Abstract
Neuroblasts generated in the adult subventricular zone (SVZ) migrate through the rostral migratory stream (RMS) to the olfactory bulb (OB). Previous work uncovered motility ranging from straight to complex, but it was unclear if directional changes were stochastic or exhibited stereotypical patterns. Here, we provide the first in-depth two-photon time-lapse microscopy study of morphological and dynamic features that accompany turning and direction reversals in the RMS. We identified three specific kinds of turning (30-90 degrees): bending of the leading process proximal to the cell body (P-bending 47% of cases), bending of the distal leading process (D-bending 30%) or branching of the leading process or lamellipodium (23%). Bending and branching angles were remarkably constrained and were significantly different from one another. Cells reversed direction (>90 degrees) through D-bendings (54%), branching (11%) or de novo growth of processes from the soma (23%), but not P-bending. Direction reversal was often composed of several iterations of D-bending or branching as opposed to novel modalities. Individual neuroblasts could turn or change direction in multiple patterns suggesting that the patterns are not specific for different lineages. These findings show that neuroblasts in the RMS use a limited number of distinct and constrained modalities to turn or reverse direction.
Introduction
Adult neurogenesis occurs in two regions of the adult rodent brain: the subventricular zone (SVZ) and the dentate gyrus (DG) of the hippocampus. In the former case, neuroblasts have to migrate 3–8 mm to their final destination, the olfactory bulb (OB). The SVZ contains the main stem cell niche in the mature brain and SVZ stem cells give rise to transit amplifying progenitor cells, which in turn generate migratory neuroblasts.Citation1–Citation3 The neuroblasts then migrate to the OB in a well-defined pathway called the rostral migratory stream (RMS).Citation4 Once in the OB, neuroblasts migrate to the granular and periglomerular layers where they differentiate into a variety of interneurons that integrate into the existing circuitry.Citation5–Citation7
Cell migration is crucial for brain development, and migratory defects are thought to contribute to neurological diseases such as epilepsy and mental retardation.Citation8 Newborn neurons adopt two main migratory strategies during brain development: radial and tangential migration. In radial migration, neurons use radial glia as a scaffold to reach their appropriate location whereas tangential migration seems to be independent of radial glia fibers.Citation9 Cells migrating radially as well as tangentially can occasionally reverse directionsCitation10,Citation11 presumably in order to sample the environment and receive fine-tuned directional information. Neuroblast migration along the adult RMS has been defined as tangential “chain migration” similar to chain migration of neural crest cells during development.Citation12,Citation13 Chain migration refers to longitudinal clusters of neuroblasts that interact with each other. However, the term is somewhat misleading as the movement is not single-file nor in a chain; instead, the cells leap-frog over each other with a significant population remaining stationary at any given time.Citation14 Although migrating SVZ neuroblasts are ensheathed by GFAP+ astrocytes that form glial tubes thought to instruct the migration,Citation15 neuroblasts can migrate along each other in vitro in the absence of glial tubes.Citation16,Citation17
How do SVZ neuroblasts find their way to the OB? A recent study using two-photon time-lapse microscopy in brain slices from postnatal and adult eGFP+ miceCitation14 showed that chain orientation remains stable, indicating that at the population level migration is deterministic. This notion is supported by the long-standing observation that almost all cells born in the SVZ migrate to the OB.Citation4 However, observations of individual cells suggested migration may be probabilistic. Cells displayed a wide range of local behaviors, from straight movements to complex local motility.Citation14 Both types of motility incorporated relatively frequent turns, which ranged from subtle to dramatic. For example, the majority of nestin-eGFP+ cells in the dorsal SVZ and in the RMS moved rostrally (71%) but a significant proportion of cells (29%) exhibited direction reversals resulting in caudal movements.Citation14 Routine changes in direction that varied from a few degrees to complete reversals have been noted in SVZ neuroblasts,Citation18–Citation20 and they exhibited remarkable right angle turns when migrating in the striatum.Citation18 Therefore we were curious whether neuroblasts migrating through the RMS turned with random or stereotypic movements. In this study, we examined whether SVZ cells turned or reversed direction in predictable ways by using stereotypical morphological changes. We previously found that many SVZ cells were highly polarized in the direction of movement with a long leading process and a short trailing process at the opposite pole. We also showed that 44% of cells in the RMS were motile but did not exhibit this classic bipolar morphology, and conversely 19% of cells in the RMS with bipolar migratory morphology were stationary.Citation14 Therefore, we also determined the relationship between the leading process and lamellipodial morphology and directional changes during SVZ neuroblast migration.
Results
Neuroblasts used distinctive patterns for turning and direction reversals.
We analyzed migration in eight movies comprising the vertical limb, elbow and horizontal limb of the RMS ( and ). The vertical limb descends ventrally from the SVZ, the elbow comprises a bend in the RMS just anterior to the lateral ventricle, and the horizontal limb extends rostrally from the elbow into the core of the OB. We never observed significant differences in morphology, turning or direction reversals in these closely spaced subregions. The direction of movement at the population level was what we expected based on the in vivo data.Citation4 All SVZ neuroblasts migrated in a stepwise saltatory manner in which elongation of the leading process is followed by nucleokinesis.Citation14,Citation21,Citation22 A representative example of nucleokinesis is presented in the sequence of frames and schematics shown in and K. The position of the cytoplasmic dilation at the proximal edge of the leading process anticipates where the centrosome and thus the nucleus will translocate next,Citation21 similar to what has been observed in migrating MGE cells.Citation22 For the period in which we had observed nucleokinesis in a quasi-linear trajectory, the average turning angle was 19°. This value is below those observed during the turns and directional reversals described below.
Turning.
We analyzed cells that turned, defined here as changes in direction between 30°–90°. We found three distinctive turning patterns schematized in . Many cells deviated from straight trajectories by bending of the leading process proximal to the cell body. We termed this pattern proximal-bending (abbreviated P-bending). In P-bending ( and ), the cytoplasmic swelling extended further into the leading process than in regular nucleokinesis and exhibited bending. The bending occasionally moved forward some distance accompanied by the dilation but without surpassing the middle point of the leading process, and then the soma translocated to the bending point. Although it sometimes appeared that the cell body had not turned during this pattern, the leading process changed its original axis of orientation. Consequently, the next movement occured in a different direction without bending.
