Abstract
Aims: Oral squamous cell carcinoma (OSCC) is the most prevalent malignancy of the oral cavity resulting in severe morbidity and mortality. To date only few proteins have been suggested as potential biomarkers or targets for this type of cancer. Cancerous inhibitor of PP2A (CIP2A) is a protein expressed in epithelial tissues that stabilizes the oncogene c-Myc and causes cell transformation. This study was designed to investigate the expression of CIP2A in OSCC cell lines and tissues representing human normal, dysplasia and OSCC. Methods: Using quantitative real time PCR, mRNA quantification for CIP2A was performed in a primary gingival cell line and OSCCs CAL 27 and SCC-25. Paraffin embedded human specimen classified as normal, dysplastic or OSCC were immunohistochemically stained for CIP2A expression. EGFR and CIP2A were also stained by immunofluorescence for co-localization. Samples of human normal oral tissue and OSCC were studied by PCR for mRNA expression of CIP2A. Results: CIP2A was significantly increased in the human carcinoma cell lines compared to the primary gingival cell line. CIP2A was overexpressed in the human oral dysplasia and OSCC tissues compared to normal oral tissues. CIP2A was also preferentially localized in the dysplastic and OSCC epithelial areas compared to EGFR that was expressed mainly in areas of relatively normal epithelium and in dysplastic tissues above the basal layers. Conclusions: CIP2A may play a significant role in oral malignant transformation and therefore, it may be a potential target for chemotherapy of OSCC.
Introduction
It is estimated that about 35,000 new cases and over 8,000 deaths related to cancer of the oral cavity and pharynx will occur annually in the USA in 2010.Citation1,Citation2 Oral cavity and pharynx cancers are currently ranked as the sixth- eight most prevalent cancers in the world, with squamous cell carcinomas (SCC) of the oral mucosa by far the most common type (83%–90%).Citation3,Citation4 It has been demonstrated that early detection of cancerous lesions generally results in reduced morbidity and mortality for some types of cancer.Citation4 This is important for oral cancers because cases that require substantial resection or radiation treatment can result in significant morbidity.Citation4 Unfortunately, oral precancerous lesions are frequently asymptomatic and can be difficult to detect at the earliest stages using current visual inspection methods.Citation5 Although the WHO classification for epithelial dysplasia is accepted by most of the oral pathologists, there is a great variability in their interpretation of the criteria.Citation6–Citation8 Furthermore, studies have demonstrated significant inter-examiner discrepancies in the assessment and grading of the histopathological features of dysplastic lesions.Citation7,Citation8 Keeping this in mind, it is obvious that the adoption of new reliable markers of epithelial dysplasia may be instrumental in the accurate and reproducible histological diagnosis of oral dysplasia.
Epidermal growth factor receptor (EGFR) is a tyrosine kinase receptor of the ErbB family, which is expressed in a variety of solid tumors, including oral cancers.Citation9 High EGFR expression has been correlated with tumor size, metastasis and survival.Citation10 In recent years, EGFR has been considered a promising target for monoclonal antibody as well as other therapy.Citation10,Citation11 However, EGFR targeted therapy is not universally applicable for oral cancers and the identification of alternate markers is needed.
In 2002, our laboratory reported the cloning and characterization of a novel autoantigen p90 which was shown to be overexpressed in hepatocellular carcinoma and gastric cancer.Citation12 In a subsequent collaborative study, p90 was discovered as protein that binds to and inhibit Protein Phosphatase 2A (PP2A) activity and shown to play a critical role in cancer progression.Citation13 Thus p90 was renamed the cancerous inhibitory protein of PP2A or CIP2A. Most interestingly CIP2A was shown to inhibit of the degradation of c-myc oncoprotein and was reported to be overexpressed in oral cancer in the same study using a Finnish cohort.Citation13
The purpose of the present study was to examine the expression of CIP2A in oral cancer cell lines and oral dysplasia and OSCC tissues and determine their distribution and co-localization in the different components of epithelial cells in relation to EGFR staining. The data generated by this study may be instrumental in developing strategies for early detection of premalignant and malignant oral lesions and identifying new attractive targeted therapies for oral cancer.
