Abstract
The zebrafish sensory lateral line system has emerged as a powerful model for the mechanistic study of collective cell migration and morphogenesis. Recent work has uncovered the details of a signaling network involving the Wnt/β-catenin, Fgf and Delta-Notch pathways that patterns the migrating lateral line primordium into distinct regions. Cells within these regions exhibit different fundamental behaviors that together orchestrate normal lateral line morphogenesis. In this review, we summarize the signaling network that patterns the migrating lateral line primordium and describe how this patterning coordinates crucial morphogenic cell behaviors.
Introduction
Understanding the mechanistic underpinnings of morphogenesis is a major focus of developmental biology. During development, groups of cells self-organize into genetically specified threedimensional geometries that are ultimately crucial for the function of tissues, organs and organisms.Citation1 Although understanding these processes at a mechanistic level is a vitally important aim of modern developmental biology, elucidating such mechanisms is often challenging due to the complex geometries of many biological structures and the redundant and interdependent nature of the molecular regulatory mechanisms involved.
Over the last ten years the zebrafish primary posterior lateral line system has emerged as a powerful model for the elucidation of molecular mechanisms underlying morphogenesis. This is due to the relatively simple morphology and accessibility of the lateral line and the amenability of the zebrafish model to detailed genetic experimentation. The cell behaviors that drive morphogenesis can be observed directly in the living embryo and correlated with specific molecular perturbations afforded by the wealth of tools available for zebrafish biology.
The lateral line is a mechanosensory system found in aquatic vertebrates that enables detection of water movements. The lateral line is composed of rosette shaped sensory organs called neuromasts distributed across the surface of the animal.Citation2,Citation3 Each neuromast contains a group of hair cells that are homologous and functionally analogous to the hair cells that underlie hearing and balance in terrestrial vertebrates.Citation4 These hair cells are encased in a population of supporting cells. In zebrafish, the lateral line can be subdivided into two main branches: the anterior and the posterior lateral line. Neuromasts of the anterior lateral line cover the head of the animal and neuromasts of the posterior lateral line are distributed on the trunk.Citation3,Citation5,Citation6 The primary posterior lateral line is the first group of neuromasts to develop along the horizontal myoseptum. During postembryonic development, repeated waves of neuromast formation occur on the trunk so that neuromasts become evenly distributed across the surface of the mature animal.Citation7–Citation9 The work reviewed here pertains to the primary posterior lateral line, which will be referred to simply as ‘the lateral line.’ Development of the anterior lateral line remains to be studied in any detail.
The lateral line originates from a migrating primordium composed of approximately 100 cells that forms just posterior to the otic vesicle and migrates posteriorly along the horizontal myoseptum toward the tip of the tail ( and B).Citation7 As it migrates, sensory organ precursors, termed proneuromasts, form within the trailing region of the primordium (). During migration, proneuromasts are deposited from the trailing (anterior) side of the migrating primordium to generate a series of five or six proneuromasts distributed along the anterior-posterior axis (). In addition, the primordium deposits a single line of cells, termed interneuromast cells, between the proneuromasts. Later in development these interneuromast cells will proliferate to generate additional neuromasts.Citation8
Upon approaching the tail tip, the primordium turns and migrates toward the ventral fin fold where migration stalls and the primordium divides into a series of 2–3 terminal proneuromasts.Citation7 Later in development, deposited proneuromasts mature into mechanosensory neuromasts. The detailed embryology of the zebrafish posterior lateral line has been extensively reviewed in references Citation10–Citation12.
Recently, there has been a rapid expansion in mechanistic knowledge of lateral line morphogenesis. Although the embryology of the lateral line has fascinated biologists for over 100 years,Citation13–Citation15 it has only been within the last ten years that detailed mechanistic knowledge has been gained. This review will focus on how primordium patterning via cell-cell signaling interactions coordinates the fundamental cell behaviors that drive lateral line morphogenesis. Each cell behavior will be discussed in detail. We will describe the cell behaviors and how they are regulated by the cell-cell signaling based patterning mechanisms and discuss potential avenues for future research.
Patterning the Primordium
The migrating primordium is divided into a leading region that has a disorganized, ‘pseudo-mesenchymal’ appearance and a trailing region containing two or three rosette shaped proneuromast ().Citation16 It has been shown that these different regions are patterned by a signaling feedback system involving the Wnt/β-catenin and Fgf signaling pathways.Citation17 Wnt/β-catenin signaling is active in the leading region where it induces expression of secreted Fgf ligands, as well as the membrane bound Fgf inhibitor sef (il17rd).Citation17 Fgf ligands are free to diffuse out of the inhibitory leading region where they stimulate signal transduction in the trailing region. Simultaneously, Fgf signaling in the trailing region leads to the expression of dkk1, a Wnt/β-catenin repressor.Citation17 The consequence of this signaling mechanism is to pattern the primordium into two distinct gene expression domains referred to here as the leading region and the trailing region. In the leading region, comprising approximately the leading 1/3 of the primordium, Wnt/β-catenin signaling is active and Fgf signaling is largely repressed. In the trailing region, Fgf signaling is active and Wnt/β-catenin signaling is largely repressed.Citation17 It is important to note that, despite this regionalization of gene expression, there is likely to be significant overlap between these signaling activities in the central portion of the primordium. This signaling feedback system has important consequences for coordinating cell behaviors that drive morphogenesis of the lateral line, as will be detailed below.
A recent study of lef1 mutants concluded that Wnt/β-catenin signaling does not affect primordium patterning based on the analysis of lef1 mutants that were co-injected with a morpholino targeting the redundantly acting tcf7.Citation46 However, these manipulations most likely result in a Wnt/β-catenin hypomorphic condition. Wnt/β-catenin signaling is only reduced and not entirely turned off as Wnt/β-catenin signaling target gene expression is unaffected in lef1 morphants and mutants,Citation37,Citation46,Citation47 while expression of these genes is lost in the primordium following induction of Wnt/β-catenin repressor dkk1 or the dominant repressor Δ-tcf.Citation17 Therefore, morpholino knockdown of the co-expressed Tcf/Lef factor tcf7 in lef1 mutants leads to a milder phenotype than the one reported for the complete loss of Wnt/β-catenin signaling by induction of dkk1 and Δ-tcf. It is possible that the particular tcf7 morpholino injected is not able to abrogate tcf7 translation completely, as no evidence was provided in the studies.Citation46,Citation47 Analysis of a tcf7/lef1 double mutant could help clarify this issue. As mutations in both genes are now available, this experiment is within our reach.
