Abstract
The utility of MORF/cMORF pretargeting for the radiotherapy of cancer requires further validation in tumored mice before clinical trials. We now report on a therapeutic study in mice pretargeted with MORF-CC49 (an anti-TAG-72 antibody CC49 conjugated with MORF, a phosphorodiamidate morpholino oligomer) and then targeted by 188Re-cMORF (a 188Re labeled complementary MORF). Before the dose-escalating therapeutic study, a pretargeting study in LS174T tumored mice was performed at tracer levels. By both necropsy and imaging, the tracer study showed that the whole body radioactivity was largely restricted to tumor in the mice pretargeted 48 h earlier with MORF-CC49 and the tumor radioactivity was retained over 90 h. After decay correction, a best-fit to the biodistribution provided the areas under the radioactivity curves (AUCs) used for the radiation dose estimates. The tumor to normal organ AUC ratios in all cases were greater than unity and ranged from 3 (kidneys) to 48 (muscle). Tumor growth was inhibited in the therapy study. At the highest 188Re dose of 1.40 mCi, a complete but temporary tumor remission was evident in 3 out of the 5 animals. Histological examination of tissues from these animals showed no evidence of cytotoxicity to normal tissues but obvious radiation damage to tumor. In conclusion, effective radiotherapy was achieved in a mouse model by MORF/cMORF pretargeting using 188Re as the therapeutic radionuclide and CC49 as the pretargeting antibody.
Introduction
As an alternative to conventional radiotherapy using radiolabeled antitumor antibodies, radiotherapy by pretargeting can reduce the radiation burden to normal organs while maximizing toxicity to tumor by separating the antibody from its radiolabel.Citation1–Citation5 The MORF/cMORF pretargeting approach uses two complementary phosphorodiamidate morpholino oligomers as the recognition pair. A number of imaging studies using technetium-99m as the radiolabelCitation6–Citation9 have demonstrated sufficiently rapid tumor accumulations and normal tissue clearance, suggesting that, if a therapeutic radionuclide is used, effective radiotherapy may be possible. This laboratory has already reported on a therapeutic response using a rhenium-188 (188Re) labeled cMORF and a MORF conjugated antiCEA antibody MN14.Citation10 Recently, again using 99mTc as the label, another antibody CC49 targeting TAG-72 has been shown to be a suitable alternative antibody for this therapeutic application.Citation11,Citation12
The attractive properties of 188Re for radiotherapy have been previously appreciated and the 188Re has been used in tumored mice either directly labeled to antibodies and peptidesCitation13–Citation20 or by pretargeting.Citation21–Citation23 Thus far, few instances of size reduction have been reported.Citation10,Citation13–Citation20 We now report that a temporary tumor remission was achieved using the anti TAG-72 antibody CC49 and the MORF/cMORF pretargeting strategy.
Results
Pharmacokinetics and radiation dose estimates.
The 188Re accumulations in tumor and eight normal tissues obtained in the tracer study are listed in and are plotted against time in with the solid lines showing the non-linear best fits. When presented in %ID/g, the tumor accumulation (, part I) shows a steady decrease. However, this decrease is due to tumor growth rather than loss of label, as is evident from the virtually unchanged tumor accumulation when presented in % ID/organ (, part J).
Since the radioactivity decreased rapidly in blood while the accumulation in tumor remained fairly constant, the tumor to blood (T/B) ratio increased rapidly as shown in . The T/B ratio reached 5 immediately and increased steadily to 20 over 90 h. The tumor to normal tissue (T/NT) ratios increased also fairly rapidly for most organs except for liver and spleen (data not presented). Fortunately, the accumulations in these two organs were minimal.
After decay correction, the AUCs for tumor and organs of interest were calculated from the best fits to the biodistribution data. As shown in , the AUC ratios of tumor to normal tissues were always greater than unity, ranging from 3 (kidneys) to 48 (muscle). The absorbed radiation doses calculated from these AUC values are listed in in rads per µCi of 188Re. It should be noted that since tumor accumulations are strongly related to tumor size, the AUC ratios and the radiation dose ratios will also be related to tumor size. In addition to any differences in initial tumor sizes, the tumor size reduction resulting from a therapeutic effect is another factor adding to the uncertainty inherent in absorbed radiation dose estimates.