We found a second major pattern of turning termed D-bending (distal-bending) ( and ). In contrast to P-bending, D-bending was not associated with the cytoplasmic dilation and could initially occur at any point of the leading process distal to the soma ( and ). Whereas P-bending was characterized by a single angle that appeared in the cytoplasmic dilation proximal to the cell body and a straight leading process, D-bending was often characterized by multiple shallow angles, thereby conferring to the leading process a curved shape (). Similar to P-bending, the leading process bent in D-bending and the soma translocated to the bending point. Bending at right angles through a similar pattern was previously described for orthogonal turning in SVZ glioblasts.Citation18 Most D-bending (88%) (N = 8 slices, 100 cells, 30 bending observations) occurred through bending of the leading process. A minority of cells (12%) exhibited D-bending at the intersection of the leading process and lamellipodium (). This latter subpattern of directional change has not been previously described and may be analogous to growth cone steering during axon pathfinding.
The third turning behavior was via branch formation, which consisted of de novo growth and subsequent biased selection of the most stable branch (). In about half the cases of branch formation (46%) (N = 8 slices, 100 cells, 25 branching events), the lamellipodium bifurcated and each new branch was tipped with its own lamellipodium (). Inevitably, one of the lamellipodia collapsed and the branch shortened until complete retraction occurred (). Thus, formation of a new lamellipodium by migrating neuroblasts is another way in which to change direction. Some cells (25%) formed side branches at the leading process for turning (), and others formed somal branches (8%) or multiple types of branches (8%). Somal branching was different from polarity reversal (see below) in that the new branch did not emanate from the soma opposite to the pre-existing leading process but, rather, near its proximal base, and the cell did not reverse direction.
We next quantified the different morphological patterns underlying turning in RMS cell migration. Amongst neuroblasts that turned without reversing direction, P-bending occurred most frequently (47% ± 6), then turning via D-bending (30% ± 6) and branching (23% ± 5) (). The predominant frequency of P-bending and its cyclic nature (see below) suggest that it is probably the main mechanism for reorientation along the migratory pathway.
Dynamic observation of migrating neuroblasts also revealed that these patterns did not necessarily result in a changed direction. Sometimes P-bending occurred in a sequence that conferred a zigzag or sigmoid appearance to the cell movement. In such cases there was no net change in direction but a displacement of the cell body along a straight axis of movement. In addition, we noticed that branching of the leading process and bifurcation at the lamellipodium could occur transiently without a subsequent change in direction. The same applied to growth of a new process from the soma at the opposite pole of the pre-existing leading process (see below). We did not observe obvious differences in any of the migration parameters described above between the vertical and horizontal limbs or elbow of the RMS.
Direction reversals.
Many cells exhibited distinctive direction reversals, switching from rostral to caudal migration (shifts in direction of 90 to 180°, see Materials and Methods). Similar to previous observations,Citation14 we observed this event in 23% of migrating neuroblasts (N = 8 slices, 100 cells). We found at least three different patterns for direction reversal (DR): DR-bending, DR-branching and polarity reversal (). Unexpectedly, we never observed P-bending in direction reversals.
About half the cells (54% ± 15) that reversed direction did so through sequential D-bending of the leading process (, and ). A variant of this pattern was based on two consecutive bendings at right angles of the leading process. Polarity reversal was the next most common form of direction reversal (23% ± 12) (, and ). It was defined by the formation of a single new process from the soma opposite the pre-existing leading process. Similar to previous observations,Citation14 we showed that as the new process elongated, the pre-existing leading process retracted. The new process persisted and sent the cell in the opposite direction. Only cells that reversed direction exhibited polarity reversal. Finally, a pattern for direction reversal used by a minority of neuroblasts migrating in the RMS was via branching (11% ± 5) (, and ). Branches emanated exclusively from the lamellipodium at the same time that the leading process turned radially to come in close apposition to the cell body. Next, a branch that formed at right angles with respect to the leading process and pointing backwards became dominant (ex. , time point 00:57). Once the rest of the branches had withdrawn, the soma translocated to the branching point poising it for backward migration. A similar scenario for reversing direction has been described for granule cerebellar cells,Citation20 but not for SVZ neuroblasts.
Duration of behaviors.
We next calculated the length of time taken for each pattern of turning and reversing direction (N = 8 slices, 100 cells, 132 observations) and found statistical differences [One-way ANOVA, F(5,126) = 12.58, p < 0.05, ]. Within turning behaviors, D-bending (17.8 ± 1.5 min) and branching (17.5 ± 2.1 min) lasted longer than P-bending (11.7 ± 0.7 min). However, only the difference between D-bending and P-bending was statistically significant. Both D-bending (31.8 ± 4.1 min) and branching (24.0 ± 2.7 min) lasted longer in cells that reversed direction than in those that did not, suggesting that direction reversals are serial iterations of either branching or D-bending rather than a novel mechanism. Turning by D-bending took significantly less time than D-bending during direction reversal (). In cells reversing direction, polarity reversal (13.3 ± 2.7 min) took the least time and was significantly faster than the other two patterns of reversal direction. Turning by D-bending took significantly less time than D-bending during direction reversal ().
Individual neuroblasts could exhibit multiple patterns of direction changes.
A subset of migrating neuroblasts (25%) (N = 8 slices, 100 cells) underwent several directional changes via two or three different patterns (). There did not seem to be restrictions to the combinations of turning and direction reversals; they could occur in any order and could be spaced at a variety of time intervals. The three turning patterns combined with each other and with reversing direction via D-bending (which is the most common pattern for direction reversal). The combination with the highest percentage (24%) was P-bending with branching. This was unanticipated, as we expected P-bending and D-bending to cluster. However, in the category with the second highest percentage (16%), P-bending and reversing direction through D-bending clustered together. Additionally, we identified cells in which the three turning patterns appeared together (8%) or two turning behaviors in association with direction reversal via D-bending (4%). A subset of neuroblasts exhibited several turnings by iterations of the same pattern (13%) (N = 8 brain slices, 100 cells). For most of these (77%), the repeated pattern was P-bending, whereas repeated branching accounted for the remaining cells.
Morphological angles of turning cells are constrained.