Another important aspect of primordium patterning involves the expression of Fgf and Delta ligands in small groups of cells located at the center of forming proneuromasts in the trailing region ().Citation19,Citation20 These ‘central cells’ are formed by a classical lateral inhibition mechanism involving the increasingly restricted expression of Delta ligands.Citation19 delta expression in central cells is crucial to induce and restrict Fgf ligand expression and atoh1a dependent hair cell specification to cells at the center of the proneuromasts.Citation20–Citation22 Mosaic experiments involving small clones of Fgf ligand producing cells in an otherwise Fgf ligand deficient primordium have shown that short range Fgf signaling from central cells is sufficient to induce the differentiation of hair cells, implying that short range Fgf signaling from these cells is a key event in hair cell development.Citation21
Therefore, in addition to the Wnt/β-catenin-Fgf signaling system described above, Delta and Fgf expression in central cells constitutes a second, crucial source of signaling ligands in the primordium. These two signaling centers interact, as Fgf signaling and Fgf-dependent dkk1 expression is lost in Delta-Notch deficient primordia, allowing expansion of Wnt/β-catenin target gene expression.Citation20 Therefore, Delta-Notch signaling in central cells is necessary for dkk1 expression and restriction of Wnt/β-catenin signaling to the leading region, likely via short range Fgf signaling from central cells.Citation20 Interestingly, the Wnt/β-catenin target gene axin2 is also expressed in proneuromast central cells,Citation17 however, the consequence of this expression remains unknown. It is tempting to speculate that Wnt/β-catenin and Fgf signaling in developing proneuromasts interact in a similar fashion as in the primordium and that Fgf ligand expression in central cells requires Wnt/β-catenin signaling in these cells. In support of this hypothesis, it appears that central cell expression of Fgf ligand is lost from the most newly formed proneuromasts within the primordium when Wnt/β-catenin signaling is inhibited.Citation17 However, central cell expression of Fgf ligand is maintained in more mature proneuromasts that are about to be deposited from the trailing edge of the primordium, suggesting that different interactions between Fgf and Wnt/β-catenin might exist in mature proneuromasts (Aman A and Piotrowski T, unpublished observation). The detailed connection between these two crucial primordium signaling centers, the leading zone and proneuromast central cells, represents an important problem for future research.
With this sketch of the cell-cell signaling patterning mechanism present in the primordium we now turn our attention to how this patterning coordinates the fundamental cell behaviors that drive lateral line morphogenesis.
Collective Migration
Perhaps the most conspicuous cell behavior underlying lateral line morphogenesis is collective cell migration. In this process, the approximately 100 cells of the lateral line primordium migrate in concert along the horizontal myoseptum. Because of this behavior, the lateral line primordium has emerged as an excellent model to study molecular regulation of collective cell migration in a vertebrate system. Although this process and its regulation has been extensively reviewed in references Citation10, Citation23 and Citation24, for completeness, we will provide a brief discussion here.
It has been shown that migration of the lateral line primordium depends critically on the expression of the chemokine receptor cxcr4b in the leading region of the primordium and its ligand cxcl12a (formerly called sdf1a) along the presumptive migratory path.Citation25 Embryos with disruptions in the expression of either molecule exhibit severe defects in collective cell migration.Citation25,Citation26 Furthermore, ectopic expression of an orthologous chemokine ligand, cxcl12b, in cxcl12a-deficient embryos is capable of directing the primordium along ectopic migratory paths.Citation26
A second cxcl12a receptor, cxcr7b, is expressed in the trailing region of the migrating primordium. Like cxcr4b, cxcr7b is necessary for normal collective migration. cxcr7b loss of function via morpholino injection leads to a failure of primordium migration similar to loss of cxcr4b.Citation27,Citation28 Loss of cxcr7b correlates with migration failure in other conditions, such as when Wnt/β-catenin signaling is expanded in the primordium,Citation17,Citation21,Citation22 and in other examples of cell migration, such as primordial germ cell migration.Citation29
Asymmetric expression of chemokine receptors in the primordium is regulated by the Wnt/β-catenin-Fgf signaling system described above. Specifically, primordia with Wnt/β-catenin signaling expanded into the trailing region show a complete loss of detectable cxcr7b expression and a failure of migration.Citation17 Conversely, loss of Wnt/β-catenin signaling leads to an expansion of cxcr7b expression into the leading region.Citation17 Interestingly, although loss of Wnt/β-catenin signaling causes a robust expansion of cxcr7b, this expansion is not complete. A few cells at the extreme leading edge do not express cxcr7b under conditions of Wnt/β-catenin loss.
It has recently been proposed that a second signaling system involving the zebrafish estrogen receptor esr1 regulates the distribution of chemokine receptor expression in the primordium. Disruption of esr1 expression leads to cxcl12a-dependent expansion of cxcr4b and downregulation of cxcr7b expression.Citation18 These gene expression changes closely resemble the pattern of chemokine receptor expression when Wnt/β-catenin signaling is expanded in the primordium. Specifically, even though cxcr7b expands toward the leading edge in esr1 morphants, this expansion is incomplete. Although the authors rule out a direct role of esr1 in repressing Wnt/β-catenin signaling,Citation18 it remains to be tested whether this signaling system operates independently and in parallel to Wnt/β-catenin signaling or whether the Esr1 pathway participates in a more complicated regulatory network with Wnt/β-catenin and Fgf signaling. Manipulating Esr1 signaling and Wnt/β-catenin signaling in the same embryo would be useful for clarifying this issue.
Although loss of cxcr7b correlates with failure of collective migration, there is no consensus on the molecular nature of how cxcr7b controls cell migration in the lateral line. It has been proposed that the anisotropic expression of cxcr4b and cxcr7b imbue the primordium with intrinsic polarity that allows migration across a homogenous stripe of Cxcl12a protein.Citation27 In this model, Cxcr7b at the trailing edge serves to remove Cxcl12a protein from extracellular space at a greater rate than Cxcr4b in the leading region thereby creating a traveling gradient of Cxcl12a across the length of the primordium. This is consistent with the molecular nature of Cxcr7b having a 10-fold higher affinity for Cxcl12a.Citation48 In addition, this mechanism for Cxcr7b function has recently been demonstrated in migrating primordial germ cells (PGCs) where Cxcr7b-Cxcl12a binding leads to internalization and proteosomal degradation of Cxcl12a protein and the establishment of instructive Cxcl12a gradients that guide PGCs toward the presumptive gonad.Citation29
However, mosaic experiments demonstrated that only a few cells at the leading edge of the primordium must express cxcr4b to orient migration of the entire tissue.Citation16 This result suggests that there may be additional signals between cells at the leading edge and the remainder of the primordium. These signals could take the form of secreted signaling ligands or mechanically transduced signals.Citation30 The finding that only a few leader cells need to express cxcr4b for proper migration seems to cast doubt on the model that intrinsic polarity of chemokine receptors underlies directional migration of the primordium as cells in the central portion of such mosaic primordia express no chemokine receptors and therefore would be unable to function as a Cxcl12a sink. In this case, no instructive gradient of Cxcl12a protein would be formed across mosaic primordia.
A recent mathematical modeling study proposes that Cxcl12a adsorption by Cxcr4b receptors in the primordium is capable of generating differential ligand concentration in front and behind the primordium. Specifically, the forward migration of the primordium, coupled with Cxcl12a adsorption by Cxcr4b binding and internalization, leads to a lower level of Cxcl12a behind the primordium than in front of the primordium that is capable of sustaining directed migration. Therefore, the primordium leaves behind a wake of lower Cxcl12a concentration as it migrates.Citation31 However, this model is based on the premise that the chemokine receptor Cxcr4b is homogenously expressed in the primordium and it is therefore difficult to reconcile with the ability of mosaic primordia to migrate normally. In mosaic primordia, in which only a few wild type leader cells express cxcr4b, Cxcl12a would only be absorbed by a few cells at the leading edge allowing the accumulation of ligand near the central portion of the primordium. It is unclear if such a low number of cells would be able to generate an instructive Cxcl12a gradient. In addition, the model is too simplistic in its current form, as it presumes that only one chemokine receptor functions in the primordium. Since cxcr7b expression in trailing cells is also crucial for primordium migration, this mathematical model needs to modified.