Tumor imaging.
Two animals were imaged on a NanoSPECT/CT small animal camera (Bioscan, Washington DC) at 3.5 and 10 h post 188Re administration and the acquisitions were reconstructed using the InvivoScope 1.35 program (Bioscan). The anterior, left lateral and posterior projections of the SPECT images at 3.5 and 10 h of one animal are shown in . As shown, the 188Re was largely restricted to tumor and there was no important change in tumor accumulation over the imaging period. Similar results were obtained for the other animal. As shown in , the biodistribution results by necropsy of the two imaged animals are in general agreement with those of other animals sacrificed at similar time points.
Dose escalation study.
The results in indicated that administering 30 µg of the MORF-CC49 antibody had no detectable influence on tumor size. Analysis of variance for repeated measures (RMANOVA) showed no significant difference in tumor growth between the animals receiving MORF-CC49 alone and the untreated animals (p = 0.921). We had earlier shown that the 188Re-cMORF has no effect on tumor growth.Citation10 The subsequent dose escalation study was therefore performed without either of these controls.
Prior to radioactivity administration, the average tumor weights were generally similar among the five groups (estimated at 0.26 ± 0.15, 0.46 ± 0.16, 0.35 ± 0.09, 0.46 ± 0.14 and 0.26 ± 0.14 g respectively for n = 0–4). and B present the average increases in tumor weight at day 5 and day 11 respectively post radioactivity administration. Indicated by the increase in tumor weight at day 5, the tumor growth was clearly slower in all animals receiving radioactivity but more evident in animals receiving 0.7 mCi or greater (tumors were barely palpable in three of the five mice in the highest dose group). Tumor growth in animals receiving either no radioactivity or the lowest dose was sufficiently rapid such that two untreated animals had to be euthanized at day 5 as the tumors had reached their size limit. The remaining untreated animals and most of the animals receiving the lowest dose were sacrificed prior to day 11 for the same reason. At day 11, the tumors in animals of the three remaining groups increased in size but at a rate inversely correlated to the dose (0.70 mCi group vs. 1.4 mCi group, p < 0.05). In , the estimated average size of tumor over time for untreated animals and animals receiving the highest dose are presented. The figure shows a definite dose-response relationship for the highest dose group. Tumor growth inhibition for this group extended to at least day 20.
Histopathology.
presents representative H&E stained sections obtained from untreated mice and mice receiving the highest 188Re dose. The percentage of live cells in tumor was reduced to about 30% in the treated mice from about 90% in untreated mice. Furthermore, while necrosis in the untreated mice was mainly localized to tumor cells remote from blood vessels, suggestive of ischemic necrosis resulting from insufficient blood supply in a rapidly growing tumor, the necrosis in the treated mice were in addition localized to cells in close proximity to the blood vessels, suggestive of 188Re toxicity. In the femur of treated mice, a slight decrease in the overall bone marrow cellularity to about 70% was evident compared to about 95% in untreated mice. However, there was no evidence of toxicity in either myeloid, erytroid or megakaryocytic cell lines in the bone marrow. Similarly, no morphologic abnormality was evident in the white and red pulp of the spleen. The histopathologic examination of tissues with the highest radioactivity levels (kidney, liver and lung) in the treated mice showed no significant morphologic alterations.
Discussion
Given the wide variety of tumor-associated antigens, clinical relevance requires that a variety of antibodies be available for pretargeting. For this reason, the antiTAG-72 CC49 antibody was earlier considered for imaging as an alternative to the antiCEA MN14 antibody for MORF/cMORF pretargeting.Citation12 By both imaging and necropsy, the CC49 antibody was considered to be equally useful to the MN14 antibody in the LS174T tumor model. These previous results also confirmed our earlier prediction that the maximum percent tumor accumulation of the radiolabeled cMORF is independent of the pretargeting antitumor antibody,Citation11 despite the differences in pharmacokinetic properties.
