Abstract
Purpose: HPV16 is associated with ~50% of all cervical cancers worldwide. The E6 and E7 genes of oncogenic HPV types, such as HPV16, are necessary for the HPV transforming function and tumorogenesis making them ideal targets for novel treatments. Radioimmunotherapy employs systemically administered radiolabeled monoclonal antibodies (mAbs) that bind to tumor-associated antigens. Previously we demonstrated in mice that radioimmunotherapy targeting viral antigens with mAb to HPV16 E6 suppressed CasKi cervical tumors expressing high levels of E6 (~600 copies of HPV per cell). However, that study opened the question whether radioimmunotherapy can suppress the growth of cervical tumors with low E6 and E7 expression, such as may be seen in patients.
Experimental Design: We evaluated the expression of E6 in patients' tumors and in the SiHa cell line expressing low levels of E6 and E7 (1-2 copies of HPV per cell) and found them comparable. We initiated SiHa tumors in nude mice, radiolabeled C1P5 mAb to E6 with a beta-emitter 188-Rhenium (188Re) and treated tumor-bearing mice with: (1) 200 μCi 188Re-C1P5 alone; (2) proteasome inhibitor MG132 alone; (3) MG132 followed by 200 μCi 188Re-C1P5; (4) unlabeled C1P5; (5) 200 μCi 188Re-18B7 (isotype-matching control mAb); (6) no treatment. 188Re-C1P5 alone and in combination with MG-132 significantly retarded tumor growth compared to all control groups.
Conclusions: Our data demonstrate the possibility to suppress tumor growth by targeting viral antigens even in cervical tumors with low E6 expression and provide additional evidence for the potential usefulness of radioimmunotherapy targeting HPV-related antigens in the clinic.
Introduction
More than 95% of all cervical cancers are associated with the human papillomavirus (HPV),Citation1,Citation2 with over 500,000 women being diagnosed with cervical cancer per year worldwide (WHO data, http://www.who.int/research/en/). The viral oncoproteins E6 and E7 immortalize epithelial cells in culture and increase cellular transformation in concert with other oncoproteins.Citation3–Citation5 E6 oncoproteins are located intracellularly and bind to p53, promoting its rapid degradation via the ubiquitin-dependent pathway, while E7 oncoproteins bind to the retinoblastoma (Rb) gene, thus causing ineffective regulation of cell growth and deregulates mitosis.Citation6 In addition, these oncogenes minimize the effects of the tumor suppressor genes p53 and Rb, so that more random mutations can occur, which can potentially lead to malignant transformation. Thus, targeting E6 and E7 oncoproteins appears to be logical for the development of novel therapies for cervical cancer.Citation7
Radioimmunotherapy (RIT) uses tumor antigen-specific monoclonal antibodies (mAbs) for targeted delivery of cytocidal ionizing radiation to the tumor cellsCitation8 and radiolabeled mAbs have been approved for treatment of primary, recurrent or refractory non-Hodgkin lymphoma (NHL). Previously we demonstrated the feasibility of targeting E6 and E7 oncoproteins in experimental cervical cancer by using radiolabeled mAbs to E6 as selective mediators of tumor destruction.Citation9 Targeting viral antigens within the tumors is fundamentally different from traditional RIT, which aims for cell surface associated tumor markers. The distinctive features of this approach are: (1) the targets are of viral origin as opposed to “self” human antigens, which minimizes cross-reactivity with host tissues and (2) the viral proteins normally reside in intracellular compartments, such as the intranuclear location of the E6 and E7 oncoproteins. Although intracellular proteins are normally outside the reach of immunogloblulins, this approach works because in tumors there are many non-viable and necrotic cells with permeable membranes that allow mAbs access to intracellular antigens. After the radiolabeled mAb binds to its respective antigen, it mediates destruction of viable tumor cells through long range beta emission of a radionuclide such as 188-Rhenium (188Re), an effect which has been termed “cross-fire” (). Our group recently demonstrated that pre-treatment of CasKi cervical tumor-bearing mice with proteasome inhibitor MG-132 elevated the levels of E6 target protein in the tumor, which resulted in the increased uptake of E6-specific mAb in the tumors,Citation10 thus potentially making them more susceptible to E6-targeted cell kill.