Future experiments in primordium collective cell migration should be aimed at understanding the molecular functions of Cxcr4b and Cxcr7b. For example, it has been proposed that Cxcr7b is a non-signaling, scavenger receptor and therefore serves as a Cxcl12a sink.Citation23,Citation27,Citation29 However, this hypothesis remains to be experimentally tested for primordium migration. Additionally, it will be very interesting to determine if there are mechanotactic signals emanating from the leading edge cells of the migrating primordium as proposed by Lecaudey and Gilmour.Citation30 Such signals could potentially explain the ability of small numbers of cxcr4b-positive cells to rescue migration of an otherwise cxcr4b deficient primordium.
Cell Shape Changes
The central role of the migrating primordium is to form and distribute proneuromasts along the trunk of the animal. These proneuromasts are generated within the migrating primordium and deposited from its trailing edge (). Proneuromast formation is characterized by the alignment of cells into radially organized cellular rosettes. Rosettes are formed by the apical constriction of a ring of cells surrounding a small group of central cells.Citation21,Citation22,Citation32 The result of this process is a ring of pear shaped cells with the central cells displaced basally ().Citation21,Citation22,Citation32 The central cells express delta and fgf ligands and represent the presumptive hair cell progenitors.Citation20–Citation22,Citation32
The process of apical constriction of proneuromast cells correlates with the accumulation of apical markers such as ZO-1,Citation22,Citation32 β-cateninCitation20,Citation32 and Protein Kinase c proteins (PKRC).Citation32 During rosette formation, these apical markers accumulate strongly in the constricted cell apices along with F-actin.Citation32 This apical constriction requires the action of the cell polarity regulators lgl1 and lgl2.Citation32 As the formation of cellular rosettes requires Wnt/β-catenin-dependent Fgf signaling,Citation17,Citation21,Citation22 it is likely that Fgf signaling might directly regulate lgl1/lgl2 and/or prkci.Citation32 However, this connection remains to be experimentally verified. Importantly, the feedback loop between Wnt/β-catenin-Fgf signaling restricts apical constriction and rosette formation to the trailing region leaving the leading region largely devoid of Fgf signaling and proneuromast formation. Interestingly, a lineage tracing analysis experiment involving photoconversion of Kaede protein revealed that the first row of cells in the leading edge of the primordium is capable of giving rise to cells in deposited proneuromasts, as well as new leading region cells suggesting that the leading region serves as a progenitor pool for continued proneuromast formation.Citation21 Therefore, patterning the primordium into a trailing, organ forming region and a leading, progenitor region is important to ensure that enough cells can be generated to produce a sufficient number of proneuromasts along the anterior-posterior axis.
Work by Matsuda and Chitnis demonstrates that a strong loss of Delta-Notch signaling in the primordium also leads to the failure of apical constriction of proneuromast cells and primordium fragmentation.Citation20 This finding contrasts with earlier work that proposed that this morphogenic process was independent of Delta-Notch signaling.Citation21,Citation22 However, the Delta-Notch signaling reduction in these earlier studies was not complete.Citation20 The failure of rosette formation described in Matsuda and Chitnis was interpreted to result from the reduction in central cell Fgf signaling, which is consistent with other reports that correlate loss of Fgf signaling with failure of apical constriction and rosette morphogenesis.Citation17,Citation20–Citation22
Recent work on isolated chick otic ectoderm has revealed an Fgf-dependent morphogenic signaling mechanism.Citation33 This study uncovered that in the chick otic epithelium Fgf ligands bind to receptors localized to the basal aspect of the cells. This Fgf signaling mechanism occurs in the absence of Mek1/2 phosphorylation and protein translation; therefore, it does not involve canonical Fgf signaling leading to gene expression. Instead, this signaling mechanism results in the membrane accumulation of PKCalpha and the basal depletion of actin via a myosin-dependent mechanism, thereby driving apical constriction of the otic ectoderm into the otic cup.Citation33
It will be very interesting to explore whether a similar mechanism links Fgf signaling to apical constriction in proneuromasts. Of note, Nechiporuk and Raible found in mosaic experiments that only a few centrally positioned Fgf-positive cells rescue apical constriction and proneuromast formation in mosaic animals that lack Fgf ligand expression in the primordium.Citation21 It is reasonable to hypothesize that a short range Fgf signal from central cells is capable of driving proneuromast cell shape changes. According to this hypothesis, Fgf produced by central cells acts on basallateral Fgf receptors located in the presumptive supporting cells within the primordium. It is interesting to note that the central, Fgf ligand producing cells in the developing proneuromasts are basally displaced, positioning them to signal to the basal aspect of the surrounding support cells. Fgf receptor activation in basal parts of these cells might lead to depletion of actin in the basallateral aspect by a similar mechanism as occurs in the chick otic epithelium.Citation33 This could result in the radial apical constriction of presumptive supporting cells to generate the rosette morphology of the proneuromast (). Of course, this is but one of many mechanistic hypotheses for the connection between Fgf signaling and rosette morphogenesis and it may be that Fgf dependent apical constriction in proneuromasts is achieved by a very different mechanism than in the otic epithelium.
If the above hypothesis is correct, Fgf deficient primordia might be expected to have failed rosette formation due to lack of polarized actin depletion resulting in failed apical constriction. Detailed imaging of the distribution of F-actin, Fgf receptors and components of epithelial apical-basal polarity machinery in wild type primordia and primordia with manipulated Fgf and Delta-Notch signaling would aid in evaluating this hypothesis.
Interestingly, Fgf signaling regulates hair cell specification and apical constriction/rosette formation independently. While Fgf signaling is necessary for expression of the proneural gene atoh1 and the specification of sensory hair cells, loss of atoh1 function does not prevent the Fgf-dependent formation of cellular rosettes.Citation20–Citation22,Citation32 Therefore, Fgf signaling controls both proneuromast morphogenesis and neurogenesis by parallel pathways, similar to the roles of Fgf signaling during otic development.
Cell Proliferation
Cell proliferation is crucial for normal lateral line morphogenesis. The migrating primordium is composed of approximately 100 cells at any given time, yet the final posterior lateral line is comprised of approximately 300 cells.Citation34 It has been observed by time lapse analysis that the rate of proliferation measured in the primordium is such that it will generate enough cells to constitute all the cells of the lateral line.Citation34 This implies that there must be coupling between the generation of cells in the primordium and the rate the primordium migrates and deposits proneuromasts.