Prior to a therapy study with the CC49 antibody in this investigation, it was necessary to ensure that the 188Re label on the cMORF was retained in the pretargeted tumor for at least several half lives of 188Re. As shown in part J, there was no detectable release from tumor over 90 h, a period sufficiently long to provide valid AUCs for this radionuclide. In addition, all normal tissues except for liver and spleen showed an extensive decrease in radioactivity levels over this period, but the radio-activity levels in these two organs were low throughout. Thus except for kidney, the T/NT AUC ratios in all normal tissues considered were all over 6. Even in kidneys where a lower AUC ratio was expected because of its role in clearing the effector, the AUC ratio was 3.
The results obtained in the pharmacokinetic study also provided a set of data usable to estimate the absorbed radiation dose to tumor and normal tissues. As earlier,Citation10 a “self-absorbed” model was again used and the results are presented in . A comparison of the results in this study with those from the previous study using MN14,Citation10 shows significantly lower estimated radiation doses in all tissues apart from tumor. However, we earlier confirmed the observations of othersCitation29–Citation31 that tumor accumulation often decreases with increasing tumor size.Citation32 Thus the approximately two-fold lower radiation dose for tumor is due to the larger size (average 0.96 g) in this CC49 study compared to the earlier MN14 study (average 0.56 g).
Selecting the optimum dosages and timing in pretargeting is difficult, but can be simplified following certain rules.Citation25 Thus, a convenient antibody dosage below that just to saturate the tumor antigen sites was selected, a two day pretargeting interval was selected based on the pharmacokinetics of IgG antibodies,Citation12,Citation33 and the dosage of the radiolabeled cMORF effector was selected to just saturate the MORF-antibody in tumor. The dosage of the labeled cMORF was 2.5 µg in tumored mice pretargeted with 30 µg of MORF-CC49 (gpm = 0.68),Citation12 corresponding to a cMORF/MORF molar ratio of 3.1. If this ratio was kept constant, the percent tumor accumulation of labeled cMORF would be the same.Citation25 For this reason, a cMORF/MORF ratio as close to 3.1 as achievable was selected (2.6 for the pharmacokinetic study and 3.0 for the therapy study).
Compared to the relatively modest therapeutic response in our earlier study,Citation10 the higher doses used in this study provided more striking results. At day 5 post 188Re injection when the untreated control mice were euthanized, tumors in three mice receiving the highest dose became barely palpable. The number of reports documenting a temporary tumor remission following intravenous administration of 188Re is quite limited.Citation13–Citation20
The maximum 188Re dose in this study was confined by the injectate volume that was restricted to below 250 µL and by the age of the 188Re generator used. As shown in , a clear dose response was apparent at doses above 0.35 mCi. It was also gratifying that the results of the histopathology examination showed only minimal damage to normal tissues. In addition, no decrease in average body weight was observed at any dose (data not presented). Taken together, these data suggested radiotherapeutic efficacy coupled with minimal normal tissue toxicity, serving as a rationale for future studies with higher radiation doses.
Materials and Methods
The MORF and cMORF sequences were respectively 5′-TCT TCT ACT TCA CAA CTA-linker-amine (6,060 kDa) and 5′-TAG TTG TGA AGT AGA AGA-linker-amine (6,318 Da) and were purchased from GeneTools (Philomath, OR). The C6-SANH [N-(6′-succinimidyl hexanoyl) 6-hydrazinonicotinamide acetone hydrazone] (403 Da) and C6-SFB [N-(6′-succinimidyl hexanoyl) 4-formylbenzamide] (360 Da) were from Solulink (San Diego, CA). Commercial 9-mL Sephadex G-25 columns were from NeoRx Corp. (Seattle, WA), Sephadex G-100 was from Amersham Biosciences (Uppsala, Sweden) and P-4 Gel (medium) was from Bio-Rad Laboratories (Hercules, CA). The 188W/188Re generator was purchased from Oak Ridge National Laboratory (Oak Ridge, TN).
