Abstract
Commentary to:
SU11248, A selective tyrosine kinases inhibitor suppresses breast tumor angiogenesis and growth via targeting both tumor vasculature and breast cancer cells
Emily Young, Lucio Miele, Kevan B Tucker, Min Huang, Jeremy Wells and Jian-Wei Gu
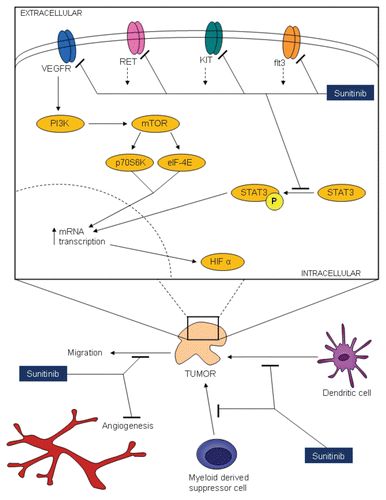
The utility of targeted agents in breast cancer has been recognized for decades. In 1952, Huggins reported modulation of endocrine axes via adrenalectomy as a means of inducing tumor regression.Citation1 Since then, selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs) have dramatically improved clinical outcome in patients with hormone-receptor positive disease.Citation2 Similarly, identification of the HER2/neu proto-oncogene in approximately 25% of patients with breast cancer ushered in agents, such as trastuzumab and lapatinib, directed at this moiety.Citation3 Within the past several years, vascular endothelial growth factor (VEGF) targeted therapies have also been incorporated into the treatment paradigm of advanced breast cancer. Several phase III trials combining the VEGF-directed antibody bevacizumab with cytotoxic chemotherapy have demonstrated significant clinical benefit.Citation4,Citation5 In contrast to bevacizumab, the clinical utility of VEGF receptor tyrosine kinase inhibitors (VEGFR-TKIs) is less understood in the setting of breast cancer. A key to optimizing the potential use of these agents is to better understand their mechanism of action—already, multiple alternate mechanisms to direct VEGFR antagonism have been proposed (). In this issue, Young et al. provide preclinical evidence suggesting the role of the VEGFR-TKI, sunitinib, in mediating both autocrine and paracrine effects in a heterogeneous array of breast cancer cell lines.Citation6
Young et al. first demonstrate the antitumor efficacy of sunitinib in murine xenograft models.Citation6 Female immunocompetent mice were injected with mouse breast adenocarcinoma (E0771) cells, and one of two cohorts received sunitinib via oral gavage. Tumor cross-sectional area was reduced by 48% in the sunitinib-treated group as compared to the control group. Morphometric analyses further suggest a decrease in microvessel density with sunitinib therapy. Illustrating a distinct antitumor mechanism of sunitinib, migration assays performed on the E0771 breast cancer cell line demonstrated impaired tumor motility with sunitinib therapy. Does the antitumor effect of sunitinib extend beyond the estrogen receptor (ER)-positive E0771 cell line? To this end, Young et al. further assessed ER-positive MCF-7 and ER-negative MDA-MB-231 breast cancer cell lines. In comparing the two, MDA-MB-231 cells had higher VEGF and VEGFR-1 expression. While higher doses of sunitinib (10 µmol/L) led to similar growth inhibition in both MCF-7 and MDA-MB-231 cells, treatment with lower doses of sunitinib (5 µmol/L) had a more substantial effect on MCF-7 cells. These results suggest a differential antitumor effect of sunitinib based on ER status; certainly, further validation is necessary to confirm this finding.
In line with the report by Young et al. a substantial literature has amassed suggesting that a direct antitumor effect of sunitinib complements an effect on the microenvironment—a unifying hypothesis may be inhibition of signal transducer and activator of transcription 3 (Stat3). Xin et al. have demonstrated a correlation between sunitinib-induced Stat3 inhibition and tumor apoptosis in the Renca human renal cell carcinoma (RCC) cell line.Citation7 Furthermore, sunitinib appears to inhibit Stat3 in Renca tumor-associated myeloid-derived suppressor cells (MDSCs). Perhaps as a consequence, accumulation of MDSCs (along with regulatory T cells and other immune cells) is blunted at the site of tumor. Clinical support of these observations is derived from a report by Finke et al. examining metastatic RCC patients treated with sunitinib.Citation8 Peripheral blood collected on days 1 and 28 of therapy show a diminution in regulatory T-cell percentage with a concomitant increase in the type-1 T-cell cytokine response. Several reports utilizing non-RCC models suggest a similar effect of sunitinib on inhibitory immune cells, thereby possibly potentiating an anti-tumor immune response.