After having identified morphologically distinct patterns for turning in migrating neuroblasts, we wondered if these patterns could be established in terms of the angle formed by the leading process and lamellipodium (). For the definition of angle trajectory and how it was measured in 3D, please consult the Materials and Methods section. We measured angles in the three patterns for turning and remarkably found that in each case, the angles did not span across a broad range but were instead restricted. The angle during P-bending was 43° ± 2 (n = 22), of D-bending was 52° ± 6 (n = 8) and of branching was 79° ± 5 (n = 16). The differences were statistically significant between the branching angle and either D-bending (Mann-Whitney U test, p = 0.002) or P-bending angles (Mann-Whitney U test, p < 0.0005) ().
To further examine the different variations in branching behavior, we compared the branching angle during bifurcation of the lamellipodium (79° ± 5; n = 16) and with side branch formation (82° ± 10, n = 6), but they were not statistically different (Mann-Whitney U test, p = 0.606). Finally we wanted to test if there was a significant difference in branching and D-bending angles during reversing direction as compared to turning. Branching during direction reversal (86° ± 5, n = 3) showed no significant differences (Mann-Whitney U test, p = 0.576) with respect to branching for turning (79° ± 5; n = 16). Similarly, we found no significant differences (Mann-Whitney U test, p = 0.247) between the D-bending angle during reversing direction (67° ± 9; n = 7) or during turning (52° ± 6; n = 8). These data support the idea that bending and branching represent fundamental behaviors underlying both turning and direction reversal.
Relationships between turning angle, speed and complexity of movement.
Neuroblasts migrating in the RMS exhibit different local motile behaviors.Citation14 These differences in directionality can be classified using the migratory index (MI) which is the ratio of the net distance between the original and final points and the total distance traveled by the cell.Citation14,Citation23 A subset of neuroblasts called “migratory” cells had a high MI and moved in a relatively straight directed fashion, whereas “exploratory” cells had a low MI and moved more locally and randomly. Here we further differentiated these two subpopulations in terms of their turning angles. We calculated the turning angle ϑk (N = 3 slices, 39 cells; ) for migratory, intermediate and exploratory cells for 14–40 frames. The mixed population showed a Gaussian distribution with an average angle of 80° ± 1 (). The average turning angle for migratory cells was 70° ± 2 (), for intermediate cells 83° ± 3 () and for exploratory cells 94° ± 2 (). These values are similar to those obtained in the SVZ (Szele lab, unpublished observations).
We then calculated changes in speed over time and examined if there was an association between turning angles and speeds at each step. A bivariate correlation analysis revealed the existence of a negative correlation between these two parameters in migratory (Pearson correlation coefficient = −0.35, p < 0.0005, ), intermediate (Pearson correlation coefficient = −0.24, p = 0.001, ) and exploratory cells (Pearson correlation coefficient = −0.20, p < 0.0005, ).
To examine angles in more detail and to compare them with overall turning and direction reversal behaviors, we generated representative 3D plots () that uncovered interesting examples of cell dynamics during both behaviors. In the value observed for D-bending was 115° and in the average turning angle for P-bending was 94°. shows an angle for reversing direction via D-bending was 142° and via polarity reversal of 76°. shows the one case of direction reversal via branching. Interestingly, the orthogonal turning occurred in two steps of ϑ19 = 50° and ϑ20 = 45°. By adding up these two steps the neuroblast performs an overall turning angle of 95°, which roughly corresponds to the orthogonal turning observed in the two-photon time-lapse movies. This example illustrates how the branch geometry may relate to the turning angle during a directional change. We also measured the P-bending and D-bending angles of the leading process and the corresponding turning angle. A D-bending angle of 68° was associated with a direction reversal of 115° () and a P-bending angle of 31° resulted in an 88° turning (). Finally, we measured the D-bending angle at the leading process for direction reversals ( and E). A D-bending of 55° corresponded to a direction reversal of 142° (). This suggests that bending geometry is not different for turning and reversing direction and reinforces the idea that the latter may occur by a combination of D-bending steps.
Discussion
In this study we sought to determine the extent of variation in turning behaviors of neuroblasts migrating in the RMS. We identified six stereotypic patterns by which neuroblasts either turn or reverse direction. P-bending and polarity reversal were exclusively observed in turning and direction reversal, respectively. D-bending and branching on the other hand could result in either turning or in direction reversals. P-bending was the most common pattern for turning and often mediated the smooth direction changes that predominate in migratory neuroblasts. In cells that turned via branching without reversing direction we observed that branching of the lamellipodium occurred in about half the cases. As the lamellipodium possesses a high concentration of receptors, we speculate that this bifurcation may serve to increase the accuracy in sensing the environment. We report for the first time curving of the lamellipodium, which could be analogous to growth cone steering in extending axons. We also provide evidence for other turning behaviors that involve the leading process including D-bending and branching. With regard to direction reversals, the present work not only confirms previous observations that suggest mechanisms based on curving of the leading process and new process formationCitation14 but it also describes an additional pattern via branching.
