) in Hank's Balanced Salt Solution (HBSS) at room temperature for 1 h prior to use. ES-D3 cells at 70% confluency were non-enzymatically dislodged by 5 mM EDTA in HBSS without calcium, magnesium and sodium bicarbonate for 20 min. The cells (11 × 106 cells/ml) were washed in HBSS to remove trace quantities of EDTA and resuspended in 5 ml HBSS. The cells were incubated at 37°C for 30 min with 12.5 µM CMFDA (5-choloromethylfluorescein-diacetate) cell-tracker dye. The labeled cells were then centrifuged at 1,000 rpm and washed twice with HBSS containing 1% BSA to remove excess CMFDA. Then, 100 µl of the cell suspension was added to each well (1.1 × 105 cells/well) and the plate was incubated at 37°C for 30 min. The wells were gently washed three times with 1% (w/v) BSA in HBSS to remove unattached or loosely bound cells. Cells that had firmly adhered to the wells were lysed by the addition of 0.5% Triton X-100 (diluted in HBSS). The amount of fluorescence in each well was quantified with a FLx800 Fluorescence plate reader (Bio-Tek, Winooski, VT) at λex = 485 nm and λem = 530 nm. To determine the number of adhered cells from the fluorescence values, a standard curve was generated by serial dilutions of known numbers of CMFDA-labeled ES-D3 cells.Citation20
Inhibition assay.
Inhibition assays were essentially the same as the adhesion assays, except that they were performed in the presence of various disintegrins. In brief, 96-well plates were coated with human dermal fibronectin (Sigma-Aldrich, St. Louis, MO) or bovine placental collagen IV (Chemicon International, Temecula, CA) at concentrations of 1 µg/well overnight at 4°C. On the day of experiment, the coating solution was aspirated and non-specific binding was reduced by incubation with 1% BSA in HBSS for 1 h. The cells were gently harvested non-enzymatically, washed with HBSS and labeled with CMFDA dye, as above. The disintegrins were dissolved in HBSS at a concentration of 2 µg protein/well and incubated with cells at room temperature for 20–25 min, while ensuring that the final concentration of cells was kept constant at 100,000/well. As a control for this experiment, the same number of cells was allowed to bind in parallel to ECM protein-coated wells in absence of the disintegrins.
Microscopy.
Phase contrast images were obtained using an inverted Nikon TE 2000 U microscope and QImaging CCD camera. Fluorescent images were obtained using a Leica DMRX microscope and Leica DC300F CCD camera using the appropriate excitation and emission filter set combinations to measure Alexa 488 and Alexa 594, DAPI and rhodamine fluorescence.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR).
Total RNA was isolated using Trizol LS reagent (Invitrogen) according to the manufacturer's instructions. RNA gels were run using 2% agarose (RNAse free) to ensure that RNA was intact prior to complementary (cDNA) synthesis reaction. RNA concentration was determined using NanoDrop 3300 Fluorospectrometer with ND-3300 software (Thermo Scientific, Wilmington, DE). 5 µg of total RNA was then reverse-transcribed into cDNA with random primers using the high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Quantitative PCR (qPCR) analysis of cDNA was performed in a StepOne Real-Time PCR System with fast thermal cycling (Applied Biosystems) using the following Taqman inventoried primers and probes (Applied Biosystems): Oct 3/4 (Assay ID: Mm03053917_g1), FoxA2 (Assay ID: Mm00839704_mH) and Sox17 (Assay ID: Mm00488363_m1). Reactions were performed in triplicate and repeated independently at least three times. Gene expression levels were normalized to an endogenous gene PPIA (Assay ID: Mm02342430_g1) and relative expression levels or fold changes compared to those of undifferentiated ES cells (day 0 control) were quantified using the comparative CT method (2−ΔΔCT), as detailed in the user bulletin # 2 of the Applied Biosystems documentation.
Flow cytometry.