In 2005, the first systematic analysis of proliferation in the lateral line was conducted based on a series of BrdU incorporation assays.Citation35 The authors described that proliferation is not evenly distributed in time and space in the migrating primordium. Rather, there was a bias of BrdU incorporation toward the leading portion of the primordium. This bias fluctuated according to the phase of the deposition cycle. Primordia that were in the process of depositing a proneuromast showed a reduction in BrdU incorporation frequency in the trailing 1/3 of the tissue. Primordia that had just deposited a proneuromast showed no such bias.Citation35 However, the authors did not take into account that the length of the primordium fluctuates with the deposition cycle.
Recent work has uncovered a mechanistic basis for spatial and temporal pattern of proliferation within the primordium. Manipulation of the Wnt/β-catenin and Fgf signaling pathways revealed that these signaling activities are crucial regulators of primordium proliferation.Citation36 Because of feedback between these signaling pathways, experiments were designed to test the role of each signaling pathway in isolation. The results demonstrate that both Wnt/β-catenin and Fgf signaling are necessary for normal rates of proliferation in the primordium.Citation36 This finding provides an explanation for the previously reported spatial and temporal bias in proliferation. The size of the domain where these pathways overlap in the leading and central portions of the primordium remains constant, although the size of the primordium fluctuates as proneuromasts are deposited.Citation36 Therefore, the previously observed reduction in proliferation in the trailing proneuromast that is about to be deposited correlates with cells that have been displaced out of the Wnt/β-catenin and Fgf signaling co-expression domain.
Analysis of cell proliferation in the primordium suggests a model explaining the rate of proneuromast deposition.Citation36 Key to this model is the above mentioned finding that due to proliferation the primordium continuously elongates and cells in the central portion of the primordium are progressively displaced toward the trailing edge. Eventually, cells will be displaced entirely out of the region where Wnt/β-catenin and Fgf coincide and will become quiescent. Because cells that are displaced into the trailing region no longer receive Wnt/β-catenin signals, they exhibit a distinct gene expression profile from the remainder of the cells in the primordium. For example, these cells express the chemokine receptor cxcr7b and downregulate expression of cxcr4b. Cells that occupy this region slow down and deposit from the primordium.Citation17,Citation27,Citation28 Following deposition, the primordium is shorter and all cells lie within the proliferative region where Wnt/β-catenin and Fgf signaling is active until a sufficient number of cells accumulate for the cycle to repeat (). In support of this idea, cxcr7b expression begins at a set distance from the leading edge of the primordium regardless of the phase of the deposition cycle and the primordium length. Therefore, cxcr7b is a marker of the deposition domain at the trailing end of the primordium.Citation36
The ‘elongation dependent deposition model’ predicts that cells within the Wnt/β-catenin-free trailing region will express genes that are necessary for proneuromast deposition. These genes could potentially repress the ability of trailing cells to migrate, increase adhesion between trailing cells and the local substrate or decrease adhesion between trailing cells and the remainder of the primordium. Of course, these are not mutually exclusive possibilities and it is likely that some combination of these cellular activities represents the cellular mechanism of proneuromast deposition.
The initial evidence that the proliferation rate sets the rate of proneuromast deposition came from two types of experiments. In the first, the proliferation rate was directly reduced by treating the embryos with proliferation inhibiting drugs. This treatment leads to the deposition of a reduced number of more widely spaced proneuromasts without affecting the speed of migration or the size of the deposited proneuromasts.Citation36 In the second line of experimentation, embryos with elevated rates of apoptosis were analyzed. Similarly to proliferation inhibited embryos these primordia generated more widely spaced proneuromasts.Citation36 Taken together, these results demonstrate that the neuromast deposition frequency is tightly coupled to the rate of cell addition within the primordium.
Analysis of lateral line development in embryos with elevated apoptosis also raises a critical caveat pertaining to future lateral line research. The injection of morpholino antisense nucleotides to knock down gene function frequently elevates the level of apoptosis.Citation40 As an increased level of apoptosis causes the deposition of fewer proneuromasts, morpholino injection experiments aimed at evaluating molecular mechanisms of lateral line development have to be well controlled for their effect on apoptosis and the cell cycle. Several reports have relied on morpholino injection experiments to elucidate molecular mechanisms regulating proneuromast deposition without testing possible effects on cell death.Citation41–Citation43 In light of these recent findings, these functional studies should be re-examined.
The model of proliferation dependent proneuromast deposition has been subsequently reinforced by a mathematical modeling study. In this study the primordium was modeled as an elastic rod traveling on a homogenous stripe of Cxcl12a protein. As the rod grows, cells at the trailing edge are exposed to less and less Cxcl12a due to absorption of Cxcl12a by cells in the leading and central portions of the primordium. Eventually, the primordium reaches a critical length where the cells at the trailing edge are no longer exposed to enough Cxcl12a to migrate and they deposit from the primordium.Citation31
Additional experimental evidence for the mitogenic role of Wnt/β-catenin signaling has recently been provided by the analysis of lef1 morphants and mutants.Citation37,Citation46,Citation47 lef1 is a TCF/LEF class transcription factor that functions downstream of Wnt/β-catenin signaling.Citation38,Citation39 In lef1 morphants and mutants, BrdU incorporation frequency is reduced relative to control embryos.Citation37,Citation46,Citation47 Analysis of lef1 mutant embryos has led to a subtly distinct model of proneuromast deposition.Citation47 This model posits that proliferation sets the length of time it takes for a new proneuromast to form within the migrating primordium. When a proneuromast accumulates a sufficient number of cells, it deposits from the trailing edge of the primordium. Several experiments could be performed to distinguish between these two possibilities. First, if proneuromast maturation/growth and not proneuromast displacement is mediated by cell proliferation, inhibiting normal rates of proliferation would be expected to generate primordia harboring fewer proneuromasts on average. Conversely, if proliferation is not necessary for proneuromast formation and instead for primordium elongation, the average number of proneuromasts per primordium will be the same as in untreated primordia. Therefore, primordia with reduced rates of proliferation should be analyzed for proneuromast number. Second, the hypothesis that cell proliferation drives the rate of proneuromast formation could be tested by following the formation of proneuromast rosettes in primordia via timelapse microscopy. The proneuromast formation model predicts that in proliferation-deficient embryos the average time to form a proneuromast rosette will be longer while the length dependent deposition model predicts that proneuromasts will form at a normal rate and then take longer on average to transit through the primordium and be deposited.
Analysis of cell number in wild type primordia that harbor three proneuromasts raises problems with the neuromast maturation/growth model of proneuromast deposition (unpublished data). This analysis was performed by counting DAPI labeled nuclei in individual proneuromast rosettes of Tg(-8.0cldnb;lynEGFP) embryos, in which cells of the primordium are tagged with membrane localized GFP fluorescence enabling identification of individual proneuromast rosettes. This analysis reveals that there is no significant difference in cell number between the trailing proneuromast that is about to deposit and the proneuromast that resides immediately posterior to it. These proneuromasts do not exhibit a significant difference in cell number and yet only the trailing proneuromast will deposit. This argues against a model where proneuromast deposition is triggered by an increase in proneuromast size.