Results
Up-regulation of CIP2A expression in oral cancer cell lines. CIP2A protein and mRNA expression levels in two oral cancer cell lines, SCC-25 and CAL 27, were examined and compared to normal primary gingival epithelial cells (pGECs) by Western blot analysis and quantitative real-time PCR, respectively (). Using tubulin as a loading control, the CIP2A protein levels in both SCC-25 and CAL 27 cells were clearly demonstrated to be up-regulated compared to pGECs (). Moreover, upon normalizing to 18S rRNA levels, CIP2A mRNA levels were found to be approximately 6 and 4-fold higher in CAL 27 and SCC-25 cells, respectively, compared to pGECs ().
Up-regulation of CIP2A expression in oral cancer tissues. CIP2A and EGFR expression were analyzed in oral squamous cell carcinomas by IIF analysis ( and ). As shown in , CIP2A expression in OSCC tissue exhibited a cytoplasmic staining pattern as previously reported.Citation12 Pre-immune rabbit serum yielded no staining of OSCC tissues (data not shown), where as rabbit anti-CIP2A serum yielded strong staining of OSCC tissues. To further demonstrate the specificity of the anti-CIP2A antibodies, we pre-incubated the antibodies with either rCIP2A or rGiantin proteins for one hour, and then performed IIF analysis. demonstrated that after pre-incubation with rCIP2A, CIP2A staining was abolished compared to anti-CIP2A antibodies pre-incubated with rGiantin. This data also showed that this rabbit anti-CIP2A serum specifically recognized CIP2A protein.
Co-staining of EGFR and CIP2A in the oral squamous cell carcinomas demonstrated interestingly similar staining patterns, but also clearly distinguishing characteristics ( and ). CIP2A expression was generally found higher in differentiated epithelium compared to the undifferentiated basal layer ( and ). In contrast, EGFR staining was strongest in basal layers ( and ). Overlapping CIP2A and EGFR expression was clearly observed in somel samples ( and ). CIP2A and EGFR staining were negative in a normal human epithelial control tissue (data not shown).
Immunohistological analysis of CIP2A expression in oral cancer tissues. In addition to the IIF analyses above, standard immunohistological methods were also performed. and show representative CIP2A staining of oral dysplasias and OSCCs, respectively. Strong CIP2A staining was noted in the basal and parabasal cells in mild to moderate epithelial dysplasias (). In OSCCs, strong scattered CIP2A reactivity was noted in groups of malignant epithelial cells. Well differentiated OSCCs often displayed clusters of CIP2A positive cells. Some of the lymphocytic infiltrates also demonstrated CIP2A staining. Additionally, some CIP2A staining was also detected in keratin pearls and the squamous eddies often observed in carcinoma biopsies ().
summarizes the CIP2A protein staining intensity data from a total of 30 human tissues. Eight out of eight cases of OSCCs were graded for a maximal score of 3 per each slide, where as seven out of the eight severe dysplasia cases were graded as 2 and one as 1. From the moderate dysplasias, four out of six were graded 1 and two scored 0. All eight sections from normal oral tissues were scored 0. Moreover, the four tissue types were collapsed into “normal” and “abnormal” categories for further analysis. Results of a Mann-Whitney U test indicated that graded staining intensity was significantly higher for abnormal than for normal tissue samples (p<0.001). In subsequent specific comparisons, staining intensity grades for each of the three abnormal groups considered separately were also found to be significantly higher than those for the normal group (p<0.01 in all cases).
Discussion
In the present study we have demonstrated that CIP2A is abundantly expressed in OSCC cell lines as well as dysplastic and malignant human oral epithelium tissues. These data confirms similar findings by Juntilla et al.Citation13 who reported high expression of CIP2A protein in 11 out of 14 head and neck cancer samples, whereas 9 normal non-malignant tissues from the oral cavity were all negative for CIP2A.
CIP2A expression in our study was generally high in differentiated epithelium where the tumor cells were present compared to the less differentiated basal layers (). In contrast, EGFR staining was strongest in basal layers ().