Flow cytometry was used to quantify the fraction of cells expressing a particular marker protein, as previously described.Citation30 Briefly, the cells grown on 10 µg/ml fibronectin for three days in absence of LIF (differentiation, Method I) were harvested non-enzymatically counted in a hemocytometer, washed in 1X PBS without calcium and magnesium and lighty fixed in suspension with 4% paraformaldehyde for 15 min at room temperature. Then the cells were permeabilized in ice-cold methanol for 20–30 min, rinsed with 1X PBS and pelleted by centrifugation at 800 rpm in a Heraeus centrifuge. After a second wash, the cells were suspended in 200 µl of 1X PBS, loaded in individual wells of a 96-well plate and centrifuged again at 800 rpm for 10 min. The cells were then resuspended in 4% FBS blocking solution for 10 min on ice. After another centrifugation, the cells were incubated with anti-Flk-1 primary antibody (1:50 dilution) for 1 h on ice. This was followed by three washes and incubation with anti-rabbit or anti-mouse secondary antibody (1:2,000) on ice for 30 min on ice. The optimal antibody dilutions were determined in preliminary studies by titration. Finally the cells were washed again and suspended in 200 µl of FBS solution; cell associated fluorescence was measured in a Canto flow cytometer (Beckton Dickinson, San Jose, CA) using a CellQuest software.
Immunoprecipitation and western blotting.
ES-D3 cells were washed two times with PBS on ice. The cells were then scraped in 1 ml of RIPA extraction buffer (1% NP-40 lysis buffer, 150 mM NaCl, 50 mM Tris pH 7.5, 1% Nonidet P-40, 2 mM EDTA, 50 µg/mL leupeptin, 10 µg/mL aprotinin, 200 µM sodium orthovanadate, 4 mg/mL p-nitrophenyl phosphate). The scraped cells were briefly vortexed and then incubated on ice for 10 min. Aliquots of the cell extracts for immunoprecipitation were pre-cleared using 10 µl packed Protein A/G (1:1) (Upstate Biotechnology/Millipore, Billerica, MA) and mixing on a rotator at 4°C for 30 min. The extracts were then centrifuged at 14,000 rpm in a cold microcentrifuge for 15 min and the pre-cleared cell extract supernatant was collected. Immunoprecipitations were performed with 3 µg mouse monoclonal antibody anti Flk-1 (SC-6251, Santa Cruz Biotechnology, Santa Cruz, CA) using 1 mg (the protein content was determined using the Bradford microassayCitation31) protein lysate followed by antigen-antibody complex isolation with Protein A/G agarose beads. Immunoprecipitation was carried out in a rotator at 4°C for 2 h. The immune complexes were washed three times with 1% NP-40 lysis buffer. Samples for western blotting were boiled for 5 min in 1x Laemmli's sample buffer, cooled and centrifuged. The supernatant was collected, and resolved on 7.5% SDS-PAGE. The proteins were then transferred to Immobilon membranes and incubated with the rabbit polyclonal antibody anti Flk-1 (SC-315, Santa Cruz Biotechnology) at a dilution of 1:500. The membranes were washed and then incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Specific antibody binding was detected by enhanced chemiluminescence (ECL) (Pierce, Rockford, IL). Densitometric analysis of the films was performed using the Quantity One 1-D Analysis Software program, 2003 version (Bio-Rad, CA).
Immunostaining.