Comparison of lef1 mutants and globally proliferation-deficient primordia reveals some interesting differences. In embryos treated with general proliferation inhibitors, proneuromast deposition occurs more slowly and the resulting proneuromasts are deposited at greater distance from each other than wt.Citation36,Citation47 Conversely, in lef1 mutant primordia, proneuromast deposition occurs more rapidly, although the primordia migrate at a similar rate, and therefore proneuromasts are closer together then wt.Citation46,Citation47 One reason for this discrepancy is that lef1 mutant primordia have specific reduction of proliferation in the leading region and not in the central portion of the primordium. This leads to the early depletion of leading zone progenitor cells.Citation46,Citation47 Additionally, cells in the leading zone of lef1 mutant embryos are unable to maintain progenitor identity and are recruited into proneuromasts at a higher rate than in wt primordia.Citation46 The elongation dependent deposition model would predict that the distance between the proneuromast deposition domain and the leading edge of the primordium will be sensitive to reduction in the strength of Wnt/β-catenin signaling. This is consistent with the finding that cxcr7b, a marker for the deposition zone, is repressed by Wnt/β-catenin singling.Citation36 Therefore, reducing the strength of Wnt/β-catenin signaling in the primordium by removal of a single tcf/lef factor would lead to expansion of the deposition zone toward the leading edge of the primordium due to loss of inhibition. Although the deposition zone marker cxcr7b remains restricted to trailing tissue in lef1 mutants,Citation46,Citation47 it will take detailed measurements to determine if this domain extends closer to the leading edge in lef1 mutants. The consequence of the shortening of the Wnt/β-catenin domain and shift of the deposition domain is that it will take fewer cell divisions to displace a proneuromast into this zone and therefore proneuromasts will be deposited closer together. Simultaneously, failure to renew the progenitor pool in the leading zone of lef1 mutants leads to the depletion of cells and early arrest and fragmentation of the primordium.Citation46,Citation47
This is consistent with the conclusions drawn by Valdivia et al. where in the absence of lef1 the leading edge diminishes and the strength of a ‘hypothetical factor’ produced in the leading zone is diminished. It is interesting to speculate that the ‘hypothetical factor’ hypothesized is the Wnt ligand responsible for driving Wnt/β-catenin singling signaling in the primordium. In this case, diminishing the leading region would lead to reduction in Wnt ligand concentration thereby reducing the range of Wnt ligand into the trailing region. This, in turn, would exacerbate the expansion of the deposition zone toward the leading region. Additional evidence for the leading region being the source of Wnt ligands in the primordium stems from analysis of the expression patterns of Wnt/β-catenin target genes, such as lef1, axin2 and fgf3. These genes show graded expression patterns strongest at the leading edge consistent with a leading edge source of Wnt ligand.Citation17,Citation46,Citation47 It will be very interesting to establish the identity and expression pattern of this Wnt ligand.
Differential Cell Adhesion
While the primordium maintains a remarkable level of cohesion during migration, proneuromasts are deposited periodically and interneuromast cells are deposited continuously from the trailing edge. Therefore, dynamic regulation of cell-cell adhesion between primordium cells is crucial for lateral line morphogenesis. Differential adhesion during lateral line morphogenesis is a largely unexplored area of research. Mechanistic connections that remain to be explained are the differential adhesion among cells of a proneuromast rosette within the primordium and the lack of adhesion to continuously deposited interneuromast cells.Citation8,Citation32 Additionally, to fully understand periodic proneuromast deposition, we need to characterize how adhesion molecules are differentially regulated in the leading region of the primordium and the deposition zone.
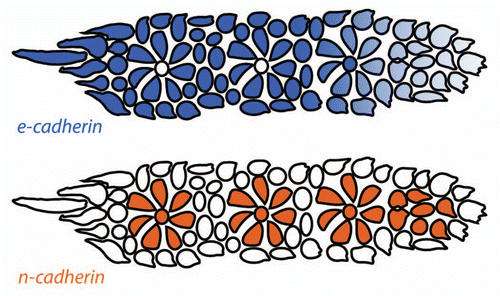
Only very few adhesion molecules have been described in the developing lateral line thus far. n-cadherin (cdh-2) is expressed in the primordium, however, it does not appear to be necessary for primordium migration or the formation and deposition of proneuromasts.Citation43 Interestingly, in situ hybridization against n-cadherin shows reduced expression in cells at the periphery of the primordium and in cells that lie between proneuromasts.Citation20,Citation43 As these cells likely represent the presumptive interneuromast cells,Citation32 this expression suggests that lack of n-cadherin in these cells might facilitate the continuous deposition of interneuromast cells.
While n-cadherin disruption by morpholino injection experiments does not lead to a failure in migration or proneuromast deposition, interneuromast cell deposition was not investigated.Citation43 Although this study detected a reduction in the number of deposited proneuromasts, this reduction was within the normal range to be expected from morpholino toxicity, therefore should be reexamined.Citation36 Detailed time lapse analyses of n-cadherin deficient embryos would be useful to evaluate possible defects in interneuromast cell deposition.
e-cadherin (cdh1), is expressed throughout the primordium but appears to be more strongly expressed in cells at the periphery.Citation20,Citation44e-cadherin expression appears to be downregulated in Delta-Notch deficient embryos by in situ hybridization. Loss of e-cadherin expression in this context correlates with fragmentation of the primordium and proneuromasts suggesting a role for e-cadherin in primordium cohesion.Citation20 Interestingly, e-cadherin expression is rescued in Delta-Notch deficient embryos by atoh1 knockdown which in turn rescues cohesion in proneuromasts within primordia, but not deposited proneuromasts.Citation20 This finding suggests that an uncharacterized set of atoh1-dependent adhesion molecules are required for cohesion of deposited proneuromasts.Citation20 The role of e-cadherin during lateral line morphogenesis remains to be functionally tested.
It is tempting to hypothesize that central cell Fgf signaling might be involved in the regulation of differential adhesion between proneuromasts and surrounding cells in the primordium. For example, there might be a specific set of adhesion molecules regulated by Fgf signaling emanating from central cells that is responsible for proneuromast cohesion. Presumptive interneuromast cells may lie outside the influence of these ligands and therefore express a different set of adhesion molecules and do not adhere well to the proneuromasts.Citation20,Citation32 The simplest hypothesis that can be drawn from published data are that n-cadherin-positive, e-cadherin-negative pro-interneuromast cells adhere tightly to each other and less tightly to e-cadherin-negative, n-cadherin-positive proneuromast cells (). As the pro-interneuromast cells lie at the periphery of the primordium and between the proneuromasts within the primordium, these cells are free to continuously deposit as the primordium migrates. Evaluation of this hypothesis will require functional manipulation of cadherins in the primordium. It is possible that additional adhesion molecules play important roles in the primordium that have yet to be discovered. Overall, the study of cell adhesion within the primordium is a relatively unexplored avenue that will likely yield great insights into lateral line morphogenesis in the future.