We analyzed two-photon time-lapse movies from a transgenic mouse line that expresses enhanced green fluorescent protein (eGFP) specified to neural tissue by the second intronic enhancer of the nestin promoter. Interestingly, nestin expression is found in SVZ GFAP+ stem cells, transit amplifying progenitors and neuroblasts;Citation14 however, we showed previously that only neuroblasts are motile,Citation23 thus the cells examined in this study were migratory neuroblasts. The nestin-eGFP+ cells examined in this study give rise to newborn neurons throughout the OB adult periglomerular and granular layers. In addition, nestin-GFP cells were found in all of the anatomical subregions shown to harbor different SVZ lineages.Citation24,Citation25 These GFP+ cells migrate into and intermix in the RMS, the area that we studied. Thus it is unlikely, but nonetheless possible, that the behaviors described here are segregated to specific RMS neuroblast lineages. A major advantage of this nestin-GFP line is that labeling occurs in a minority of cells therefore facilitating morphological and quantitative analyses. This nestin-GFP line labels many but not all neuroblasts, thereby allowing this detailed individual cell analysis.Citation14
We found that the leading process and lamellipodium of migrating neuroblasts were extremely dynamic; they could bend, branch, retract and grow de novo, and these changes were previously correlated with directional movement.Citation14 Similarly, Kakita and Goldman described bidirectional migration of glial progenitors in the SVZ through the growth of a new process at the opposite pole of the cell body.Citation18 These authors also identified two characteristic patterns for orthogonal turning via branching and curving of the leading process. A biased selection of leading process branches has equally been documented for tangentially migrating cortical interneurons during development.Citation26 Taken together, these observations suggest that bending and branching of the leading process and new process formation are broadly used for directional changes in different cell types and times of development. Changes in leading process and lamellipodial morphology require structural repatterning of the cytoskeleton. Cytoskeletal dynamics are essential aspects of cell migration as they influence cell directionality through a variety of mechanisms.Citation8
We used a dynamic and three-dimensional approach to visualize cell migration in the RMS during time-lapse movies. The third dimension is necessary to accurately detect morphological changes. For example, to measure branching or bending events we often needed to rotate the image because the leading process moved in 3D and was not fully visible in movies collapsed and analyzed in two dimensions. In our morphological approach, we measured the angles formed at the leading process and/or lamellipodium during P-bending, D-bending or branching behaviors. This 3D analysis revealed that when exhibiting any given mode of turning, neuroblasts did not form a continuum of angles. On the contrary, their values were tightly clustered suggesting that they are each constrained. The lack of difference in average angles between P-bending and D-bending indicates that they may be constrained by a similar subcellular mechanism, which is different from that underlying branching. Actin crosslinking proteins such as Arp2/3 may play a role in the formation of these angles. However, Arp2/3-mediated actin branching tends to occur at a fixed angle of 70°,Citation27 which does not correspond to our results. This invites speculation of a microtubule-based mechanism underpinning these turning behaviors. Indeed loss of the microtubule associated protein, doublecortin, increases leading process branching and reduces SVZ neuroblast migration.Citation28,Citation29 Similarly another microtubule associated protein, Lis1, regulates branching in migrating cortical interneurons, via interactions with platelet-activating factor (PAF) acetylhydrolase 1b.Citation30
Dynamic patterns during directional changes were established by calculating the turning angle and the velocity over time. We found a negative correlation between these parameters in migrating neuroblasts irrespective of their local motility. The main implication of this finding is that successive small steps, rather than one large step, are used to turn or change direction. During direction reversal via branching we observed that an overall orthogonal turning was composed of two successive turns which added up to approximately 90°. Similarly, reversing direction via D-bending appears to result from additional D-bending steps. Overall, our data reinforces the idea that direction reversals are composed of several iterations of D-bending and/or branching. Polarity reversal seems to be an exception to this rule: it was only observed in cells that reversed direction.
Another parameter that we examined was the overall duration for each pattern of turning. D-bending for reversing direction took significantly longer than D-bending for turning whether it involved a single step or cumulative bending steps. Surprisingly, the duration of reversing direction via polarity reversal (13.3 ± 2.7 min) was rather short compared to direction reversal via branching or D-bending. Consequently, this pattern appears to be one of the most effective ways for rapid direction reversals. The relative low frequency of direction reversal via branching (11% ± 5) may explain why this behavior was unnoticed in previous studies.Citation14
As mentioned above, several strands of research have suggested that subregions of the SVZ harbor different types of neuroblast precursors;Citation24,Citation25 therefore the possibility exists that each may exhibit different patterns of motility. Interestingly, our results show that individual neuroblasts can change direction in multiple patterns, suggesting these behaviors were not specific to individual lineages. It is possible that subregions of the RMS present migratory niches that only allow one or two types of turning behaviors, irrespective of lineage, however, this also was not the case. We found multiple types of turning and direction reversals within the relatively small volumes of tissue sampled. Although direction reversal is a relatively rare phenomenon, we observed one cell that reversed direction twice via branching and D-bending, further supporting this idea. We favor a scenario in which at small spatiotemporal scales migration is probabilistic. It is likely that variations in both niche and intrinsic molecules regulate the multiple turning and reversing behaviors seen here. For example, because Slit1/2 is a chemorepellent that regulates directional migration in the SVZ,Citation31 future studies analyzing the expression of Slit receptors in those neuroblasts switching to rostrocaudal migration may prove important.
Our data analysis was based on a sample of 100 cells that we followed over an average recording period of 02:48 (hh:mm). This sample came from eight independent experiments and the imaged area was in the vertical limb, elbow and horizontal limb of the RMS (). Of course, having more data from different locations and ages would be important to validate our conclusions. This is especially important for studying combinations of rare patterns. An increase in the sample size would contribute to strengthening our definition of directionality in terms of the turning angle involved and subsequent quantifications of linear versus non-linear movement. However, it is important to point out that the small standard errors (ex. angles) indicate that we had more than a sufficiently large sample.
Another possible criticism of our analysis is that exploratory behavior or other observations we have made about migration in slicesCitation14,Citation23 may be epiphenomena caused by disruption of molecular gradients in our in vitro protocol. A large number of diffusible molecules impact SVZ migration,Citation32 and these could be diluted by the rapidly circulating aCSF in our experiments. We imaged slices within a few hours after removal from the mouse, since several days growth of slice cultures on millipore filters would more likely change endogenous molecular gradients. Another potential confounding factor, however, is cell death and associated toxicity at the slice surface, thus we examined this in a previous study and found minimal evidence for apopotosis.Citation23 It is also important to point out that we observed cells well below the surface of the tissue, which is where most tissue damage occurs in slices. We imaged approximately 50–100 microns into the slice and therefore we do not know if any of the behaviors described here would be found at shallower or greater depths. In addition, similar results were observed in all slices and in different transgenic linesCitation14,Citation23 and multipolar migration in the embryonic cortexCitation10 closely resembles SVZ exploratory behavior. This lends confidence to the notion that exploratory migration and the turning behaviors described in this study are bona fide biological processes and not in vitro epiphenomena. Nevertheless, we cannot completely rule out that some of these behaviors are in vitro artifacts, and an ultimate goal is to confirm them in vivo. Recent implementations of gradient refractive index (GRIN) lens or high-resolution microlens technologyCitation33 have opened the door to in vivo two-photon studies and promising prospects for analysis of cell migration in the adult SVZ/RMS.
In embryonic multipolar migration, cells retract their leading processes, grow new ones and change directions.Citation10 In this respect they resemble the polarity reversal described in this study. Cortical neuronsCitation34 as well as interneurons migrating from the medial ganglionic eminence into the cerebral cortexCitation11 can exhibit direction reversals and move towards the ventricle before resuming radial migration towards the pial surface. This is very similar to the direction reversals observed in the RMS in this and our previous studiesCitation14,Citation23 and suggests that temporary direction reversals are more universal in cell populations with directed migration than previously thought. Turning during embryonic migration can also be mediated by branching of the leading process,Citation35 similar to what we saw in the postnatal RMS. These similarities suggest that patterns and cellular machinery of turning during migration is conserved across brain regions and developmental time periods.