Though CaSki cells and tumors were used in the original proof-of-principle experiments that demonstrated efficacy,Citation9,Citation10 there was a concern that this efficacy was artificially enhanced by the fact that these cells possess a very high number of HPV16 copies (600 copies per cell) resulting in high expression of E6 protein, and consequently providing abundant target even if there were few dead cells in the tumor. This concern was highly relevant to the proposed clinical use of this therapy because many cervical tumors are heterogeneous and may contain areas with minimal E6 expressing cells. We hypothesized that low HPV16-expressing human cervical tumors such as SiHa (∼1–2 HPV16 copies per cell) will still be treatable with RIT due to the “cross-fire” irradiation of distant viable tumor cells by beta-particles emanating from radiolabeled mAb. Here we report the results of RIT of mice bearing SiHa cervical tumors, which express less E6 than CaSki cells, with and without pre-treatment with proteasome inhibitor MG-132.
Results
The levels of E6 oncoprotein expression in SiHa tumors were comparable to those in tumors from patients with metastatic cervical cancer.
We analyzed the E6 and E7 expression in tumor tissue of 4 consecutively resected cervical cancer tumors in patients who consented to have their tumors studied. In every tissue the E6 and E7 oncoproteins were clearly expressed (). When E6 levels in patients' tumors were compared to those in CaSki tumors in nude mice, the former were significantly lower (). However, the levels of E6 in SiHa tumors in nude mice approximated the levels of E6 in patient tumors ().
Treatment with MG132 proteasome inhibitor elevated E6 in SiHa cells in vitro and in vivo.
Treatment of SiHa cells in vitro with 0–20 µg/ml MG132 elevated the levels of E6 in SiHa cells (). The same effect was observed in vivo when nude mice bearing SiHa tumors were treated with IP 20 µg MG132, their tumors removed 3 hrs later and analyzed by western blot for E6 expression ().
RIT was efficient in treating SiHa tumors in nude mice.
Untreated SiHa tumors grew very aggressively with one mouse needing to be sacrificed because of the tumor diameter exceeding 1 cm on day 12 post-treatment, and the rest of the mice in the untreated group had to be sacrificed for the same reason on day 21. 188Re-C1P5 mAb to E6 administered as a 200 µCi dose significantly decreased the tumor size up to day 18 post-treatment and slowed it down until the end of the observation period compared to all control groups ( and ). The combination of pre-treatment with MG132 followed by 188Re-C1P5 mAb administration 3 hrs later was also effective in slowing down the tumor growth ( and ) but not as pronounced as 188Re-C1P5 mAb alone especially when compared to the non-specific 188Re-18B7 mAb and the “cold” (unlabeled) C1P5 control groups ( and ). In fact, the pre-treatment with MG132 was antagonistic to 188Re-C1P5 mAb.
The histological evaluation of the tumors at the end of the observation period showed that the RIT-treated tumors in both 188Re-C1P5 mAb alone and combined MG132 and 188Re-C1P5 mAb groups had significantly more necrosis and hemorrhage than tumors from control mice ( and B). The morphologic appearance of the tumor cells in these groups often had a more vacuolated cytoplasm suggesting degeneration. The tumors in untreated, “cold” C1P5 mAb and the non-specific 188Re-18B7 mAb groups consisted mostly of coherent sheets of intact tumor cells (, D and F) with some islets of non-viable cells observed in the MG132 alone group ().