Conjugations and radiolabeling.
The antiTAG-72 antibody CC49 (160 kDa) was prepared by Strategic Biosolutions (Ramona, CA) from the CC49 murine hybridoma cell line (a gift from Dr Jeffrey Schlom, National Cancer Institute, NIH). Conjugation of MORF to the CC49 was performed as described previously.Citation12,Citation24 The MAG3-cMORF effector was prepared and radiolabeled with 188Re as previously described.Citation25 A shift assay by size exclusion HPLC, in which the labeled cMORF was analyzed before and after adding excess MORF-CC49, showed a 90% shift of the radioactive peak to that of a higher molecular weight as evidence of effective radiolabeling and preserved hybridization ability.
Pharmacokinetics of 188Re-cMORF in pretargeted mice.
For any therapeutic nuclide, the areas under the radioactivity curves (AUCs) over about six half-lives are required for an accurate estimates of the radiation doses to tumor and organs. Therefore a 188Re (T1/2 = 17 h) tracer study was performed prior to the therapy study. Tumored animals were sacrificed at regular time points over the 90 h period and the results subjected to a non-linear regression analysis to provide more accurate radioactivity-time curves for tumor and organs of interest.
Since the pharmacokinetics of 99mTc and 188Re labeled cMORFs are similar,Citation25 optimal pretargeting parameters (the dosage of the MORF-CC49, the pretargeting interval and the dosage of labeled cMORF) obtained from earlier pretargeting studies with 99mTc-cMORF and MORF-CC49 were again used.Citation12,Citation26 To initiate the study, about 106 LS174T colon cancer cells were inoculated in the left thigh of each of the 16 NIH Swiss nude mice (Taconic Farms, Germantown, NY). On day 12, each mouse received via a tail vein 30 µg MORF-CC49 (MORF groups per molecule, gpm = 0.68) and 48 h later 2.1 µg (182 µCi) of 188Re-cMORF. The pharmacokinetics of the 188Re-cMORF in normal organs and tumor of the pretargeted mice was followed by necropsy after cardiac puncture under halothane anesthesia. The excised tissues were prepared and counted as previously.Citation9 To estimate the AUCs, the percent of injected dosage per gram (%ID/g) values were fitted over time using SigmaPlot data processing program (San Jose, CA).
Radiation dose estimates.
The AUCs for tumor and organs of interest were calculated from the %ID/g-time best-fit curves after decay correction. A self-absorbed model was again used in which only beta radiation was considered and 100% of the radiation energy was assumed to be deposited in the source organ.Citation10,Citation21,Citation27 The absorbed radiation doses in Rads were calculated using 2.143* 0.766 (MeV)*AUC (µCi*h/g), where 2.143 is a unit conversion factor and 0.766 MeV is the average energy of the 188Re beta particles.
Tumor imaging.
To supplement the biodistribution results of the tracer study, two pretargeted animals receiving 188Re-cMORF were imaged on a NanoSPECT/CT camera (Bioscan, Washington DC) at 3.5 and 10 h post radioactivity administration. To facilitate the imaging, the dosages were increased to 60 µg for MORF-CC49 and 4.8 µg (1.4 mCi) for 188Re-cMORF with the antibody/effector dosage ratio held constant and equal to that in the tracer study. We earlier showed experimentally and theoretically that the biodistribution of the effector remains unchanged if this ratio (and the timing schedule) is not varied (provided that the tumor was not saturated by the antibody).Citation25,Citation28 After imaging, each animal was sacrificed for biodistribution.
Dose escalation study.
The 188Re-cMORF effector had earlier been shown to have no observable influence on tumor growth in the LS174T model.Citation10 Prior to the dose escalation study, it was also necessary to determine whether the MORF-CC49 itself had any therapeutic potential. One group of four tumored mice were each administered 30 µg of the MORF-CC49 while another group of four mice were left untreated as control. Tumor sizes were measured over time. Differences were evaluated using analysis of variance for repeated measures (RMANOVA).