A unique feature of the adult is that RMS neuroblasts migrate along each other in chains and through glial tubes.Citation15,Citation36 Our approach did not allow us to correlate cell turning behaviors in relation to these other cells as they were non-fluorescent. Several questions arise: are individual turning behaviors associated with the cell's position in the interior versus exterior of the chain? Do they correlate with contacting the surrounding glia versus neurons? Future studies using different color fluorochromes such as in the Brainbow mouseCitation37 may help elucidate these issues. Another important issue would be to ascertain how these directional patterns in the RMS could change after injury or in cells emigrating towards the injury.Citation38 Given that neuroblasts have been shown to migrate ectopically to injured areas and that this could be related to brain repair,Citation39 it would be particularly interesting to examine whether certain patterns of turning could be predictive of emigration.
Adult neurogenesis and proliferation in the human dentate gyrus and SVZ has been documented.Citation40 The human SVZ is composed of a hypocellular layer flanked by an “astrocytic ribbon” that contains few neuroblasts.Citation41 However, RMS cell composition and neuroblast density remains controversial.Citation41,Citation42 Huntington's disease, stroke and multiple sclerosis seem to activate SVZ progenitor cells and induce their emigration towards injury. Explants of human RMS or SVZ could be obtained postmortem or in the context of callosectomies and neuroblast migration studied in vitro. It is likely that turning and reversal directions would increase as a prelude to neuroblast emigration to injury.
Materials and Methods
Brain slices, two photon microscopy and image processing were conducted according to published protocols.Citation14 Cells were imaged at least 50 and usually within 100 microns of the surface, to avoid disrupted molecular gradients and potential cell death at the slice/aCSF interface. The imaged fields included the vertical limb of the RMS near the rostral SVZ, the elbow and the horizontal limb of the RMS (). The average age of animals was 56 ± 10 postnatal days (P) and the average recording time was 02:48 ± 00:14 (hh:mm). Further details are shown in .
Quantification.
We followed 100 individual cells from eight independent two-photon time-lapse movies. The cells with the clearest visibility were selected. When the complete cell morphology was not visible in Quicktime (Apple), we examined it in 3D using Volocity (Improvision) software. We included cells that were observable for no fewer than 10 frames of the movie's duration (30 min.). Although multipolar cells can be motile,Citation14 we only considered cells with initial bipolar morphology. This probably excluded many exploratory cells, many of which are multipolar, though these were included in 3D tracking and subsequent calculations of cell body movement, velocity and turning angle. A total of 106 turning events and 26 direction reversals were analyzed. A turning event was defined as that in which the associated turning angle is 30°–90° from the axis of orientation. This cutoff value was established retrospectively. We calculated the turning angle in a sample of neuroblasts (N = 39 cells) and using 3D plots of seven representative neuroblasts we confirmed that the maximum turning angle observed during quasi-linear nucleokinesis was 30°. A direction reversal was defined as an event in which the cell body reversed migratory direction from rostral to caudal by greater than 90°. It is worth noting that this does not necessarily imply an 180° turning angle (see 3D plots in ). The percentage for each pattern was calculated as the number of events for that particular pattern (either turning or direction reversal pattern) divided by total turning or direction reversal events in that movie and these values were averaged from the eight two photon time-lapse movies.
Duration of patterns.
The duration of each behavior was established in minutes. To ensure consistency we followed several criteria. For reversing direction via branching or D-bending we took the frame prior to radial turns of the leading process as the origin and the last frame as that in which turning via branching or bending ended. For reversing direction via new process formation we took the first frame in which the two processes were visible as the origin and the last frame as that when only the new process remained. For D-bending we took the frame previous to the formation of the curve in the leading process as the origin and the last frame as that in which the leading process regained its linear shape and the cell soma occupied the curving point. For branching we took the first frame as that prior to the formation of the branch and the last frame as that with a single process restored. A branch was defined as a process emanating from the lamellipodium, the cell soma or the side of the leading process that persisted for more than two consecutive frames (6 min). For P-bending we took the first frame as that in which the soma was round prior to elongation of the process and the last frame as that in which the soma translocated to the bending point and regained its round morphology.
Multiple directional changes in single cells.
We quantified those cells that underwent several changes in direction either by repetitive use of a single pattern or by combination of two or three different patterns. Here repetition refers to the occurrence of at least two identical patterns in the same neuroblast. Consecutive iterations of turning patterns were those in which no quasi-linear nucleokinesis occurred between repetitions. Percentages of cells with multiple changes in direction via iteration or combination of patterns were calculated with reference to total number of cells (N = 100). The proportion for each iterated pattern or combination of patterns was compared to the other percentages.
Morphological angles.
We followed individual cells from three independent two-photon time-lapse movies () and measured angles associated with each pattern for turning, namely branching, D-bending and P-bending, and with bending and branching for reversing direction. A total of 56 measurements were taken from 43 cells that exhibited measurable angles. To ensure consistency, neuroblasts were rotated in 3D using Volocity software until the structure under study was contained in a single plane and the maximal angle viewed. This allowed us to use the 3D data to construct 2D angles. The angles were measured in degrees using the angle tool in ImageJ (NIH, rsb.info.nih.gov/ij). We calculated the complementary angle with the formula 180-x, with “x” being the angle in degrees (°). During branching we considered side branches as well as bifurcation of the lamellipodium. For bifurcation at the lamellipodium we took the measurement when the two lamellipodia were visible. Multiple branching at the lamellipodium and somal branches were not included due to the difficulty in measuring them. An image of each measurement was saved for reference. Values are given as average degrees (°) ±SEM (Standard Error of Mean).
3D tracking.
Each cell was tracked in 3D in Volocity for 14–40 time points. Cells were identified for tracking by scrolling through images in the z- and t-dimensions and finding the clearest cells to track, i.e., the ones that remained recognizable over time. The x, y and z coordinates were obtained from the center of the cell body.
Cell body movement.
The x, y, z coordinates of cell bodies were imported into a spreadsheet (Microsoft Excel). The distance that the cell body moved between images was then calculated using a 3D distance equation distance = sqrt[(dx)2 + (dy)2 + (dz)2]. The collective displacements for each cell were summed to yield the total distance moved.