Differential adhesion between proneuromasts and the rest of the primordium plays a crucial role in the periodic proneuromast deposition process. In contrast to interneuromast cells, proneuromasts only deposit once the entire proneuromast is displaced into the deposition zone, thus leading to periodic deposition. In addition, only the last proneuromast loses contact to the rest of the primordium, likely because in the Wnt/β-catenin free deposition zone, adhesion is differentially regulated. The differential expression of adhesion molecules in the Wnt/β-catenin free trailing region could cause cells in that region to become increasingly adhesive to the underlying extracellular matrix, perhaps by the increased expression or activity of integrin receptors. It would be worthwhile to investigate if such extracellular matrix adhesion molecules are expressed in the primordium and are repressed in the leading region by Wnt/β-catenin signaling. If the differential adhesion of proneuromasts to primordium cells in the leading region and the deposition zone is indeed regulated by Wnt/β-catenin signaling, this would provide further evidence for a central role of Wnt/β-catenin signaling in regulating multiple aspects of lateral line morphogenesis. In this scenario, Wnt/β-catenin signaling would regulate chemokine dependent cell migration, Fgf dependent apical constriction/rosette formation, cell proliferation, as well as cell-cell adhesion.
Outlook
Although great strides have been made into understanding lateral line morphogenesis, much work remains. Now that we understand the basic links between cell-signaling based primordium patterning and the fundamental cell behaviors that drive lateral line morphogenesis, the door is open to understanding these links at a deeper, more mechanistic level. For example, it has been shown that Wnt/β-catenin and Fgf signaling are together required to drive proliferation within the primordium.Citation36 However, little is known about the downstream molecular effect that underlies this co-requirement.
Since Wnt/β-catenin and Fgf signaling regulate migration, the formation of proneuromasts and cell proliferation within the primordium, these signaling interactions provide an elegant mechanism of coordinating these crucial morphogenic cell behaviors. Regulating all of these cell behaviors with a common upstream patterning system represents an elegant way of coordinating these activities during lateral line morphogenesis to ensure the correct number and spacing of mechanosensory organs. It is likely that such coordination of cell behaviors by pleiotropic patterning mechanisms, where common upstream signaling systems affect numerous cell behaviors across space and time, represents a common aspect of morphogenesis. However, structures with more complicated morphologies than the lateral line will likely require more complex patterning mechanisms to exert even finer control and coordination of a wider suite of cell behaviors.
This coupled cell behavior mechanism described above also allows an inroad to speculation about evolutionary change to the embryonic lateral line. Different species of aquatic vertebrates have widely varying numbers of primary proneuromasts.Citation45 The model presented above can be used to derive some mechanistic predictions about these differences. For example, one could hypothesize that animals with a greater number of primary proneuromasts than found in zebrafish might have a higher rate of proliferation within the primordium and/or a slower rate of migration. Conversely, animals with fewer proneuromasts than zebrafish likely have less proliferative and/or more quickly migrating primordia. One complicating factor could be that different species might also have differences in proneuromast size. Mechanisms that regulate proneuromast size within the primordia are poorly understood.
Although use of the lateral line system has proven very productive for studying collective cell migration,Citation7,Citation25 the lateral line also has become a very productive model for the study of epithelial morphogenesis.Citation20–Citation22,Citation32 The advantage of the lateral line system for such studies is that the molecular regulation of epithelial cell shape changes can be readily perturbed and analyzed in a living embryo.Citation17,Citation20–Citation22,Citation32 One challenge that must be overcome to further this line of research is the early requirement of many fundamental epithelial morphogenesis proteins. However, with the increasing sophistication of molecular genetics tools and the ease of generating mosaic zebrafish embryos these challenges are far from insurmountable.
In general, we are entering an exciting time when more and more detailed analysis will become possible. Now that the general rules of primordium patterning and the cellular basis of lateral line morphogenesis are coming into focus, we will be able to address increasingly detailed aspects of morphogenesis. Some of the lessons learned from these studies promise to shed light on other disparate areas of biology as coordinating fundamental cell behaviors such as proliferation, migration and cellular morphology are common features of most developmental events.
Figures and Tables
Figure 1 Lateral line embryology visualized using Tg(-8.0cldnb:lyneGFP) embryos. (A) At 24 hpf the primordium (asterisk) has fully migrated onto the somites from its origin near the otic vesicle (OV). (B) The primordium has recently deposited the first proneuromast. (C) Higher magnification of the primordium in (A). Note the rosette shaped proneuromasts forming in the trailing region and the lack of rosettes in the leading region. (D) The post embryonic lateral line is comprised of 4–6 proneuromast in a row along the horizontal myoseptum.
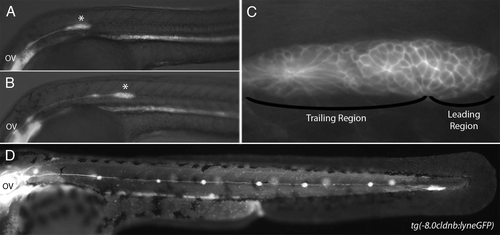
Figure 2 (A) Primordium polarity is maintained by Wnt/β-catenin signaling. The leading region expresses Wnt/β-catenin target genes (red) and the trailing region expresses Fgf target genes (green). Both signaling pathways are active in the central portion of the primordium (yellow). Wnt/β-catenin signaling dependent sef expression represses Fgf signaling in the leading region and Fgf dependent dkk1 expression represses Wnt/β-catenin signaling in the trailing region. Solid lines represent genetic interactions. Dashed lines represent diffusion of secreted factors. (B) Cells in the center of rosette shaped proneuromasts within the primordium express delta and fgf ligands (blue). The remainder of the primordium, with the exception of the leading edge expresses notch receptors (orange). Expression of delta and fgf ligands is restricted to central cells though Delta-Notch mediated lateral inhibition. Cells that constitute the leading edge progenitor pool are outlined in green. Cells in forming proneuromasts are outlined in pink.
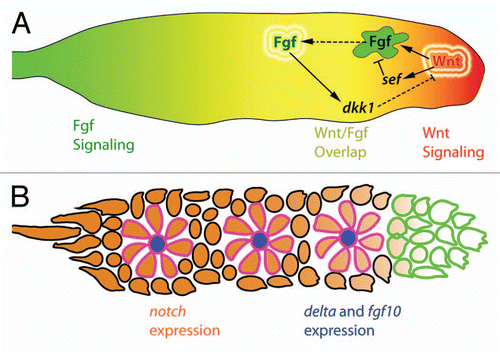
Figure 3 Hypothetical mechanism of apical constriction in forming proneuromasts. Fgf signals emanating from the leading region stimulate the expression of fgf and delta ligands. This expression is restricted to centrally located hair cell progenitors (HC, blue) by Delta-Notch mediated lateral inhibition. Fgf signals from the central hair cell progenitor could potentially interact with Fgf receptors in support cell progenitors (SC, green) leading to the depletion of cortical actin filaments (purple) and radial apical constriction. Apical adherens junction proteins such as ZO-1 and β-catenin (red) accumulate at the center of the forming proneuromast. The forming proneuromast is surrounded by interneuromast cell progenitors (INMC, white) that are outside the range of central cell fgf ligand and therefore do not apically constrict (adapted with permission from Hava et al.Citation32).