Discussion
Cervical cancer is the second leading cause of cancer death among women worldwide despite the major advances in early detection and treatment of pre-cancerous lesions and, more recently, with the availability of preventative type-specific prophylactic HPV vaccines. In women with metastatic cervical cancer the impact of surgery and the efficacy of radiation therapy, even when concomitant with chemotherapy, are limited. Thus, novel therapeutic options with potentially less toxicity than chemotherapy are urgently needed. Naked mAbs have been evaluated in the treatment of gynecologic malignancies, such as ovarian cancer; however, the success of these therapies has been limited.Citation12 Currently, there are no clinical trials of mAbs therapies for patients with cervical cancer.
We recently suggested that RIT targeting viral antigens could be used in the treatment of virus-associated tumorsCitation13,Citation14 and performed proof-of-principle experiments in experimental HPV16-related cervical cancer.Citation9,Citation10 Many virus-associated cancer cells express viral antigens either on their surfaces or intracellularly. Intracellular viral antigens are also potential targets for RIT, since tumor cell turnover is likely to result in the release of these proteins into tumor interstitial space. It is likely that in patients with HPV-related cancers the tumors are heterogeneous in terms of their levels of E6 expression, and may contain the areas devoid of E6 expressing cells. Consequently the E6 levels in clinical tumors is unlikely to reach the levels seen in CasKi tumors (600 HPV16 copies per cell).Citation9,Citation10 In preparation for translating the RIT targeting viral proteins in cervical cancer into the clinic, we sought a more realistic experimental cancer model and selected SiHa, which expresses only low levels of E6 (1–2 HPV 16 copies per cell). The RIT of SiHa tumors in nude mice worked very similar to the results observed previously with 1.5 higher radioactivity dose of the same 188Re-C1P5 mAb administered to nude mice bearing CaSki tumors with high levels of E6 expression. It is important to emphasize that when treating virally-associated cancers by targeting viral antigen, not every cell in the tumor needs to express viral antigens for a therapeutic effect. Long range emitters such as 188Re (emission range in tissue 10 mm) emit radiation in a 360° sphere and consequently can kill viable tumor cells in the vicinity of the antigen location via the so-called “cross-fire” effect (). We have recently demonstrated by performing computer modeling of RIT of melanoma tumors with 188Re-labeled mAb to melanin—also an intracellular antigen—that even in tumors with low concentration of target antigen the radiation dose to the tumor will be almost equal to the dose delivered to the tumor with high levels of targeted antigen.Citation15 This is due to the complex interplay of mAb binding to the antigen in tumor periphery with penetration into the deeper levels of the tumor.Citation15
The proteasome inhibitor MG-132 reduces the degradation of ubiquitin-conjugated proteins in mammalian cells without affecting ATPase or isopeptidase activities. MG-132 has been reported to result in increased levels of E6 and E7 proteins in cervical cancer cells.Citation16,Citation17 In our previous study with CasKi cells, MG-132 elevated the levels of E6 in the cells in vitro.Citation10 However, we did not observe any therapeutic advantage of pre-treating tumor-bearing mice with MG132 prior to radiolabeled mAb. On the contrary, the pre-treatment with MG-132 was antagonistic to the effect of 188Re-C1P5 mAb, which is obvious from the comparison of 188Re-C1P5 mAb alone and combination therapy to the control groups especially 188Re-18B7 mAb and “cold” C1P5 (). As E6 and E7 are important oncoproteins, one possible explanation could be that the elevation in their levels with MG132 might have affected the tumor suppressor genes p53 and Rb downstream. In the future it might be worthwhile to pre-treat tumor-bearing mice with a “classical” chemotherapeutic agent such as cisplatin or a second dose of radiolabeled mAb, which would result in killing some of the tumor cells and lead to more of E6 target protein becoming accessible to E6-specific mAb. In this regard, we have recently shown that pre-treatment of experimental melanoma tumors with dacarbazine (DTIC) rendered some tumor cells non-viable, which made intracellular melanin pigment more accessible for the radiolabeled melanin-binding antibody.Citation18
In our previous study we observed a significant tumor retardation effect of the “cold” C1P5 mAb on CaSki tumors in nude mice when the mAb was given in the amount of 30 µg per mouse.Citation9 In this study some retardation of SiHa tumor growth with 6 µg “cold” C1P5 mAb was still observed though radiolabeled 188Re-C1P5 mAb was significantly more effective (). The nature of this tumor retardation may be due to the complement deposition caused by the mAb and the ensuing inflammation which we observed in the CaSki tumors (Phaeton R, Jiang Z and Dadachova E, unpublished observations). Hence, for the “cold” antibody effect the lower amount of E6 target could have translated into fewer immunoglobulin binding sites with a concomitant reduction in the amount of antibody available for pro-inflammatory activities.