Velocity calculation.
We used the x, y and z coordinates and the cell body movement to calculate velocity. As Z-stacks were captured every 3 minutes, the timestep is a constant (0.05 h). We divided the distance (X) between successive positions (step length) by the timestep dt and calculated the speed denoted by vk, where k = 2,…,N. The speed unit is µm/h.
Turning angle.
We were interested in the reorientation of the cell at a given point. We calculated the turning angles, θk, for the trajectory of each cell via the expression: where k = 3,…, N. The angle θk is shown in and it is given by inverting this equation. The angles are measured in degrees (°).
Cell trajectories in 3D.
3D plots (Cartesian coordinates) were created in Graphis software (Kylebank Software) by importing the x, y and z coordinates of each cell from a spreadsheet (Microsoft Excel). We related each point in the plot with its corresponding turning angle and identified the exact time points in which the directional change was observed in Quicktime movies. This allowed us to relate each defined pattern with the turning angle in the migratory pathway. In some cases one point was enough to determine the cell's orientation for that pattern such as in nucleokinesis. In others we took several points in which the change in direction occurred and averaged the turning angle for that pattern. The timepoints considered for average turning angles within the same pattern are denoted by k-kn. Values are given as average turning angle of all observations of a certain pattern.
Statistics.
To determine whether an ANOVA test would apply to our data, we analyzed it using Shapiro-Wilk and Kolmogorov-Smirnov tests for normality and Levene test for homogeneity of variance. The dependent variable (duration) was transformed with the natural logarithm to achieve homogeneity of variance. Statistical differences were determined by one-way ANOVA and a post hoc Bonferroni test. Significance level was set at 5%. Values are given as average duration ± SEM. Statistical tests were carried out in SPSS. Statistical differences between the patterns were determined by use of the Kruskal-Wallis (KW) test. We performed post-hoc pair-wise comparisons with a Mann-Whitney U test and a Bonferroni correction. General significance level was set at 5%; the adjusted significant level after Bonferroni correction was 2%. We also used the Mann-Whitney U test for comparisons between bifurcation of the lamellipodium and side branch formation; branching for turning and direction reversal and D-bending for turning and direction reversal. Statistical tests were carried out in SPSS.
Figures and Tables
Figure 1 (A–H) First frames of eight two-photon time-lapse movies used in this study with a schematic of a parasagittal section showing the SVZ/RMS pathway. The duration of each movie is indicated in the top right corner (h:min). The colored rectangles represent the location of the imaged area. Scale bar = 50 µm. (I) Schematic showing the approximate location in the RMS of the vertical limb (black arrow), elbow (red arrow) and horizontal limb (green arrow). cc, corpus callosum; ctx, cerebral cortex; dl SVZ, dorsolateral subventricular zone; LV, lateral ventricle; OB, olfactory bulb; RMS, rostral migratory stream; str, striatum. (J) Straight movement in a neuroblast exhibiting nucleokinesis. Red asterisk: stationary cell. Arrowhead shows the movement of the cytoplasmic dilation in the proximal leading process, and in the final frame, the movement of the cell body into it. Scale bar = 50 µm. (K) Schematic of cell undergoing nucleokinesis without turning.
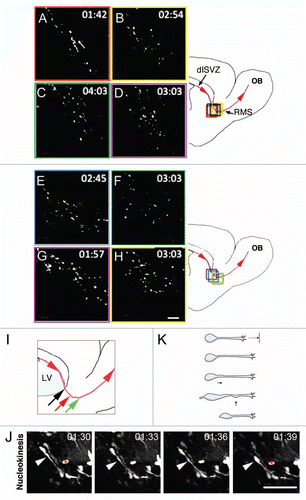
Figure 2 Schematic showing types of turning and direction reversals. (A) P-bending is characterized by a bend in the leading process close to the soma, followed by nucleokinesis and reorientation of the cell's long axis. (B) Turning via D-bending is associated with bending of the leading process closer to the lamellipodium. (C) Branching frequently occurs at the lamellipodium and is characterized by retraction of the less dominant branch leading to a subtle shift in direction. (D) Polarity reversal involves generation of a new process on the opposite pole from the direction of migration. (E and F) Direction reversals via branching and bending are defined by successive iterations of these behaviors. Blue arrows point to the direction of migration. C, caudal; R, rostral.
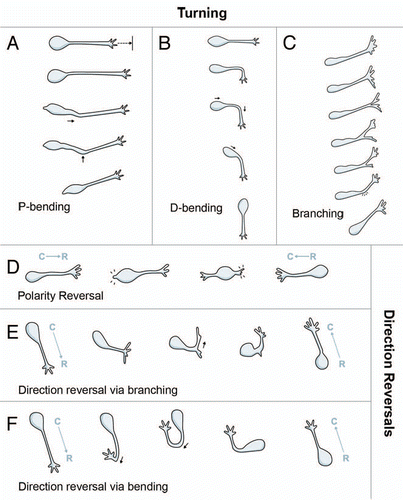
Figure 3 Typical examples of turning. (A) P-bending of the leading process near the cell body. 2-dimensional frames do not show maximum angle of this cell. Arrowhead: bending introduced at the cytoplasmic dilation. Asterisk: stationary cell. Scale bar = 25 µm. (B) D-bending of the leading process. Scale bar = 25 µm. (C) Bending of the lamellipodium (arrowhead at 00:06). Scale bar = 12.5 µm. (D) Branching of the lamellipodium (arrowhead at 1:03) and retraction of the less stable branch. Scale bar = 12.5 µm.
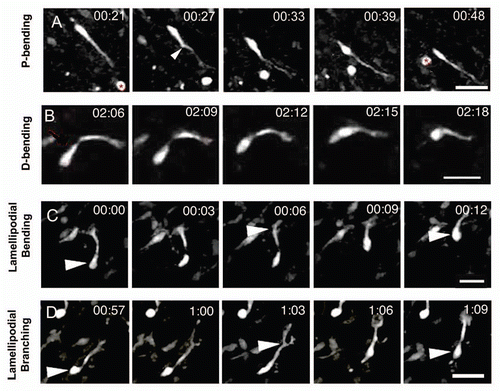
Figure 4 Quantification of turning and direction reversals. (A) Percentage of neuroblasts turning and reversing directions in different modes. P-bending was the most common pattern for turning and D-bending of the leading process was the most common for direction reversal. (B) Duration in minutes of the different patterns for turning and direction reversal (DR). Direction reversal (DR) usually required more time than turning. Compare the means between D-bending vs. DR-bending. *p < 0.05. (C) Combinations of different patterns were observed for multiple direction changes (RD: reversing direction). Values are given as percentage of each pattern combination with respect to the total proportion of neuroblasts which exhibited multiple direction changes by combining different patterns.