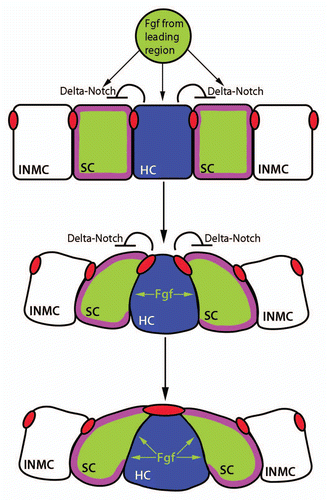
Figure 4 The proliferation-dependent primordium lengthening model of periodic proneuromast deposition. Proliferation in the leading zone (green) displaces cells into the deposition zone (blue) where they begin slowing down and depositing from the primordium. When an entire proneuromast is displaced into the deposition zone it deposits. This leads to a shortened primordium (middle part). Proliferation continues, another proneuromast is displaced into the deposition zone, and the cycle repeats (reproduced with permission from Aman et al.Citation36).
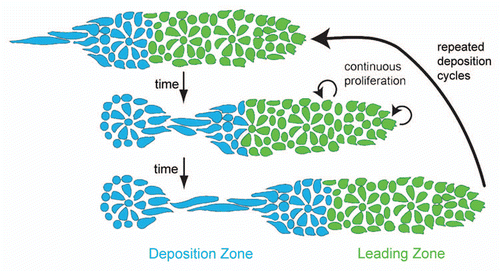
Figure 5 Distribution of classical cadherins in the primordium. e-cadherin (blue) is expressed broadly in the primordium but is downregulated in the leading region and in proneuromast central cells. n-cadherin (orange) is expressed in apically constricted proneuromast cells and in proneuromast central cells. Cells at the primordium periphery and between the proneuromasts within the primordium express e-cadherin but not n-cadherin and represent the presumptive interneuromast cells (adapted with permission from Matsuda and ChitnisCitation20).
References
- Aman A, Piotrowski T. Cell migration during morphogenesis. Dev Biol 2010; 341:20 - 33; PMID: 19914236; http://dx.doi.org/10.1016/j.ydbio.2009.11.014
- Stone LS. Experiments on the development of the cranial ganglia and the lateral line sense organs in Amblystoma punctatum. J Exp Zool 1922; 35:420 - 496; http://dx.doi.org/10.1002/jez.1400350403
- Metcalfe WK, Kimmel CB, Schabtach E. Anatomy of the posterior lateral line system in young larvae of the zebrafish. J Comp Neurol 1985; 233:377 - 389; PMID: 3980776; http://dx.doi.org/10.1002/cne.902330307
- Nicolson T. The genetics of hearing and balance in zebrafish. Annu Rev Genet 2005; 39:9 - 22; PMID: 16285850; http://dx.doi.org/10.1146/annurev.genet.39.073003.105049
- Webb JF. Gross morphology and evolution of the mechanoreceptive lateral-line system in teleost fishes. Brain Behav Evol 1989; 33:205 - 222; PMID: 2655823
- Gompel N, Cubedo N, Thisse C, Thisse B, Dambly-Chaudiere C, Ghysen A. Pattern formation in the lateral line of zebrafish. Mech Dev 2001; 105:69 - 77; PMID: 11429283; http://dx.doi.org/10.1016/S0925-4773(01)00382-3
- Sapède D, Gompel N, Dambly-Chaudiere C, Ghysen A. Cell migration in the postembryonic development of the fish lateral line. Development 2002; 129:605 - 615; PMID: 11830562
- Grant KA, Raible DW, Piotrowski T. Regulation of latent sensory hair cell precursors by glia in the zebrafish lateral line. Neuron 2005; 45:69 - 80; PMID: 15629703; http://dx.doi.org/10.1016/j.neuron.2004.12.020
- Nuñez VA, Sarrazin AF, Cubedo N, Allende ML, Dambly-Chaudière C, Ghysen A. Postembryonic development of the posterior lateral line in the zebrafish. Evol Dev 2009; 11:391 - 404; PMID: 19601973; http://dx.doi.org/10.1111/j.1525-142X.2009.00346.x
- Ghysen A, Dambly-Chaudiere C. Development of the zebrafish lateral line. Curr Opin Neurobiol 2004; 14:67 - 73; PMID: 15018940; http://dx.doi.org/10.1016/j.conb.2004.01.012
- Ghysen A, Dambly-Chaudière C. The three-sided romance of the lateral line: Glia love axons love precursors love glia. Bioessays 2005; 27:488 - 494; PMID: 15832385; http://dx.doi.org/10.1002/bies.20225
- Dambly-Chaudière C, Sapède D, Soubiran F, Decorde K, Gompel N, Ghysen A. The lateral line of zebrafish: a model system for the analysis of morphogenesis and neural development in vertebrates. Biol Cell 2003; 95:579 - 587; PMID: 14720460; http://dx.doi.org/10.1016/j.biolcel.2003.10.005
- Platt J. Ontogenetic differentiations of the ectoderm in Necturus. Anat Anz 1894; 8:51 - 56
- Bailey SW. An experimental study of the origin of lateral-line structures in embryonic and adult teleosts. J Exp Zool 1937; 76:187 - 233; http://dx.doi.org/10.1002/jez.1400760203
- Northcutt RG, Catania KC, Criley BB. Development of lateral line organs in the axolotl. J Comp Neurol 1994; 340:480 - 514; PMID: 8006214; http://dx.doi.org/10.1002/cne.903400404
- Haas P, Gilmour D. Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Dev Cell 2006; 10:673 - 680; PMID: 16678780; http://dx.doi.org/10.1016/j.devcel.2006.02.019
- Aman A, Piotrowski T. Wnt/β-catenin and fgf signaling control collective cell migration by restricting chemokine receptor expression. Dev Cell 2008; 15:749 - 761; PMID: 19000839; http://dx.doi.org/10.1016/j.devcel.2008.10.002
- Gamba L, Cubedo N, Ghysen A, Lutfalla G, Dambly-Chaudiere C. Estrogen receptor ESR1 controls cell migration by repressing chemokine receptor CXCR4 in the zebrafish posterior lateral line system. Proc Natl Acad Sci USA 2010; 107:6358 - 6363; PMID: 20308561; http://dx.doi.org/10.1073/pnas.0909998107
- Itoh M, Chitnis AB. Expression of proneural and neurogenic genes in the zebrafish lateral line primordium correlates with selection of hair cell fate in neuromasts. Mech Dev 2001; 102:263 - 266; PMID: 11287207; http://dx.doi.org/10.1016/S0925-4773(01)00308-2
- Matsuda M, Chitnis AB. Atoh1a expression must be restricted by Notch signaling for effective morphogenesis of the posterior lateral line primordium in zebrafish. Development 2010; 137:3477 - 3487; PMID: 20876657; http://dx.doi.org/10.1242/dev.052761
- Nechiporuk A, Raible DW. FGF-dependent mechanosensory organ patterning in zebrafish. Science 2008; 320:1774 - 1777; PMID: 18583612; http://dx.doi.org/10.1126/science.1156547
- Lecaudey V, Cakan-Akdogan G, Norton WHJ, Gilmour D. Dynamic Fgf signaling couples morphogenesis and migration in the zebrafish lateral line primordium. Development 2008; 135:2695 - 2705; PMID: 18599504; http://dx.doi.org/10.1242/dev.025981
- Perlin JR, Talbot WS. Signals on the move: Chemokine receptors and organogenesis in zebrafish. Sci STKE 2007; 2007:45