After confirming that the MORF-CC49 had no therapeutic effect similar to that of 188Re-cMORF, a study with five groups of mice (n = 0–4, N = 5) at five doses was initiated 11 days post tumor inoculation. Mice in group n (n = 1–4) each received (n × 15) µg of MORF-CC49 intravenously and 48 h later (n × 1.Citation20) µg and (n × 350) µCi of 188Re-cMORF. The control group (n = 0) received neither MORF-CC49 nor 188Re-cMORF. Thus, except for the control group, mice each received 15–60 µg of MORF-CC49 and 1.2–4.8 µg (up to 1.40 mCi) of labeled cMORF. Once again, the ratio of antibody/effector dosage was held constant and equal to that used in the tracer and imaging studies. The tumor size and body weight were monitored and each mouse was sacrificed when the square root of the product of width and thickness of the tumor thigh exceeded 1.50 cm. The tumor weight was estimated from this product by the empirical formula: tumor weight (g) = 0.94* the width*thickness of tumor thigh (cm2) − 0.63.Citation28
Histopathology.
Preliminary information about toxicity to tissues and tumor was collected after euthanization from the mice receiving the highest radiation dose of 1.40 mCi by histopathological examination of liver, lung, kidney, spleen and tumor. The femur was also included because of the radiosensitivity of bone marrow. Except for the femur, all specimens were first fixed in 10% neutral buffered formalin and then embedded in paraffin blocks. The femur was decalcified and fixed overnight in a commercial solution (Surgipath Decalicifier I, Surgipath Medical Ind. Inc., Richmond, IL) before being embedded in a paraffin block. Tissues were then cut into 5 µm thick slices and fixed on glass slides for Hematoxylin and Eosin (H&E) staining. The findings were compared to those from the untreated control mice.
Conclusion
The predominant localization and sustained retention of 188Re in tumor by MORF/cMORF pretargeting observed in the tracer study and the successful subsequent radiotherapy in mice suggest that further therapeutic studies of MORF/cMORF pretargeting are justified.
Abbreviations
| MORF | = | an 18 mer phosphorodiamidate morpholino oligomer |
| cMORF | = | another oligomer complimentary to the MORF |
| AUC | = | area under the radioactivity-time curve |
Figures and Tables
Figure 1 Biodistributions of the 188Re-cMORF effector from plotted individually in %ID/g (parts A to I) and, in the case of tumor only, also in %ID (part J)
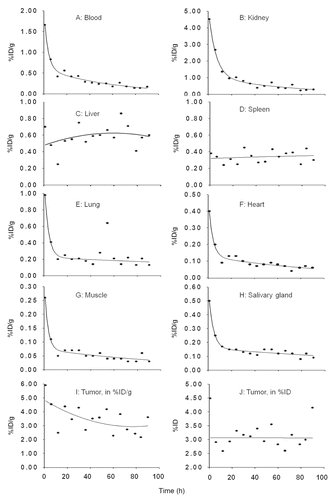
Figure 2 (A) The tumor to blood ratios with time since radioactivity administration. The solid line represents the linear best fit. (B) Histograms showing tumor to normal tissue AUC ratios for listed organs.
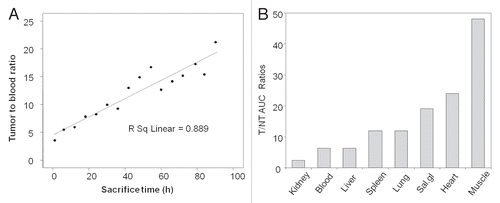
Figure 3 Anterior, left lateral and posterior projections for each SPECT/CT acquisition of one pretargeted mouse imaged at 3.5 h (A) and 10 h (B) after injection of 188Re-cMORF. Except for the intense urine radioactivity at 3.5 h, the radioactivity is essentially restricted to tumor at both time points.