Figure 5 Typical examples of direction changes. (A) Polarity reversal. Arrowhead at time point 00:30 points to the new leading process. The other arrowheads to the cell body. (B) Direction reversal via branching. The leading process rotates to come in close apposition to the cell body. Simultaneously the lamellipodium bifurcates and branches by approximately 90°. The portion pointing backwards persists. The cell body eventually translocates to the point of branching, between 01:09 and 01:12, and migration resumes. Note the bending of the leading process prior to rotation. Arrowheads point to position of cell body. (C) Direction reversal via D-bending. A neuroblast reversing direction through D-bending of the leading process (red arrow). Arrowhead shows cell soma and asterisk shows stationary cell. Scale bars = 25 µm.
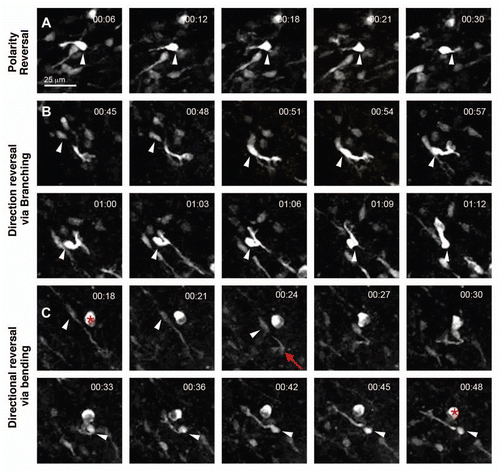
Figure 6 Different morphological angles are formed by neuroblasts during turning. Cells were rotated in 3D in Volocity to reveal maximum turning angle. The angle in the leading process (A) during D-bending was measured as indicated by the superimposed angle in red. The same procedure applies to P-bending (B) and branching of the lamellipodium (C). Scale bars = 35 µm. (D) Turning patterns occurred through formation of characteristic angles by migrating neuroblasts. ***, p < 0.0005, Mann-Whitney U test between the angles formed during branching, P-bending and D-bending.
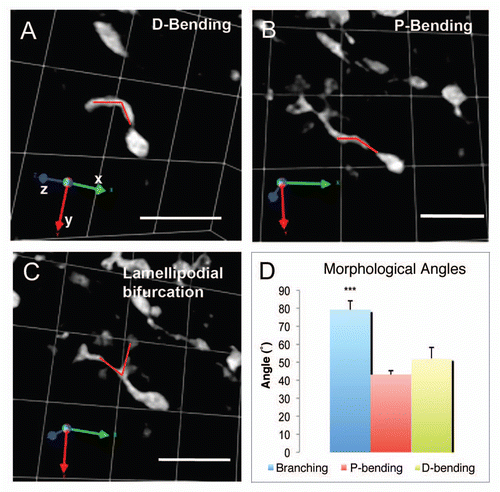
Figure 7 Distribution of turning angles in migratory versus exploratory cells. (A) Schematic illustrating the concept of turning angle ϑk. For simplicity we have represented it in two dimensions. However, it should be considered a turning kernel since it is defined by the x, y and z coordinates. (B–E) Distribution of turning angles for the mixed population (B), migratory (C), intermediate (D) and exploratory (E) neuroblasts. (F–H) Scatter diagrams plotting the turning angle (X axis) and the corresponding speed at that point (Y axis) in migratory (F), intermediate (G) and exploratory (H) neuroblasts.
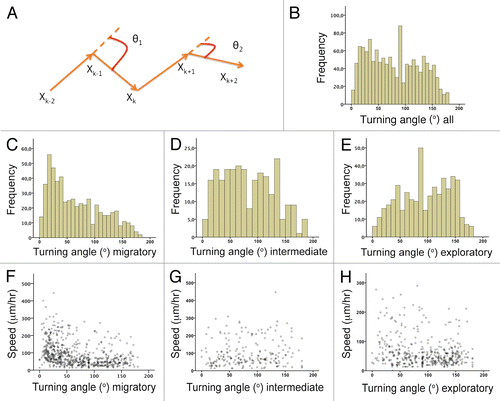
Figure 8 Examples of individual cells exhibiting multiple turning behaviors. X, Y and Z axis show the coordinates imported to Graphis. (A) A neuroblast changing direction through multiple D-bending of the leading process (purple ellipse). The value of the average turning angle for the time points (ϑ7–17) in which we observed this pattern is 115°. The cell's trajectory is indicated by the arrowheads. (B) A neuroblast undergoing nucleokinesis (green ellipse) followed by a direction reversal via branching (purple ellipse). Arrows point to two consecutive turning steps to achieve an orthogonal turning (coordinate xk-1 for the calculation of ϑ19 and ϑ20 respectively). This is an illustrative example of the close correspondence between branch geometry (91° ≈ 90°) and the overall turning angle (50° + 45° = 95° ≈ 90°). (C) A neuroblast reversing direction via D-bending (purple ellipses) followed by nucleokinesis (green ellipse). Direction reversal occurred through two main turning steps whose values are 100° (ϑ12) and 133° (ϑ16). Arrows point to the xk (nucleokinesis, ϑ24) and xk-1 (reversing direction via D-bending, ϑ12) coordinate for the calculation of the turning angle. The initial point in the cell's trajectory is in the center. (D) A neuroblast undergoing nucleokinesis (green ellipse) and two iterated P-bendings (purple ellipses). The values of the average turning angle for the time points (ϑk-kn) in which we observed these patterns have been indicated. Arrow points to the xk coordinate for the calculation of the turning angle ϑ28. (E) A neuroblast undergoing direction reversal via D-bending (purple ellipse) followed by nucleokinesis (green ellipse). The values of the turning angle associated with these patterns have been indicated. Arrow points to the xk coordinate for the calculation of the turning angle ϑ9. (F) A neuroblast undergoing direction reversal via new process formation from the soma at the opposite pole of the leading process (purple ellipse). Arrow points to the xk coordinate for the calculation of the turning angle ϑ13.