In addition to analysis by flow cytometry and western blotting, cell-associated markers for pluripotency, early and late differentiation were also visualized by immunostaining, essentially as previously described.Citation15 In brief, the cells were washed twice in 1X PBS, fixed in 4% paraformaldehyde for 15 min at room temperature, and washed twice more with 1x PBS. The samples were permeabilized for 15 min in 0.1% Triton in 1x PBS. Non-specific binding was reduced by blocking with 10% chicken serum, 1% BSA, 0.1% Triton in 1X PBS (blocking solution) for 1 h at room temperature followed by incubation with primary antibodies overnight at 4°C. Primary antibodies were FoxA2 (1:100, Abcam, Cambridge, MA), Oct4 (1:100, Abcam), Vimentin (1:20, Abcam), alpha-Smooth muscle actin (1:100, Abcam) and wide-spectrum Cytokeratin (1:100, Dako, Carpinteria, CA). As a negative control, the primary antibodies were omitted or species-specific IgG (1:100) was used to assess nonspecific binding. Cells were then washed three times for 10 min each before incubation for 1 h at room temperature in the dark with a secondary antibody labeled with either Alexa 488 (1:1,000) or Alexa 594 (1:1,000). All primary and secondary antibodies were diluted in the blocking solution. The samples were washed again three times for 15 min each and mounted with Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI, Vector, Burlingame, CA) as a nuclear counterstain. All fluorescent images were acquired using a Leica DMRX upright microscope equipped with a DFC 300F CCD camera (Leica) with Leica imaging software and processed using Adobe Photoshop CS5 and Adobe Illustrator CS5 (Adobe Systems, San Jose, CA).
Statistics.
Where appropriate, all data are expressed as mean ± SEM (n = 6–16) from three to six independent experiments. Comparisons between groups were analyzed by Student's t-test or one-way analysis of variance (Microsoft Office Excel, 2003) to determine significant differences. p values less than 0.05 were considered statistically significant.
Abbreviations
| bitisgabonin-1 | = | Bitis gabonicus disintegrin |
| EC3 | = | Echis carinatus disintegrin 3 |
| ECM | = | extracellular matrix protein |
| ES-D3 | = | a mouse embryonic stem cell (mES) D3 line |
| FACS | = | fluorescence activated cell sorter |
| Flk-1 | = | fetal liver kinase |
| VEGFR2 | = | VEGF receptor type-2 |
| FoxA2 | = | forkhead box A2 |
| LIF | = | leukemia inhibitory factor |
| Oct 3/4 | = | octamer-binding protein-3/4 |
| PECAM-1 | = | platelet endothelial cell adhesion molecule-1 |
| qRT-PCR | = | quantitative reverse transcription-polymerase chain reaction |
| RGD | = | arginine-glycine-aspartic acid |
| Sox17 | = | SRY-related high-mobility-group (HMG) box gene 17 |
| SSEA-1 | = | stage specific embryonic antigen-1 |
| VCAM-1 | = | vascular cell adhesion molecule-1 |
| VLO4 | = | Vipera lebetina obtusa disintegrin 4 |
| VP12 | = | Vipera palestinae |
| CLP | = | C-lectin type protein |
Figures and Tables
Figure 1 LIF-deprivation increased adhesion of ES-D3 cells grown on fibronectin to α5 disintegrins and anti-α5 antibody. (A) 1.5 × 106 cells were differentiated on flasks coated with 0.1% gelatin (control), 30 µg/ml collagen IV or 10 µg/ml fibronectin for three days (Method I), harvested with 0.5 mM EDTA, loaded with CMFDA dye and submitted for adhesion assay on plates coated with 2 µg/ml per well of VLO4 (white bar), Bitisgabonin-1 (gray bar), EC3 (light gray bar) and VP12 (black bar). The data represents mean ± SEM of three experiments, each performed in triplicates. *p < 0.05 compared to cells grown on gelatin. (B) 1.5 × 106 cells grown on flasks coated with 10 µg/ml fibronectin were cultured for three days in the presence (undifferentiated) or absence (differentiation, Method I) of LIF. The cells were harvested tested for adhesion to plates coated with the different monoclonal anti-α and anti-β antibodies (10 µg/ml per well). White bars, undifferentiated; gray bars, differentiated. The data represent mean ± SEM of six experiments, each performed in triplicates. *p < 0.05 compared to α1; **p < 0.05 compared to α5; +p < 0.05 compared to β3.