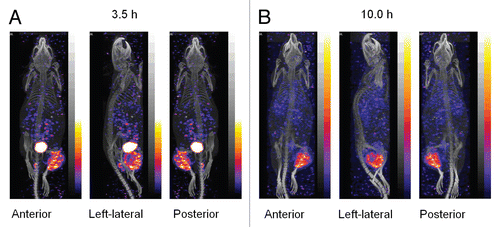
Figure 4 Histograms presenting the increase in tumor weight at day 5 (A) and day 11 (B) after 188Re administration. The estimated tumor dimensions over time for groups 0 (0 mCi) and 4 (1.40 mCi) are also presented (C). Average, N = 5. Error bars signify one standard deviation.
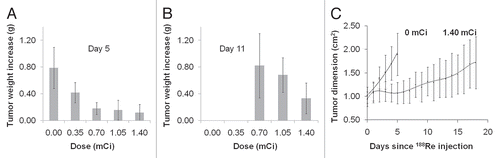
Figure 5 Representative H&E stained tissue sections of tumor and several normal tissues from untreated mice and mice receiving the highest 188Re dose.
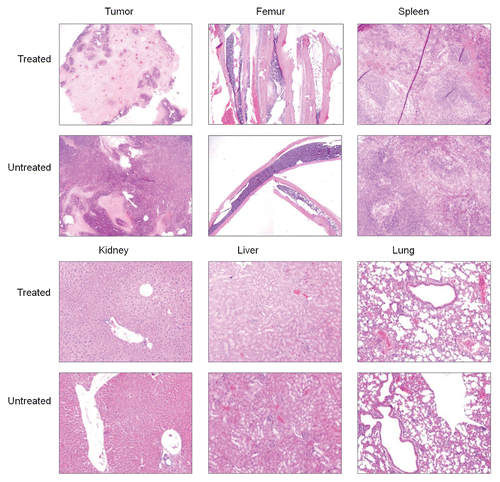
Table 1 Individual biodistributions in %ID/g and, for stomach and intestines, %ID/organ from 1–90 h post IV injection of 188Re-cMORF to tumored mice pretargeted 48 h earlier with MORF-CC49
Table 2 The AUCs and absorbed radiation doses for tumor and organs
Table 3 Tumor dimension (width × thickness of the tumor thigh, cm2) of mice receiving only 30 µg of the MORF-CC49 compared to untreated mice
Acknowledgements
The authors are grateful to Dr. Jeffery Schlom (Laboratory of Tumor Immunology and Biology, Center for Cancer Research, NCI, NIH, Bethesda, MD) for providing the CC49 hybridoma. Financial support was from the National Institutes of Health (CA94994 and CA107360).
References
- Karacay H, Brard PY, Sharkey RM, Chang CH, Rossi EA, McBride WJ, et al. Therapeutic advantage of pretargeted radioimmunotherapy using a recombinant bispecific antibody in a human colon cancer xenograft. Clin Cancer Res 2005; 11:7879 - 7885
- Sharkey RM, Cardillo TM, Rossi EA, Chang CH, Karacay H, McBride WJ, et al. Signal amplification in molecular imaging by pretargeting a multivalent, bispecific antibody. Nat Med 2005; 11:1250 - 1255
- Pagel JM, Hedin N, Subbiah K, Meyer D, Mallet R, Axworthy D, et al. Comparison of anti-CD20 and anti-CD45 antibodies for conventional and pretargeted radioimmunotherapy of B-cell lymphomas. Blood 2003; 101:2340 - 2348
- Subbiah K, Hamlin DK, Pagel JM, Wilbur DS, Meyer DL, Axworthy DB, et al. Comparison of immunoscintigraphy, efficacy and toxicity of conventional and pretargeted radioimmunotherapy in CD20-expressing human lymphoma xenografts. J Nucl Med 2003; 44:437 - 445
- Magnani P, Paganelli G, Modorati G, Zito F, Songini C, Sudati F, et al. Quantitative comparison of direct antibody labeling and tumor pretargeting in uveal melanoma. J Nucl Med 1996; 37:967 - 971
- Liu G, Mang'era K, Liu N, Gupta S, Rusckowski M, Hnatowich DJ. Tumor pretargeting in mice using 99mTc-labeled morpholino, a DNA analog. J Nucl Med 2002; 43:384 - 391
- Liu G, Zhang S, He J, Liu N, Gupta S, Rusckowski M, et al. The influence of chain length and base sequence on the pharmacokinetic behavior of 99mTc-morpholinos in mice. Q J Nucl Med 2002; 46:233 - 243