Table 1 Movie features
Acknowledgements
We thank Dr. Chris Young for guidance with the Volocity software and Rosa Martinez for her help with the schematics. F.G.S. supported by NIH grant RO1 NS/AG42253-01.
References
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci 1997; 17:5046 - 5061
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci USA 1999; 96:11619 - 11624
- Cesetti T, Obernier K, Bengtson CP, Fila T, Mandl C, Holzl-Wenig G, et al. Analysis of stem cell lineage progression in the neonatal subventricular zone identifies EGFR(+)/NG2(−) cells as transit-amplifying precursors. Stem Cells 2009; 27:1443 - 1454
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol 1969; 137:433 - 457
- Belluzzi O, Benedusi M, Ackman J, LoTurco JJ. Electrophysiological differentiation of new neurons in the olfactory bulb. J Neurosci 2003; 23:10411 - 10418
- Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci USA 1996; 93:14895 - 14900
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci 2003; 6:507 - 518
- Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell 2007; 128:29 - 43
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 2009; 32:149 - 184
- Tabata H, Nakajima K. Multipolar migration: The third mode of radial neuronal migration in the developing cerebral cortex. J Neurosci 2003; 23:9996 - 10001
- Nadarajah B, Alifragis P, Wong RO, Parnavelas JG. Ventricle-directed migration in the developing cerebral cortex. Nat Neurosci 2002; 5:218 - 224
- Kulesa PM, Fraser SE. Neural crest cell dynamics revealed by time-lapse video microscopy of whole embryo chick explant cultures. Dev Biol 1998; 204:327 - 344
- Kulesa PM, Fraser SE. In ovo time-lapse analysis of chick hindbrain neural crest cell migration shows cell interactions during migration to the branchial arches. Development 2000; 127:1161 - 1172
- Nam SC, Kim Y, Dryanovski D, Walker A, Goings G, Woolfrey K, et al. Dynamic features of postnatal subventricular zone cell motility: A two-photon time-lapse study. J Comp Neurol 2007; 505:190 - 208
- Peretto P, Merighi A, Fasolo A, Bonfanti L. Glial tubes in the rostral migratory stream of the adult rat. Brain Res Bull 1997; 42:9 - 21
- Wichterle H, Garcia-Verdugo JM, Alvarez-Buylla A. Direct evidence for homotypic, glia-independent neuronal migration. Neuron 1997; 18:779 - 791
- Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci USA 1999; 96:7526 - 7531
- Kakita A, Goldman JE. Patterns and dynamics of SVZ cell migration in the postnatal forebrain: monitoring living progenitors in slice preparations. Neuron 1999; 23:461 - 472
- Suzuki SO, Goldman JE. Multiple cell populations in the early postnatal subventricular zone take distinct migratory pathways: a dynamic study of glial and neuronal progenitor migration. J Neurosci 2003; 23:4240 - 4250
- Ward ME, Jiang H, Rao Y. Regulated formation and selection of neuronal processes underlie directional guidance of neuronal migration. Mol Cell Neurosci 2005; 30:378 - 387
- Schaar BT, McConnell SK. Cytoskeletal coordination during neuronal migration. Proc Natl Acad Sci USA 2005; 102:13652 - 13657
- Bellion A, Baudoin JP, Alvarez C, Bornens M, Metin C. Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J Neurosci 2005; 25:5691 - 5699
- Kim Y, Comte I, Szabo G, Hockberger P, Szele FG. Adult mouse subventricular zone stem and progenitor cells are sessile and epidermal growth factor receptor negatively regulates neuroblast migration. PLoS ONE 2009; 4:e8122
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science 2007; 317:381 - 384
- Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci 2007; 27:8286 - 8296
- Martini FJ, Valiente M, Lopez Bendito G, Szabo G, Moya F, Valdeolmillos M, et al. Biased selection of leading process branches mediates chemotaxis during tangential neuronal migration. Development 2009; 136:41 - 50
- Mullins RD, Heuser JA, Pollard TD. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping and formation of branching networks of filaments. Proc Natl Acad Sci USA 1998; 95:6181 - 6186
- Ocbina PJ, Dizon ML, Shin L, Szele FG. Doublecortin is necessary for the migration of adult subventricular zone cells from neurospheres. Mol Cell Neurosci 2006; 33:126 - 135
- Koizumi H, Higginbotham H, Poon T, Tanaka T, Brinkman BC, Gleeson JG. Doublecortin maintains bipolar shape and nuclear translocation during migration in the adult forebrain. Nat Neurosci 2006; 9:779 - 786
- Gopal PP, Simonet JC, Shapiro W, Golden JA. Leading process branch instability in Lis1+/− nonradially migrating interneurons. Cereb Cortex 2010; 20:1497 - 1505
- Wu W, Wong K, Chen J, Jiang Z, Dupuis S, Wu JY, et al. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature 1999; 400:331 - 336
- Young C, Brooks K, Buchan AM, Szele F. Cellular and molecular determinants of stroke induced changes in subventricular zone cell migration. Antioxid Redox Signal 2010; In press
- Barretto RP, Messerschmidt B, Schnitzer MJ. In vivo fluorescence imaging with high-resolution microlenses. Nat Methods 2009; 6:511 - 512
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 2004; 7:136 - 144
- Tanaka DH, Yanagida M, Zhu Y, Mikami S, Nagasawa T, Miyazaki J, et al. Random walk behavior of migrating cortical interneurons in the marginal zone: time-lapse analysis in flat-mount cortex. J Neurosci 2009; 29:1300 - 1311
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science 1996; 271:978 - 981
- Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 2007; 450:56 - 62
- Sundholm-Peters NL, Yang HK, Goings GE, Walker AS, Szele FG. Subventricular zone neuroblasts emigrate toward cortical lesions. J Neuropathol Exp Neurol 2005; 64:1089 - 1100
- Dizon ML, Shin L, Sundholm-Peters NL, Kang E, Szele FG. Subventricular zone cells remain stable in vitro after brain injury. Neuroscience 2006; 142:717 - 725
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med 1998; 4:1313 - 1317
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 2004; 427:740 - 744
- Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science 2007; 315:1243 - 1249