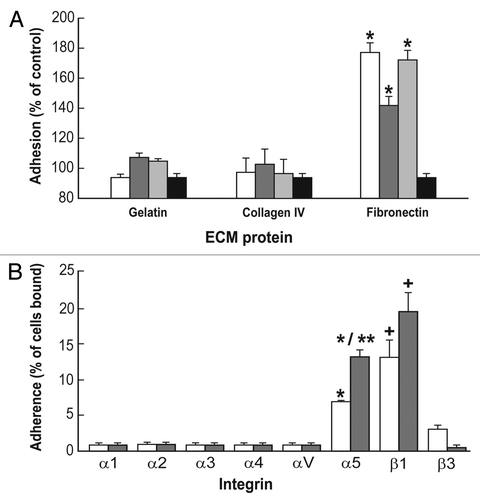
Figure 2 Growth of ES-D3 cells on fibronectin increased adhesion to anti-α5 antibody (A) and fibronectin (B). (A) 1.5 × 106 cells were cultured in flasks coated with, respectively, 0.1% gelatin, 30 µg/ml collagen I and IV, VCAM or 10 µg/ml fibronectin. Either left undifferentiated (white bars) or induced to differentiate by LIF deprivation (Method I, grey bars), the cells were harvested after three days with 0.5 mM EDTA, loaded with CMFDA and assayed for adhesion to anti-α5 antibody. The data represent mean ± SEM of three independent experiments, each performed in triplicates. *p < 0.05 compared to cells grown on gelatin; **p < 0.05 compared to undifferentiated cells grown on fibronectin. (B) The cells previously grown on either 0.1% gelatin (white bars) or 10 µg/ml fibronectin (gray bars) for three days in the absence of LIF (differentiation Method I) were harvested with 0.5 mM EDTA, loaded with CMFDA and assayed for adhesion to 30 µg/ml collagen I, 30 µg/ml collagen IV or 10 µg/ml fibronectin. The data represents mean ± SEM compared to undifferentiated control of three independent experiments, each performed in triplicates. *p < 0.05 compared to cells grown on gelatin and tested on fibronectin.
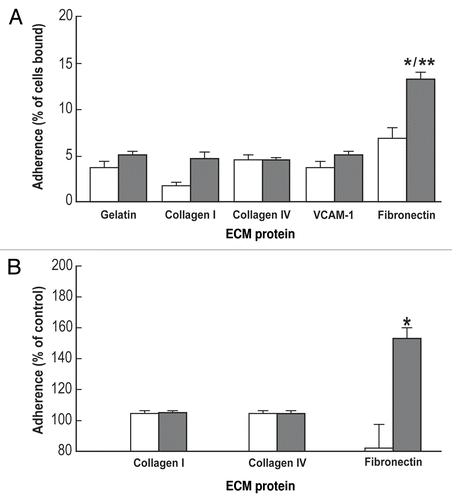
Figure 3 Light micrographs of undifferentiated and differentiated ES-D3 cells. The cells were cultured on 0.1% gelatin (A and B) or 10 µg/ml fibronectin (C and D) for 6 h (A and C) or three days (B and D) in the presence (A and B undifferentiated) or absence (C and D differentiated) of LIF (Method I). The cell morphology was evaluated by phase contrast and typical fields are presented. Bar = 20 µm. Inserts: fluorescence staining of single cells; blue -DAPI, nuclear staining; red, rhodamine-phalloidin staining of the actin cytoskeleton.
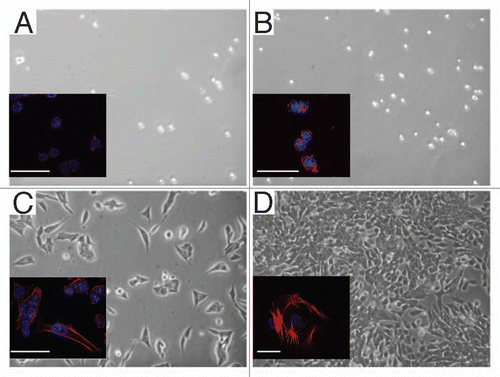
Figure 4 Time course of ES-D3 cells spreading on gelatin or fibronectin. The cells were cultured in the presence or absence of LIF (Method I) on 0.1% gelatin (white bars) or 10 µg/ml fibronectin (gray bars) for 24 h. Every 6 h the cells were photographed. % cell spreading was estimated as the ratio of spread elongated cells out of the total number of cells in the field ×100.