- Liu G, Liu C, Zhang S, He J, Liu N, Gupta S, et al. Investigations of 99mTc morpholino pretargeting in mice. Nucl Med Commun 2003; 24:697 - 705
- Liu G, He J, Dou S, Gupta S, Vanderheyden JL, Rusckowski M, et al. Pretargeting in tumored mice with radiolabeled morpholino oligomer showing low kidney uptake. Eur J Nucl Med Mol Imaging 2004; 31:417 - 424
- Liu G, Dou S, Mardirossian G, He J, Zhang S, Liu X, et al. Successful radiotherapy of tumor in pretargeted mice by 188Re radiolabeled phosphorodiamidate morpholino oligomer, a synthetic DNA analog. Clin Can Res 2006; 12:4958 - 4964
- Liu G, Dou S, Rusckowski M, Hnatowich DJ. An experimental and theoretical evaluation of the influence of pretargeting antibody on the tumor accumulation of effector. Mol Cancer Ther 2008; 7:1025 - 1032
- Liu G, Dou S, Pretorius PH, Liu X, Chen L, Rusckowski M, et al. Tumor pretargeting in mice using MORF conjugated CC49 antibody and radiolabeled complimentary cMORF effector. Q J Nucl Med Mol Imaging 2010; 54:333 - 340
- Miao Y, Owen NK, Fisher DR, Hoffman TJ, Quinn TP. Therapeutic efficacy of a 188Re-labeled alpha-melanocyte-stimulating hormone peptide analog in murine and human melanoma-bearing mouse models. J Nucl Med 2005; 46:121 - 129
- Dadachova E, Moadel T, Schweitzer AD, Bryan RA, Zhang T, Mints L, et al. Radiolabeled melanin-binding peptides are safe and effective in treatment of human pigmented melanoma in a mouse model of disease. Cancer Biother Radiopharm 2006; 21:117 - 129
- García-Garayoa E, Bläuenstein P, Blanc A, Maes V, Tourwé D, Schubiger PA. A stable neurotensin-based radiopharmaceutical for targeted imaging and therapy of neurotensin receptor-positive tumours. Eur J Nucl Med Mol Imaging 2009; 36:37 - 47
- Juweid M, Sharkey RM, Swayne LC, Griffiths GL, Dunn R, Goldenberg DM. Pharmacokinetics, dosimetry and toxicity of rhenium-188-labeled anti-carcinoembryonic antigen monoclonal antibody, MN-14, in gastrointestinal cancer. J Nucl Med 1998; 39:34 - 42
- Dadachova E, Moadel T, Schweitzer AD, Bryan RA, Zhang T, Mints L, et al. Radiolabeled melanin-binding peptides are safe and effective in treatment of human pigmented melanoma in a mouse model of disease. Cancer Biother Radiopharm 2006; 21:117 - 129
- Dadachova E, Revskaya E, Sesay MA, Damania H, Boucher R, Sellers RS, et al. Pre-clinical evaluation and efficacy studies of a melanin-binding IgM antibody labeled with 188Re against experimental human metastatic melanoma in nude mice. Cancer Biol Ther 2008; 7:1116 - 1127
- Jia ZY, Deng HF, Pu MF, Luo SZ. Rhenium-188 labelled meso-tetrakis[3,4-bis(carboxymethyleneoxy) phenyl] porphyrin for targeted radiotherapy: preliminary biological evaluation in mice. Eur J Nucl Med Mol Imaging 2008; 35:734 - 742
- Zamora PO, Bender H, Gulhke S, Marek MJ, Knapp FF Jr, Rhodes BA, et al. Pre-clinical experience with Re-188-RC-160, a radiolabeled somatostatin analog for use in peptide-targeted radiotherapy. Anticancer Res 1997; 17:1803 - 1808