Finally, the irrelevant 188Re-labeled 18B7 mAb demonstrated some retardation effect on the tumors, though the effect of E6-specific 188Re-C1P5 mAb alone was significantly more pronounced at every time point except for the last one on day 30 when tumor re-growth was observed ( and ). The same effect of non-specific radiolabeled mAb was observed by Chen et al. when the idea of targeting intranuclear antigens with radiolabeled mAbs in fast growing tumors was first suggested.Citation19 The authors explained the effect using a four-compartmental model. If this model were applied to our study, the control 18B7 isotype-matching mAb would behave in an identical fashion to C1P5 mAb within the first three compartments (blood, extravascular fluid and intracellular fluid space of permeable cells). Both 18B7 and C1P5 have IgG1 isotype and, therefore, would enter and diffuse within the extracellular fluid space (second compartment) at a comparable rate. In normal tissues, diffusion from the blood to the extracellular space is slow but in the vicinity of the tumor, where vascular permeability is increased, the mAbs enter the extracellular space at an accelerated rate. Likewise, both mAbs would penetrate permeable cells (third compartment) in a similar fashion. However, once within the tumor cells, C1P5 would show progressive binding to E6 in the non-viable cell, whereas 18B7 would remain free within the cytoplasm. With clearance of the mAb from the blood over several days, the concentration gradient would be expected to reverse and 18B7, being unbound, would diffuse from within the cells to the extracellular fluid and to the blood, while C1P5 would be retained in the non-viable tumor cell, continuously delivering cytocidal radiation to the nearby viable tumor cells by “cross-fire” effect.
In summary, our data demonstrate that it is possible to suppress tumor growth by targeting viral antigens in cervical tumors with low E6 protein expression similar to that in patients. This potentially makes RIT of HPV-related cervical cancer a clinically relevant and important therapeutic modality.
Materials and Methods
Cell line, antibodies and reagents.
CaSki and SiHa cell lines were obtained from the American Type Culture Collection (Manassas, VA). Cells were grown in RPMI-1640 medium containing 10% FBS (Sigma) and 1% Penicillin-streptomycin solution (Sigma, penicillin 10,000 U and streptomycin 10 mg/ml) at 37°C in a 5% CO2 incubator. These cell lines were derived from HPV16 positive human cervical cancers that express both E6 and E7 oncogenic proteins. A murine antibody C1P5 of IgG1 isotype which binds to E6 oncoprotein expressed by HPV16 and HPV18 types was obtained from Abcam. An isotype-matching murine control mAb 18B7 to cryptococcal polysaccharide was produced as described in.Citation11 Proteasome inhibitor MG-132 was obtained from Calbiochem; BD Matrigel™ Basement Membrane Matrix was procured from BD Biosciences.
Patients tumor tissue.
The tumor tissue from patients undergoing surgery for metastatic cervical cancer at Montefiore Medical Center was collected under an IRB-approved protocol. The tumors were placed into the cell culture flasks containing DMEM/HAM F-12K (Sigma, 1:1), 10% FBS (Sigma) and 1% penicillin-streptomycin solution (Sigma, penicillin 10,000 U and streptomycin 10 mg/ml), homogenized on ice and western blot was performed as described below.
Western blots.