Figure 5 Expression of pluripotency and differentiation markers in ES-D3 cells. The cells were cultured for eight days in the presence (undifferentiated) or absence (differentiated, Method II) of LIF and stained with specific antibodies as indicated in Materials and Methods, or incubated with 10 µg/ml FITC-isolectin B4 (Lectin) as endothelial marker. Inserts: nuclear staining (upper part) and marker staining (lower part) of undifferentiated cells. The cells were analyzed by fluorescent microscopy; typical photographs are presented. Nucleus: the nuclear staining was performed with DAPI (blue). Markers: labeled with primary antibodies followed by appropriate secondary antibodies labeled with Alexa 488 (green) and Alexa 594 (red); Merge: superposition of nuclear and specific marker staining; bars = 50 µm.
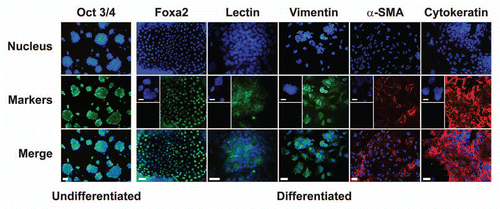
Figure 6 Expression of Flk-1 in ES-D3 cells analyzed by FACS and immunoprecipitation. The cells were grown on 10 µg/ml fibronectin for three days in presence or absence of LIF (Method I). (A) Cell extracts from undifferentiated and differentiated cells were immunoprecipitated by anti-Flk-1 monoclonal antibody and separated by 7.5% SDS-PAGE followed by western blotting with anti-Flk-1 polyclonal antibody. VEGFR-2, the position of mature glycosylated receptor. For experimental details, see Materials and Methods. (B) Flk-1expression assessed by flow cytometry in the differentiated cells. The cells were fixed, permeabilized, stained with anti Flk-1 antibody followed by second antibody (right trace) or stained only with second antibody (left trace, control).
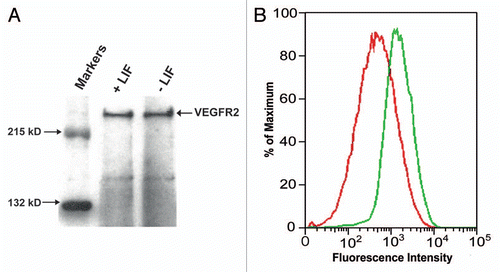
Figure 7 Time course of expression of pluripotency and meso-endodermal markers in ES-D3 cells. (A) mRNA expression levels of pluripotency marker gene Oct3/4 and meso-endodermal marker genes FoxA2 and Sox17 were evaluated in ES-D3 cells differentiated for up to eight days (Method II). Oct3/4 (triangles), FoxA2 (squares), Sox17 (diamonds). Insert: Decreased Oct3/4 mRNA level over time. Data is presented as mean ± SD of three independent experiments. *p < 0.05, **p < 0.01 compared to day 0 control. (B) Transcription factor protein expression in ES-D3 cells differentiated for 8 days (Method II), as determined by immunostaining using anti-Sox17 and anti-FoxA2 antibodies (1:100), nuclear staining by DAPI and morphology evaluated by phase contrast; bars = 50 µm.
Acknowledgements
This study was supported by grants from NTI and Coulter Foundation (P.I.L.), Stein Foundation (P.I.L., P.L.) and Royal Thai government (P.P.). We would like to acknowledge the technical assistance of Anant Chopra, Rosemary Bastian and Anusha Rajan and the editorial assistance of Gregory Botta.