Aberrant expression of CIP2A demonstrates that there is a change in the ability of a cell to regulate cell proliferation. As a phosphatase, PP2A acts as a tumor suppressor which prevents the activation of oncogenes while causing the cell to arrest while in the cell cycle. Inhibition of CIP2A can lead to detrimental effects, increasing the potential for cancerous transformations.Citation14
In malignant cells, CIP2A binds to c-Myc serine 62 (S62), an oncogenic transcription factor.Citation14 The interaction between CIP2A and c-Myc S62 inhibits a tumor suppressor known as protein phosphatase 2A (PP2A).Citation15 CIP2A therefore stabilizes c-Myc S62 through its ability to inhibit PP2A's association with c-Myc S62.Citation14 Since PP2A is a tumor suppressor, the inhibition of this molecule (due to the formation of a CIP2A/c-Myc S62 complex) causes cell transformation. CIP2A, therefore, has a definite role in tumor formation and cell growth.Citation14,Citation15
Recent studies have shown that inhibition of CIP2A in gastric cancer causes the tumor cells to undergo senescence.Citation15 In this state, the cells will no longer proliferate and the cancer growth will cease. Once the cells fail to proliferate, they will be more susceptible to treatment via radiation and chemotherapy with CIP2A as a target.Citation15 CIP2A immunopositivity is a predictor of survival for some subgroups of gastric cancer patients.Citation14 CIP2A and MYC appear to be regulated in a positive feedback loop, wherein they promote each other's expression and gastric cancer cell proliferation.Citation14,Citation16,Citation18
CIP2A is associated with clinical aggressively in human breast cancer and promotes the malignant growth of breast cancer cells.Citation17 Thus, these results validate the role of CIP2A as a clinically relevant human oncoprotein and warrant further investigation of CIP2A as a therapeutic target in epithelial cancer.
In our study CIP2A was abundantly expressed in the abnormal epithelial layers. In the dysplastic and malignant zones positively stained CIP2A cells overlapped with areas that were EGFR positive. However, EGFR was not uniformly expressed in malignant tissue whereas in dysplastic tissues staining was mainly observed in the epithelial layers above the basal layer. Whereas, CIP2A appeared to be expressed in the basal layers which tend to exhibit the most dysplastic alterations and also in the areas of frank malignancy. It is also important to note that the histologic diagnosis of dysplasia is fraught with disagreements and wide inter-observer variability.Citation6–Citation8 In view of the significantly wide variation in interpretation of oral epithelial dysplasia,Citation6–Citation8 CIP2A has the potential to be used as an adjunct tool in the aid of diagnosis of dysplastic tissues.
In conclusion, CIP2A is strongly associated with oral dysplasia and OSCC. The exact role of this protein in the oral malignant transformation is yet to be determined, however, its abundance in oral cancer cells indicates that CIP2A may present a potential new target for OSCC chemotherapy.
Materials and Methods
Cell culture.
The cell lines CAL 27 and SCC-25 were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Each of the cell lines were cultured in ATCC specified complete growth media in a 37°C incubator with 5% CO2.
Cells were then prepared for western blotting analysis of p90/CIP2A protein levels, tubulin was detected as a loading control. Real-time quantitative PCR analyses of the comparative levels of p90/CIP2A mRNA levels were then performed. The results represent the mean ± S.D. of two different experiments.
Patients and tissue samples.
OSCC, oral dysplasia and normal oral mucosa samples were obtained from the archives of the University of Florida Oral Pathology Biopsy Service. For localization of p90 and EGFR immunofluorescence (IF) was employed, 4 micron serial sections were cut, processed using established antigen retrieval protocols and immunostained with a rabbit anti-CIP2A serum. Rabbit pre-immune serum and rabbit anti-giantin antibodies were used as negative and positive staining controls, respectively. Co-staining was carried out with murine monoclonal antibodies to EGFR. The absorption assay was performed by pre-incubating anti-CIP2A antibodies with either recombinant CIP2A (rCIP2A) protein or rGiantin protein for 1 hour. Immunofluorescence was then performed as previously described.
CIP2A protein expression.
Immunohistochemistry (IHC). Eight samples diagnosed as OSCC, 8 severe dysplasia, 6 as moderate dysplasia and 8 as benign oral tissues were obtained as paraffin embedded sections and subjected to IHC staining.
The slides were evaluated by 2 calibrated board certified oral pathologists and graded for staining intensity on a scale of 0–3. Staining intensity was rated according to the following scale: no visible staining = 0, faint staining = 1+, moderate staining = 2+ and strong staining = 3+. Extensiveness was graded semi quantitatively as 0%, <10%, 10–25%, 25–50%, 50–75%, 75–90% and >90% of positively stained cells, per high-power field. To compare all of the available data, we assigned an overall score to each case by multiplying the intensity score by the mean percentage of cells stained (extensiveness). The overall score was used as the basis for statistical analysis.
Antigen retrieval was achieved using the DAKO S1699 kit. The primary antibody was rabbit anti human p90/CIP2A at dilution of 1:300. Secondary antibody was goat anti-rabbit HRP and the chromogen developed into brown staining using 3,3′-Diaminobenzidine (DAB).