- Gestin JF, Loussouarn A, Bardies M, Gautherot E, Gruaz-Guyon A, Saï-Maurel C, et al. Two-step targeting of xenografted colon carcinoma using a bispecific antibody and 188Re-labeled bivalent hapten: biodistribution and dosimetry studies. J Nucl Med 2001; 42:146 - 153
- Karacay H, McBride WJ, Griffiths GL, Sharkey RM, Barbet J, Hansen HJ, et al. Experimental pretargeting studies of cancer with a humanized anti-CEA × murine anti-[In-DTPA] bispecific antibody construct and a 99mTc-/188Re-labeled peptide. Bioconjug Chem 2000; 11:842 - 854
- van Schaijk FG, Oosterwijk E, Soede AC, Oyen WJ, McBride WJ, Griffiths GL, et al. Pretargeting with labeled bivalent peptides allowing the use of four radionuclides: 111In, 131I, 99mTc and 188Re. Clin Cancer Res 2003; 9:3880 - 3885
- He J, Liu G, Dou S, Gupta S, Rusckowski M, Hnatowich DJ. An improved method for covalently conjugating morpholino oligomers to antitumor antibodies. Bioconjug Chem 2007; 18:983 - 988
- Liu G, Dou S, He J, Yin D, Gupta S, Zhang S, et al. Radiolabeling of MAG3-morpholino oligomers with 188Re at high labeling efficiency and specific radioactivity. Appl Radiat Isot 2006; 64:971 - 978
- Liu G, Hnatowich DJ. A semiempirical model of tumor pretargeting. Bioconjug Chem 2008; 19:2095 - 2104
- Lubic SP, Goodwin DA, Meares CF, Song C, Osen M, Hays M. Biodistribution and dosimetry of pretargeted monoclonal antibody 2D12.5 and Y-Janus-DOTA in BALB/c mice with KHJJ mouse adenocarcinoma. J Nucl Med 2001; 42:670 - 678
- Liu G, Dou S, Liang M, Chen X, Rusckowski M, Hnatowich DJ. The ratio of maximum percent tumour accumulations of the pretargeting agent and the radiolabelled effector is independent of tumour size. Eur J Cancer 2009; 45:3098 - 3103
- Moshakis V, McIlhinney RAJ, Raghaven D, Neville AM. Localization of human tumour xenografts after i.v. administration of radiolabelled monoclonal antibodies. Br J Cancer 1981; 44:91 - 99
- Siegel JA, Pawlyk DA, Lee RE, Sharkey RM, Horowitz J, Goldenberg DM. Tumor, red marrow and organ dosimetry for 131I-labeled anti-carcinoembryonic antigen monoclonal antibody. Cancer Res 1990; 50:1039 - 1042
- Sharkey RM, Karacay H, Richel H, McBride WJ, Rossi EA, Chang K, et al. Optimizing bispecific antibody pretargeting for use in radioimmunotherapy. Clin Cancer Res 2003; 9:3897 - 3913
- Liu G, Dou S, He J, Liu X, Rusckowski M, Hnatowich DJ. Predicting the biodistribution of radiolabeled cMORF effector in MORF-pretargeted mice. Eur J Nucl Med Mol Imaging 2007; 34:237 - 246
- Liu G, He J, Dou S, Gupta S, Rusckowski M, Hnatowich DJ. Further investigations of morpholino pretargeting in mice-establishing quantitative relations in tumor. Eur J Nucl Med Mol Imaging 2005; 32:1115 - 1123