References
- Keller G. Embryonic stem cell differentiation: Emergence of a new era in biology and medicine. Genes Dev 2005; 19:1129 - 1155
- Burdon T, Chambers I, Stracey C, Niwa H, Smith A. Signaling mechanisms regulating self-renewal and differentiation of pluripotent embryonic stem cells. Cells Tissues Organs 1999; 165:131 - 143
- Doyle B, Caplice N. A new source of endothelial progenitor cells—vascular biology redefined?. Trends Biotechnol 2005; 23:444 - 446
- Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: Formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol 1985; 87:27 - 45
- Toumadje A, Kusumoto K, Parton A, Mericko P, Dowell L, Ma G, et al. Pluripotent differentiation in vitro of murine ES-D3 embryonic stem cells. In Vitro Cell Dev Biol Anim 2003; 39:449 - 453
- Karbanova J, Mokry J. Histological and histochemical analysis of embryoid bodies. Acta Histochem 2002; 104:361 - 365
- Powers DE, Millman JR, Huang RB, Colton CK. Effects of oxygen on mouse embryonic stem cell growth, phenotype retention and cellular energetics. Biotechnol Bioeng 2008; 101:241 - 254
- McCloskey KE, Lyons I, Rao RR, Stice SL, Nerem RM. Purified and proliferating endothelial cells derived and expanded in vitro from embryonic stem cells. Endothelium 2003; 10:329 - 336
- Messana JM, Hwang NS, Coburn J, Elisseeff JH, Zhang Z. Size of the embryoid body influences chondrogenesis of mouse embryonic stem cells. J Tissue Eng Regen Med 2008; 2:499 - 506
- Ge J, Guo Y, Wang Z, Liu H, Yang Z, Li Y, et al. Preliminary study on in vitro induced differentiation of embryonic stem cells into neurons. Yan Ke Xue Bao 2000; 16:1 - 6
- Li Y, Kang X, Guo K, Li X, Gao D, Cui J, et al. Proteome alteration of early-stage differentiation of mouse embryonic stem cells into hepatocyte-like cells. Electrophoresis 2009; 30:1431 - 1440
- Francini F, Del Zotto H, Massa ML, Gagliardino JJ. Selective effect of INGAP-PP upon mouse embryonic stem cell differentiation toward islet cells. Regul Pept 2009; 153:43 - 48
- Guo XM, Zhao YS, Chang HX, Wang CY, E LL, Zhang XA, et al. Creation of engineered cardiac tissue in vitro from mouse embryonic stem cells. Circulation 2006; 113:2229 - 2237
- Parashurama N, Nahmias Y, Cho CH, van Poll D, Tilles AW, Berthiaume F, et al. Activin alters the kinetics of endoderm induction in embryonic stem cells cultured on collagen gels. Stem Cells 2008; 26:474 - 484
- Roszell B, Mondrinos MJ, Seaton A, Simons DM, Koutzaki SH, Fong GH, et al. Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. Tissue Eng Part A 2009; 15:3351 - 3365
- Ruoslahti E. Integrins. J Clin Invest 1991; 87:1 - 5
- Giancotti FG, Ruoslahti E. Integrin signaling. Science 1999; 285:1028 - 1032
- Akiyama SK. Integrins in cell adhesion and signaling. Hum Cell 1996; 9:181 - 186
- Francis SE, Goh KL, Hodivala-Dilke K, Bader BL, Stark M, Davidson D, et al. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol 2002; 22:927 - 933
- Calvete JJ, Moreno-Murciano MP, Theakston RD, Kisiel DG, Marcinkiewicz C. Snake venom disintegrins: Novel dimeric disintegrins and structural diversification by disulphide bond engineering. Biochem J 2003; 372:725 - 734
- Marcinkiewicz C. Functional characteristic of snake venom disintegrins: Potential therapeutic implication. Curr Pharm Des 2005; 11:815 - 827
- Calvete JJ, Marcinkiewicz C, Sanz L. Snake venomics of bitis gabonica gabonica. protein family composition, subunit organization of venom toxins and characterization of dimeric disintegrins bitisgabonin-1 and bitisgabonin-2. J Proteome Res 2007; 6:326 - 336