Figures and Tables
Figure 1 p90/CIP2A is overexpressed in oral squamous cell carcinoma cell lines. (A) western blotting analysis of p90/CIP2A protein levels in two cell lines derived from oral squamous cell carcinoma of the tongue (CAL 27 and SCC-25) compared to normal primary gingival epithelial cells (pGEC). Tubulin was detected as a loading control. (B) Real-time quantitative PCR analysis of the comparative levels of p90/CIP2A mRNA levels in CAL 27, SCC-25 and pGEC cells. The results represent the mean ± S.D. of two different experiments.
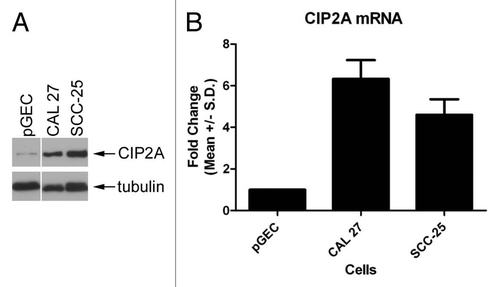
Figure 2 CIP2A/EGFR expression and localization in oral squamous cell carcinoma. CIP2A expression by immunofluorescence is highest in differentiated apical cell layers while EGFR expression is highest in basal cell layers. Serial tissue sections were co-stained with rabbit anti-CIP2A (green) and mouse anti-EGFR antibodies (red). Nuclei were counterstained with DAP I (blue). 100X (A) and 400X (B) magnification shown. (C) Specificity of anti-CIP2A staining demonstrated by absorption with recombinant CIP2A protein. Absorption was performed by pre-incubating rabbit anti-CIP2A antibodies with recombinant CIP2A or an unrelated protein giantin as a control. Immunofluorescence was then performed on serial tissue sections. Arrows indicate basement membrane. 100X magnification shown.
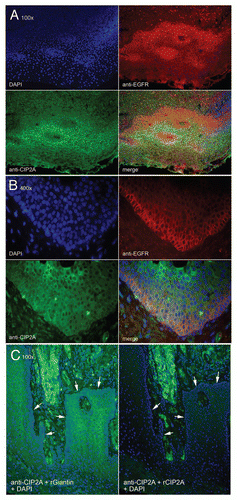
Figure 3 CIP2A expression by immunohistochemistry of oral dysplasia. Photomicrograph part for epithelial dysplasia: Figure A. Control specimen representing oral hyperkeratosis sample demonstrating only sporadic positive cells (magnification x10). Figure B. Strong reactivity for CIP2A noted in the basal and parabasal cells (black arrows) in sample from mild to moderate epithelial dysplasia (magnification X10). Figure C. Another sample of mild to moderate epithelial dysplasia displaying with numerous CIP2A positive cells at the basal and parabasal levels (magnification x20) (black arrows) Figure D. Higher magnification (x40) from previous case (C) exhibiting strongly positive CIP2A cells with prominent nuclear and lighter cytoplasmic staining (black arrows).
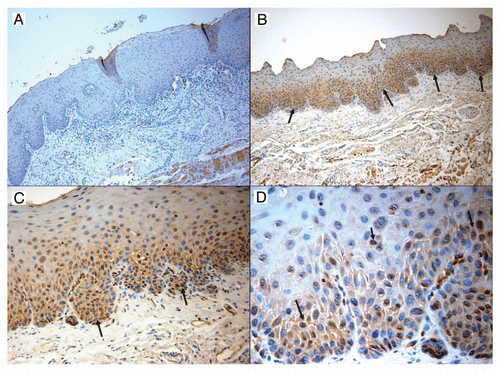
Figure 4 CIP2A expression by immunohistochemistry of oral squamous cell carcinoma. Photomicrograph part for oral squamous cell carcinoma: Figure A. Control specimen representing oral hyperkeratosis sample demonstrating total lack of any CIP2A expression (magnification x20) Figure B. Strong scattered reactivity for CIP2A noted in groups of malignant epithelial cells (yellow arrows). Some stain is also picked up by keratin pearls and the squamous eddies often seen in carcinoma biopsies (magnification ×10) Figure C. A second sample of well differentiated squamous cell carcinoma displaying numerous clusters of CIP2A positive cells. Some of the lymphocytic infiltrate also demonstrates staining (magnification ×20) (yellow arrows) Figure D. Another carcinoma sample exhibiting single malignant cells and occasional groups staining positively with CIP2A (magnification ×40) (yellow arrows). Again the staining appears localized more to the nuclei and fainter in the cytoplasm.