Cell pellets were suspended in the lysis buffer (4% SDS, 20% glycerol, 0.5 M TrisHCl (pH 6.8), 0.002% bromophenol blue and 10% β-mercaptoethanol). Protein samples were boiled in water for 15 min before running SDS-PAGE. Twenty five-thirty µl of protein solution was loaded into each well of 12% pre-cast SDS-PAGE gel (Bio-Rad). SDS-PAGE was used to monitor the relative protein amounts in the samples. Twelve percent SDS-PAGE gel was used to separate proteins and electrophoresis was performed using Mini-Protean® 3 Cell system (Bio-Rad). After electrophoresis, the gel was transferred into the PVDF transfer buffer (25 mM Tris, 190 mM glycine and 2.5% (v/v) methanol) for 5 min. Then, proteins were transferred from the gel to the Immun-Blot™ PVDF membrane (Bio-Rad) on Semi-dry Electrophoretic Transfer Cell (Bio-Rad) at 15 V for 17 min. The membrane was soaked in the blocking buffer (25 mM TrisHCl (pH 7.6), 1 mM EDTA and 150 mM NaCl) for 5 min and then transferred into the blocking solution (5% non-fat dry milk in the blocking buffer), shaking gently for an hour. The membrane was incubated in TBST solution (0.1% Tween-20, 25 mM TrisHCl (pH 7.6) and 500 mM NaCl) containing 1:3,000 diluted primary antibody at room temperature for 1 hour with gentle shaking. Then, the membrane was washed with TBST three times (10 min per wash). The membrane was hybridized by the secondary antibody conjugated with alkaline phosphatase (Rabbit polyclonal to mouse IgG H&L (alkaline phosphatase)) in TBST with a 1:100,000 dilution. After three washes with TBST, the membrane was incubated in CDP-Star™ chemiluminescent substrate solution (Sigma) for 5 min and then exposed to CL-XPosur™ film (Pierce). The film was developed as per manufacturer's instructions.
SiHa and CasKi tumor models.
All animal studies were carried out in accordance with the guidelines of the Institute for Animal Studies at the Albert Einstein College of Medicine. Six-eight week-old athymic Nu/Nu CD1 nude mice were purchased from Charles River Laboratories. Ten million CasKi or SiHa cells were mixed with 80% Matrigel and injected subcutaneously into the right flank of each mouse. Tumors measuring 0.3–0.4 cm in diameter appeared approximately in 2 weeks post injection of cancer cells.
MG132 treatment of SiHa cells in vitro and in vivo.
MG132 (Z-LLL-CHO, MW = 457.6) is a potent, reversible and cell-permeable proteasome inhibitor (Ki = 4 nM). MG132 was first dissolved in a drop of 100% ethanol followed by DMEM/HAM F-12K medium without addition of FBS and the stock solution was stored at 4°C. Cell culture was harvested and transferred into 6 sterile test tubes. Each tube contained 0.5–1 ml cell culture (cell concentration was ∼106 cells/ml) and cells were allowed to grow at 37°C in 5% CO2 incubator overnight. Then, MG132 solution was added to the tubes for the final concentrations of 0, 5, 10 or 20 µg/ml. The cells were incubated under the same conditions for another 3 hours. Finally, the cells were harvested by centrifugation and clarified by washing with PBS.
For in vivo investigation of the influence of MG-132 on the level of E6 expression in SiHa tumors, a group of 3 mice carrying the SiHa tumors was injected intraperitoneally (IP) with 20 µg MG-132 and another group of 3 tumor-bearing mice was used as control. Three hours later, all mice in both groups were sacrificed, their tumors removed, homogenized on ice and processed for western blot as described above.
Antibody radiolabeling.