While Young et al. specifically describe antagonism of VEGFR by sunitinib, targeting of other receptors may contribute to the activity of the agent. Outside of VEGFR-1 and -2, preclinical studies across multiple cell lines have demonstrated IC50 values in the nanomolar range for c-kit, flt3 and RET.Citation9 Targeting of these distinct receptors may portend increased activity in certain diseases. As an example, the response to sunitinib in imatinib-resistant gastrointestinal stromal tumor (GIST) is enhanced amongst patients who bear specific mutations in the exons coding for KIT.Citation10,Citation11 Thus, VEGFR antagonism alone may not fully explain the antitumor effect of sunitinib.Citation12
A more comprehensive understanding of sunitinib's mechanism of action lends itself to identifying patients that are more prone to benefit from therapy. This may be particularly important in the setting of breast cancer, where the efficacy of the single agent appears to be modest. In a phase II trial of sunitinib therapy in pre-treated patients with advanced breast cancer, an overall response rate of 11% was observed.Citation13 A subsequently reported trial comparing sunitinib therapy to single agent capecitabine in the setting of HER2-negative metastatic disease was terminated due to futility in reaching the primary endpoint (progression-free survival).Citation14 Furthermore, efforts to combine sunitinib with other clinically relevant targeted agents have been challenging—for example, the phase II SABRE-B trial comparing paclitaxel and bevacizumab with or without sunitinib was closed prematurely due to concerns related to toxicity.Citation15 With the fate of sunitinib in breast cancer still hanging in the balance, a mechanistic understanding may ultimately lead to biomarkers that allow the clinician to select patients best suited for therapy with this agent.
Acknowledgements
S.K.P. efforts are supported by the NIH Loan Repayment Plan (LRP), the CBCRP 15IB-0140 (California Breast Cancer Research Program Junior IDEA Award) and NIH K12 2K12CA001727-16A1.
Commentary to:
References
- Huggins C, Bergenstal DM. Inhibition of Human Mammary and Prostatic Cancers by Adrenalectomy. Cancer Res 1952; 12:134 - 141
- Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, et al. American Society of Clinical Oncology Clinical Practice Guideline: Update on Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer. J Clin Oncol: JCO 2009; 26:3756
- Pal S, Pegram M. HER2 targeted therapy in breast cancer…beyond Herceptin. Rev Endocr Metab Disord 2007; 8:269 - 277
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007; 357:2666 - 2676
- O'Shaughnessy JA, Brufsky AM. RiBBON 1 and RiBBON 2: phase III trials of bevacizumab with standard chemotherapy for metastatic breast cancer. Clin Breast Cancer 2008; 8:370 - 373
- Young E, Miele L, Tucker KB, et al. SU11248: A Selective Tyrosine Kinases Inhibitor Suppresses Breast Tumor Angiogenesis and Growth via Targeting both Tumor Vasculature and Breast Cancer Cells. Cancer Biol Ther 2010; 10:703 - 711
- Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib Inhibition of Stat3 Induces Renal Cell Carcinoma Tumor Cell Apoptosis and Reduces Immunosuppressive Cells. Cancer Res 2009; 69:2506 - 2513
- Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, et al. Sunitinib Reverses Type-1 Immune Suppression and Decreases T-Regulatory Cells in Renal Cell Carcinoma Patients. Clin Can Res 2008; 14:6674 - 6682
- Chow LQM, Eckhardt SG. Sunitinib: From Rational Design to Clinical Efficacy. J Clin Oncol 2007; 25:884 - 896
- Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006; 368:1329 - 1338
- Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D, et al. Primary and Secondary Kinase Genotypes Correlate With the Biological and Clinical Activity of Sunitinib in Imatinib-Resistant Gastrointestinal Stromal Tumor. J Clin Oncol 2008; 26:5352 - 5359
- Yang F, Jove V, Xin H, Hedvat M, Van Meter TE, Yu H. Sunitinib Induces Apoptosis and Growth Arrest of Medulloblastoma Tumor Cells by Inhibiting STAT3 and AKT Signaling Pathways. Mol Cancer Res 2010; 8:35 - 45
- Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, et al. Phase II Study of Sunitinib Malate, an Oral Multitargeted Tyrosine Kinase Inhibitor, in Patients With Metastatic Breast Cancer Previously Treated With an Anthracycline and a Taxane. J Clin Oncol 2008; 26:1810 - 1816
- Barrios C, Liu MC, Lee S, Vanlemmens L, Ferrero JM, Tabei T, et al. Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast Cancer Res Treat 2010; 121:121 - 131
- Mayer EL, Dhakil S, Patel T, Sundaram S, Fabian C, Kozloff M, et al. SABRE-B: an evaluation of paclitaxel and bevacizumab with or without sunitinib as first-line treatment of metastatic breast cancer. Ann Oncol 2010; In press