- Marcinkiewicz C, Calvete JJ, Marcinkiewicz MM, Raida M, Vijay-Kumar S, Huang Z, et al. EC3, a novel heterodimeric disintegrin from echis carinatus venom, inhibits alpha4 and alpha5 integrins in an RGD-independent manner. J Biol Chem 1999; 274:12468 - 12473
- Staniszewska I, Walsh EM, Rothman VL, Gaathon A, Tuszynski GP, Calvete JJ, et al. Effect of VP12 and viperistatin on inhibition of collagen-receptor-dependent melanoma metastasis. Cancer Biol Ther 2009; 8:1507 - 1516
- Zhu WH, Nicosia RF. The thin prep rat aortic ring assay: A modified method for the characterization of angiogenesis in whole mounts. Angiogenesis 2002; 5:81 - 86
- Okada M, Oka M, Yoneda Y. Effective culture conditions for the induction of pluripotent stem cells. Biochim Biophys Acta 2010; 1800:956 - 963
- Calvete JJ, Moreno-Murciano MP, Theakston RD, Kisiel DG, Marcinkiewicz C. Snake venom disintegrins: novel dimeric disintegrins and structural diversification by disulphide bond engineering. Biochem J 2003; 372:725 - 734
- Bidez PR 3rd, Li S, Macdiarmid AG, Venancio EC, Wei Y, Lelkes PI. Polyaniline, an electroactive polymer, supports adhesion and proliferation of cardiac myoblasts. J Biomater Sci Polym Ed 2006; 17:199 - 212
- Marcinkiewicz C, Rosenthal LA, Mosser DM, Kunicki TJ, Niewiarowski S. Immunological characterization of eristostatin and echistatin binding sites on alphaIIb beta3 and alphaVbeta3 integrins. Biochem J 1996; 317:817 - 825
- Simons DM, Gardner EM, Lelkes PI. Intact T cell receptor signaling by CD4(+) T cells cultured in the rotating wall-vessel bioreactor. J Cell Biochem 2010; 109:1201 - 1209
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248 - 254
- Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development 1998; 125:1747 - 1757
- Vittet D, Prandini MH, Berthier R, Schweitzer A, Martin-Sisteron H, Uzan G, et al. Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood 1996; 88:3424 - 3431
- Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 2000; 408:92 - 96
- Pasquinelli G, Pacilli A, Alviano F, Foroni L, Ricci F, Valente S, et al. Multidistrict human mesenchymal vascular cells: Pluripotency and stemness characteristics. Cytotherapy 2010; 12:275 - 287
- Truong H, Danen EH. Integrin switching modulates adhesion dynamics and cell migration. Cell Adh Mig 2009; 3:179 - 181
- Hayashi Y, Furue MK, Okamoto T, Ohnuma K, Myoishi Y, Fukuhara Y, et al. Integrins regulate mouse embryonic stem cell self-renewal. Stem Cells 2007; 25:3005 - 3015
- Garcia AJ, Vega MD, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell 1999; 10:785 - 798
- Iuliano DJ, Saavedra SS, Truskey GA. Effect of the conformation and orientation of adsorbed fibronectin on endothelial cell spreading and the strength of adhesion. J Biomed Mater Res 1993; 27:1103 - 1113
- Obara M, Sakuma T, Fujikawa K. The third type III module of human fibronectin mediates cell adhesion and migration. J Biochem 2010; 147:327 - 335
- Hynes RO, Lively JC, McCarty JH, Taverna D, Francis SE, Hodivala-Dilke K, et al. The diverse roles of integrins and their ligands in angiogenesis. Cold Spring Harb Symp Quant Biol 2002; 67:143 - 153
- Serini G, Valdembri D, Bussolino F. Integrins and angiogenesis: A sticky business. Exp Cell Res 2006; 312:651 - 658
- Sun X, Gulyas M, Hjerpe A. Mesothelial differentiation as reflected by differential gene expression. Am J Respir Cell Mol Biol 2004; 30:510 - 518
- Francis SE, Goh KL, Hodivala-Dilke K, Bader BL, Stark M, Davidson D, et al. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol 2002; 22:927 - 933
- Taverna D, Hynes RO. Reduced blood vessel formation and tumor growth in alpha5-integrin-negative teratocarcinomas and embryoid bodies. Cancer Res 2001; 61:5255 - 5261