The beta-emitter 188Re with a half life of 16.9 hours was produced from beta decay of 188-Tungsten parent (half life 69 days) using a 188W/188Re generator (Oak Ridge National Laboratory, Oak Ridge, TN). After 188Re was eluted in the form of sodium perrhenate, the antibodies were labeled with 188Re “directly” through binding of reduced 188Re to the generated-SH groups on the antibodies as previously described.Citation9 Briefly, the mAbs were treated (reduced) for 40 min at 37°C with dithiothreitol and subsequently purified from the unreacted dithiothreitol on Centricon-30 microconcentrators. An amount of 10 mCi 188ReO4 in saline was reduced by incubation with 20 mg Na gluconate and 20 µL 20 mg/mL SnC12 in 0.1 M HCl at 37°C for 60 min. Reduced 188Re(V)-gluconate was combined with reduced and purified 0.24 mg mAbs at 1 mg/mL concentration and kept at 37°C for 60 min. Radioactivity not bound to the mAbs was removed by centrifugal purification on Centricon-30 microconcentrators. The specific activity of radiolabeled antibodies was 33.3 mCi/mg.
Therapy of SiHa tumors in nude mice with 188Re-labeled mAbs.
For therapeutic studies mice with tumors measuring 0.5–0.7 cm in diameter were randomized into groups of five. The groups were treated IP with:
# 1–200 µCi 188Re-C1P5 mAb;
# 2-matching amount (6 µg) of “cold” C1P5 mAb;
# 3–20 µg MG-132 followed by 200 µCi 188Re-C1P5 mAb 3 hr later;
# 4–20 µg MG-132;
# 5–200 µCi 188Re-18B7 control mAb;
# 6-untreated.
Mice were observed for tumor growth and survival and for 30 days. The size of the tumor was measured every 3 days with calipers in three dimensions and the tumor volume was calculated as a product of three dimensions divided by 2. For histological analysis the tumors were removed from the mice at the completion of the experiment (for untreated control the tumors were processed on day 21 when remaining untreated mice had to be sacrificed due to large tumor diameters), fixed in buffered formalin, paraffin embedded, cut into 5 µm slices and stained with hematoxylin and eosin (H&E). The experiment was performed twice.
Statistical analysis.
The sample sizes in animal experiments were pre-planned. The differences between the tumor sizes for differently treated groups in the RIT studies were analyzed by non-parametric Mann-Whitney test using Prism software (GraphPad, San Diego, CA). Differences were considered statistically significant when p values were <0.05.
Figures and Tables
Figure 1 Diagram illustrating the mechanism of cervical cancer RIT with radiolabeled E6 or E7-binding mAbs. E6 and E7 proteins become accessible to mAbs in the non-viable cells and in the interstitial space as a consequence of cellular turnover in a fast-growing tumor. The mAbs bind to accessible E6 and E7 and deliver cytotoxic radiation to the tumor. Viable cancer cells are killed by radiation penetrating several cells diameters (so called “cross-fire” effect). CC, cervical cancer.
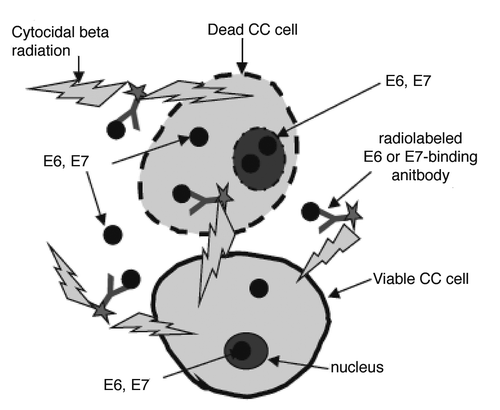
Figure 2 Western blot of cervical tumor cell lysates showing E6 and E7 expression: (A) E6 and E7 in tumor tissues from 4 patients with metastatic cervical cancer. P1–P4, Patients 1–4; (B) E6 in CasKi tumor (line 1) and in patient tumor (line 2); (C) E6 in patient tumor (line 1) and in SiHa tumor (line 2).