Table 1 Graded staining intensities of CIP2A for each of the three abnormal tissue types were significantly higher than those for normal tissue
Acknowledgements
We would like to thank Dr. Richard Lamont for providing the primary cultures of human gingival epithelial cells. This study was supported in part by the UFCD Student Summer Research Fellowship, NIDCR grant T32 DE007200, NIH grant AI47859, NIDCR grant K99DE018191, the Bankhead-Coley Cancer Research Program, Florida Department of Health grant 08BN-02, the Andrew J. Semesco Foundation and a grant from Micromedic Tech.
References
- Jamel A, Siegel R, Ward D, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. Ca Cancer J Clin 2009; 59:225 - 249
- Lingen MW. Pragmatics versus contrarians: how does one resolve their differences with respect to oral cancer screening?. Oral Surg, Oral Medicine, Oral Path 2010; 3:325 - 326
- Stewart SL, Cardinez CJ, Richardson LC, Norman L, Kaufmann R, Pechacek TF, et al. Surveillance for cancers associated with tobacco use—United States 1999-2004. MMWR Surveill Summ 2008; 57:1 - 33
- Campana JP, Meyers AD. The surgical management of oral cancer. Otolaryngol Clin North Am 2006; 39:331 - 348
- Fedele S. Diagnostic aids in the screening of oral cancer. Head Neck Oncol 2009; 30:1 - 5
- Warnakulasuriya S, Rebel J, Bouquot J, Dabelsteen E. Oral epithelial dysplasia classification systems: predictive values, utility, weaknesses and scope for improvement. J Ortal Pth Med 2008; 37:127 - 133
- Fischer DJ, Epstein JB, Morton TH, Schwartz SM. Interobserver reliability in the histopathologic diagnosis of oral pre-malignant and malignant lesions. J Oral Path Med 2004; 33:65 - 70
- Kujan O, Khattab A, Oliver RJ, Roberts SA, Thakker N, Sloan P. Why oral histopathology suffers inter-observer variability on grading oral epithelial dysplasia: an attempt to understand the sources of variation. Oral Oncol 2007; 43:224 - 231
- Hiraishi Y, Wada T, Nakatani K, Negoro K, Fujita S. Immunohistochemical expression of EGFR and p-EGFR in oral squamous cell carcinomas. Pathol Oncol Res 2006; 12:87 - 91
- Laimer K, Spizzo G, Gastl G, Obrist P, Brunhuber T, Fong D, et al. High EGFR expression predicts poor prognosis in patients with squamous cell carcinoma of the oral cavity and oropharynx: a TMA-based immunohistochemical analysis. Oral Oncology 2007; 43:193 - 198
- Cripps C, Winquist E, Devries MC, Stys-Norman D, Gilbert R. Epidermal growth factor receptor targeted therapy in stages III and IV head and neck cancer. Curr Oncol 2010; 17:37 - 48
- Soo Hoo L, Zhang JY, Chan EK. Cloning and characterization of a novel 90 kDa ‘companion’ auto-antigen of p62 overexpressed in cancer. Oncogene 2002; 21:5006 - 5015
- Junttila MR, Puustinen P, Niemela M, Ahola R, Arnold H, Bottzauw T, et al. CIP2A Inhibits PP2A in Human Malignancies. Cell 2007; 130:51 - 62
- Westermarck J, Hahn W. Multiple Pathways regulated by the tumor suppressor PP2A in transformation. Trends Mol Med 2008; 14:152 - 160
- Li W, Ge Z, Liu C, Liu Z, Bjorkholm M, Jia J, et al. CIP2A is overexpressed in gastric cancer and Its depletion leads to impaired clonogenicity, senescence or differentiation of tumor cells. Clin Cancer Res 2008; 14:3722 - 3728
- Juntila MR, Westermarck J. Mechanism of MYC stabilization in human malignancies. Cell Cycle 2008; 7:592 - 596
- Come C, Laine A, Chanrion M, Edgren H, Mattila E, Liu Z, et al. CIP2A is associated with human cancer aggressivity. Clin Cancer Res 2009; 15:5092 - 5100
- Khanna A, Böckelman C, Hemmes A, Junttila MR, Wiksten JP, Lundin M, et al. MYC-dependent regulation and prognostic role of CIP2A in gastric cancer. J Natl Cancer Inst 2009; 101:793 - 805