- Aman A, Piotrowski T. Multiple signaling interactions coordinate collective cell migration of the posterior lateral line primordium. Cell Adh Migr 2009; 3:365 - 368; PMID: 19736513; http://dx.doi.org/10.4161/cam.3.4.9548
- David NB, Sapède D, Saint-Etienne L, Thisse C, Thisse B, Dambly-Chaudière C, et al. Molecular basis of cell migration in the fish lateral line: Role of the chemokine receptor CXCR4 and of its ligand, SDF1. Proc Natl Acad Sci USA 2002; 99:16297 - 16302; PMID: 12444253; http://dx.doi.org/10.1073/pnas.252339399
- Li Q, Shirabe K, Kuwada JY. Chemokine signaling regulates sensory cell migration in zebrafish. Dev Biol 2004; 269:123 - 136; PMID: 15081362; http://dx.doi.org/10.1016/j.ydbio.2004.01.020
- Dambly-Chaudière C, Cubedo N, Ghysen A. Control of cell migration in the development of the posterior lateral line: antagonistic interactions between the chemokine receptors CXCR4 and CXCR7/RDC1. BMC Dev Biol 2007; 7:23; PMID: 17394634; http://dx.doi.org/10.1186/1471-213X-7-23
- Valentin G, Haas P, Gilmour D. The chemokine sdf1a coordinates tissue migration through the spatially restricted activation of cxcr7 and cxcr4b. Curr Biol 2007; 17:1026 - 1031; PMID: 17570670; http://dx.doi.org/10.1016/j.cub.2007.05.020
- Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, et al. Control of chemokine-guided cell migration by ligand sequestration. Cell 2008; 132:463 - 473; PMID: 18267076; http://dx.doi.org/10.1016/j.cell.2007.12.034
- Lecaudey V, Gilmour D. Organizing moving groups during morphogenesis. Curr Opin Cell Biol 2006; 18:102 - 107; PMID: 16352429; http://dx.doi.org/10.1016/j.ceb.2005.12.001
- Streichan SJ, Valentin G, Gilmour D, Hufnagel L. Collective cell migration guided by dynamically maintained gradients. Phys Biol 2011; 8:45004; PMID: 21750360; http://dx.doi.org/10.1088/1478-3975/8/4/045004
- Hava D, Forster U, Matsuda M, Cui S, Link BA, Eichhorst J, et al. Apical membrane maturation and cellular rosette formation during morphogenesis of the zebrafish lateral line. J Cell Sci 2009; 122:687 - 695; PMID: 19208766; http://dx.doi.org/10.1242/jcs.032102
- Sai X, Ladher RK. FGF signaling regulates cytoskeletal remodeling during epithelial morphogenesis. Curr Biol 2008; 18:976 - 981; PMID: 18583133; http://dx.doi.org/10.1016/j.cub.2008.05.049
- Laguerre L, Ghysen A, Dambly-Chaudiere C. Mitotic patterns in the migrating lateral line cells of zebrafish embryos. Dev Dyn 2009; 238:1042 - 1051; PMID: 19334282; http://dx.doi.org/10.1002/dvdy.21938
- Laguerre L, Soubiran F, Ghysen A, Konig N, Dambly-Chaudiere C. Cell proliferation in the developing lateral line system of zebrafish embryos. Dev Dyn 2005; 233:466 - 472; PMID: 15779042; http://dx.doi.org/10.1002/dvdy.20343
- Aman A, Nguyen M, Piotrowski T. Wnt/β-catenin dependent cell proliferation underlies segmented lateral line morphogenesis. Dev Biol 2011; 349:470 - 482; PMID: 20974120; http://dx.doi.org/10.1016/j.ydbio.2010.10.022
- Gamba L, Cubedo N, Lutfalla G, Ghysen A, Dambly-Chaudiere C. lef1 controls patterning and proliferation in the posterior lateral line system of zebrafish. Dev Dyn 2010; 239:3163 - 3171; PMID: 20981829; http://dx.doi.org/10.1002/dvdy.22469
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, et al. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 1996; 86:391 - 399; PMID: 8756721; http://dx.doi.org/10.1016/S0092-8674(00)80112-9
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, et al. Functional interaction of β-catenin with the transcription factor LEF-1. Nature 1996; 382:638 - 642; PMID: 8757136; http://dx.doi.org/10.1038/382638a0
- Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, et al. p53 activation by knockdown technologies. PLoS Genet 2007; 3:78; PMID: 17530925; http://dx.doi.org/10.1371/journal.pgen.0030078
- Haines L, Neyt C, Gautier P, Keenan DG, Bryson-Richardson RJ, Hollway GE, et al. Met and Hgf signaling controls hypaxial muscle and lateral line development in the zebrafish. Development 2004; 131:4857 - 4869; PMID: 15342468; http://dx.doi.org/10.1242/dev.01374
- Villablanca EJ, Renucci A, Sapède D, Lec V, Soubiran F, Sandoval PC, et al. Control of cell migration in the zebrafish lateral line: Implication of the gene “Tumour-Associated Calcium Signal Transducer,” tacstd. Dev Dyn 2006; 235:1578 - 1588; PMID: 16552761; http://dx.doi.org/10.1002/dvdy.20743
- Kerstetter AE, Azodi A, Liu Q. Cadherin-2 function in the cranial ganglia and lateral line system of developing zebrafish. Dev Dyn 2004; 230:137 - 143; PMID: 15108318; http://dx.doi.org/10.1002/dvdy.20021
- Liu Q, Ensign RD, Azodi E. Cadherin-1, -2 and -4 expression in the cranial ganglia and lateral line system of developing zebrafish. Gene Expr Patterns 2003; 3:653 - 658; PMID: 12972001; http://dx.doi.org/10.1016/S1567-133X(03)00109-1
- Wada H, Hamaguchi S, Sakaizumi M. Development of diverse lateral line patterns on the teleost caudal fin. Dev Dyn 2008; 237:2889 - 2902; PMID: 18816847; http://dx.doi.org/10.1002/dvdy.21710
- McGraw HF, Drerup CM, Culbertson MD, Linbo T, Raible DW, Nechiporuk AV. Lef1 is required for progenitor cell identity in the zebrafish lateral line primordium. Development 2011; 138:3921 - 3930; PMID: 21862556; http://dx.doi.org/10.1242/dev.062554
- Valdivia LE, Young RM, Hawkins TA, Stickney HL, Cavodeassi F, Schwarz Q, et al. Lef1-dependent Wnt/β-catenin signalling drives the proliferative engine that maintains tissue homeostasis during lateral line development. Development 2011; 138:3931 - 3941; PMID: 21862557; http://dx.doi.org/10.1242/dev.062695
- Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor CXCR7 in T lymphocytes. J Biol Chem 2005; 280:35760 - 35766; PMID: 16107333; http://dx.doi.org/10.1074/jbc.M508234200