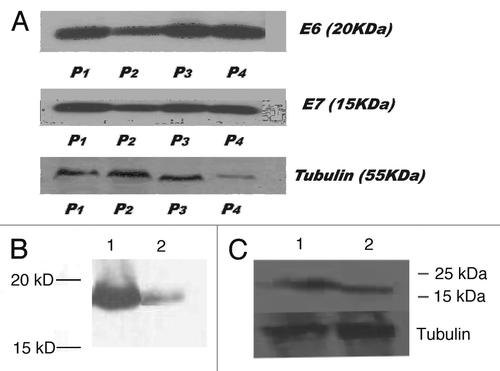
Figure 3 Influence of proteasome inhibitor MG132 on the levels of E6 in SiHa cells in vitro and in vivo: (A) SiHa cells in vitro treated with 0, 5, 10 and 25 µg/ml MG132 (lines 1–4, respectively) for 3 hrs; (B) SiHa tumors from nude mice. Tumor in line 1 is from a mouse treated with 20 µg MG132 with tumor being removed 3 hrs after is from the control mouse treatment; tumor in line 2 is from the control untreated mouse.
Figure 4 Tumor size in SiHa tumor-bearing mice treated with: 200 µCi 188Re-C1P5 mAb; matching amount (6 µg) of “cold” C1P5 mAb; 20 µg MG-132 followed by 200 µCi 188Re-C1P5 mAb 3 hr later; 20 µg MG-132; 200 µCi 188Re-18B7 control mAb; or left untreated. On day 12 one mouse in control group was sacrificed because tumor diameter exceeded 1 cm. To, tumor diameter on the day of treatment; Tn, tumor diameter on the day of measurement.
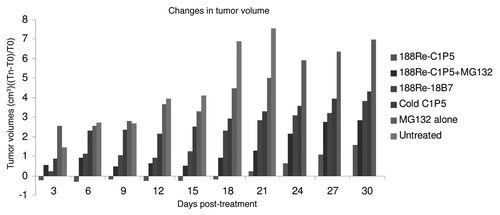
Figure 5 H&E histology of SiHa tumors from the following treatment groups in the RIT experiment: (A) 200 µCi 188Re-C1P5 mAb; (B) 20 µg MG-132 followed by 200 µCi 188Re-C1P5 mAb 3 hr later; (C) untreated; (D) “cold” C1P5 mAb; (E) 20 µg MG-132; (F) 200 µCi 188Re-18B7 control mAb. Original magnification ×100.
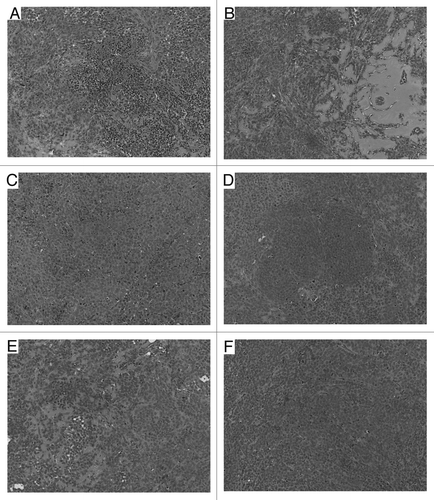
Table 1 p values for comparison of tumor sizes in E6-specific treatment groups 188Re-C1P5 mAb alone and in MG132 plus 188Re-C1P5 mAb to control groups in RIT experiment
Acknowledgements
The research was partially supported by: Mary Kay Ash Foundation grant, Einstein NIH-designated Cancer Center, Center for AIDS Research, Albert Einstein College of Medicine, Fighting Children's Cancer Foundation and NIH grant AI60507 (E.D.); NIH grants AI33774 and AI33142 (A.C.); an American Cancer Society Research Scholar Grant (115164-RSG-08-002-01-CCE) (M.E.).
References
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12 - 19
- Bosch FX, Manos MM, Muñoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst 1995; 87:796 - 802
- Kaur P, McDougall JK, Cone R. Immortalization of primary human epithelial cells coned cervical carcinoma DNA containing human papillomavirus type E6/E7 open reading frames. J Gen Virol 1989; 70:1261 - 1266
- Kaur P, McDougall JK. HPV-18 immortalization of human keratinocytes. Virology 1989; 173:302 - 310
- Percoraro G, Morgan D, Defendi V. Differential effects of human papillomavirus type 6, 16 and 18 DNAs on immortalization and transformation of human cervical epithelial cells. Proc Natl Acad Sci USA 1989; 86:563 - 567
- Nguyen CL, Münger K. The human papillomavirus E7 protein deregulates mitosis via an association with the Nuclear Mitotic Apparatus Protein-1 (NuMA). J Virol 2009; 83:1700 - 1707
- Kadish AS, Einstein MH. Vaccine strategies for human papillomavirus-associated cancers. Curr Opin Oncol 2005; 17:456 - 461
- Sharkey RM, Goldenberg DM. Targeted therapy of cancer: new prospects for antibodies and immunoconjugates. CA Cancer J Clin 2006; 56:226 - 243
- Wang XG, Revskaya E, Bryan RA, Strickler HD, Burk RD, Casadevall A, et al. Treating cancer as an infectious disease—viral antigens as novel targets for treatment and potential prevention of tumors of viral etiology. PLoS ONE 2007; 2:e114
- Phaeton R, Wang XG, Einstein MH, Goldberg GL, Casadevall A, Dadachova E. The influence of proteasome inhibitor MG132, external radiation and unlabeled antibody on the tumor uptake and biodistribution of (188)Re-labeled anti-E6 C1P5 antibody in cervical cancer in mice. Cancer 2010; 116:1067 - 1074
- Casadevall A, Cleare W, Feldmesser M, Glatman-Freedman A, Goldman DL, Kozel TR, et al. Characterization of a murine monoclonal antibody to Cryptococcus neoformans poly-saccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother 1998; 42:1437 - 1446
- Berek JS, Dorigo O, Schultes B, Nicodemus C. Specific keynote: immunological therapy for ovarian cancer. Gynecol Oncol 2003; 88:105 - 109
- Casadevall A, Goldstein H, Dadachova E. Targeting host cells harbouring viruses with radiolabeled antibodies. Expert Opin Biol Ther 2007; 7:595 - 597
- Dadachova E, Wang XG, Casadevall A. Targeting the virus with radioimmunotherapy in virus-associated cancer. Cancer Biother Radiopharm 2007; 22:303 - 308
- Schweitzer AD, Rakesh V, Revskaya E, Datta A, Casadevall A, Dadachova E. Computational model predicts effective delivery of 188-Re-labeled melanin-binding antibody to the metastatic melanoma tumors with wide range of melanin concentrations. Melanoma Res 2007; 17:291 - 303
- Kehmeier E, Rühl H, Voland B, Stöppler MC, Androphy E, Stöppler H. Cellular steady-state levels of “high risk” but not “low risk” human papillomavirus (HPV) E6 proteins are increased by inhibition of proteasome dependent degradation independent of their p53- and E6AP-binding capabilities. Virology 2002; 299:72 - 87
- Oh KJ, Kalinina A, Wang J, Nakayama K, Nakayama KI, Bagchi S. The papillomavirus E7 oncoprotein is ubiquitinated by UbcH7 and Cullin 1- and Skp2-containing E3 ligase. J Virol 2004; 78:5338 - 5346
- Revskaya E, Jongco AM, Sellers RS, Howell RC, Koba W, Guimaraes AJ, et al. Radioimmunotherapy of experimental human metastatic melanoma with melanin-binding antibodies and in combination with dacarbazine. Clin Cancer Res 2009; 15:2373 - 2379
- Chen F-M, Taylor CR, Epstein AL. Tumor necrosis treatment of me-180 human cervical carcinoma model with 13ii-labeled tnt-1 monoclonal antibody. Cancer Res 1989; 49:4578 - 4585