Abstract
The identification of secreted proteins that are differentially expressed between non-neoplastic and esophageal squamous cell carcinoma (ESCC) cells can provide potential biomarkers of ESCC. We used a SILAC-based quantitative proteomic approach to compare the secretome of ESCC cells with that of non-neoplastic esophageal squamous epithelial cells. Proteins were resolved by SDS-PAGE, and tandem mass spectrometry analysis (LC-MS/MS) of in-gel trypsin-digested peptides was carried out on a high-accuracy qTOF mass spectrometer. In total, we identified 441 proteins in the combined secretomes, including 120 proteins with >2-fold upregulation in the ESCC secretome vs. that of non-neoplastic esophageal squamous epithelial cells. In this study, several potential protein biomarkers previously known to be increased in ESCC including matrix metalloproteinase 1, transferrin receptor, and transforming growth factor beta-induced 68 kDa were identified as overexpressed in the ESCC-derived secretome. In addition, we identified several novel proteins that have not been previously reported to be associated with ESCC. Among the novel candidate proteins identified, protein disulfide isomerase family a member 3 (PDIA3), GDP dissociation inhibitor 2 (GDI2), and lectin galactoside binding soluble 3 binding protein (LGALS3BP) were further validated by immunoblot analysis and immunohistochemical labeling using tissue microarrays. This tissue microarray analysis showed overexpression of protein disulfide isomerase family a member 3, GDP dissociation inhibitor 2, and lectin galactoside binding soluble 3 binding protein in 93%, 93% and 87% of 137 ESCC cases, respectively. Hence, we conclude that these potential biomarkers are excellent candidates for further evaluation to test their role and efficacy in the early detection of ESCC.
Introduction
Esophageal squamous cell carcinoma (ESCC) is among the top ten malignancies worldwide. The major risk factors for ESCC include alcohol and tobacco usage, diets deficient in certain vitamins or antioxidants, extremely hot beverage consumption, lye ingestion and exposure to toxic chemicals such as nitrosamines and mycotoxins.Citation1 Patients are often diagnosed with ESCC when the cancer is already at an advanced stage, owing to a lack of early clinical symptoms as well as effective biomarkers for early detection. Failure of these tumors to respond to chemoradiotherapy at advanced stages usually results in a poor outcome. The efficacy of currently available biomarkers has been limited due to limited specificity and/or sensitivity, which is evident from an overall 5-year survival rate of < 20%.Citation2 Thus, there is an immediate need for early diagnostic markers for ESCC.
The secretome of a given type of cancer cell or tissue reflects its overall pathologic state and constitutes an ideal source for discovery of candidate biomarkers. Although these secreted proteins ultimately reach the bloodstream and other bodily fluids, it is not a trivial task to identify these proteins directly from proteomic analyses of serum. This difficulty occurs largely because proteins secreted from tumors comprise only a minuscule component of the serum proteome and are often masked by highly abundant proteins not secreted by the cells under investigation. Furthermore, the protein constituents of serum may derive not just from tumor cells, but from any distant tissue or organ, inclusive of secondary effects.Citation3 Therefore, identifying candidate biomarkers from enriched conditioned medium of tumor cells in culture represents a simple and reliable approach toward screening for the cancer secretome. Mass spectrometry is an excellent technology to directly identify proteins from body fluids. Cell culture-based models are suitable tools to identify biomarkers at the initial discovery phase, especially because body fluids such as plasma or serum are highly complex at the protein level.
The discovery of early detection biomarkers for ESCC remains a challenge for cancer biologists and clinicians, since there are not yet any biomarkers suitable for routine clinical use. Thus, proteins present in body fluids constitute promising candidate biomarkers.
Although the tumor tissue per se can be analyzed for biomarkers, it contains fibroblasts, inflammatory cells and other nonmalignant cells in addition to tumor cells. Thus, it is difficult to assess from primary tissues the contribution made by tumor cells.Citation4 Moreover, whole cell proteomics analysis is plagued by a high abundance of many irrelevant proteins, such as cytoskeletal proteins, making this type of analysis less than ideal from a biomarker standpoint. Nevertheless, studying the secretomes derived from tumor cell lines gives an idea about proteins secreted by a given tumor type. Therefore, we chose to study and compare the secretomes derived from normal and ESCC cells.
A number of studies have analyzed the secretomes of normal cell types including endothelial,Citation5 myeloid,Citation6 adipocytes,Citation7 microglia,Citation8 retinal pigment epithelial cellsCitation9 and MDCK cells.Citation10 The cancer secretome has also been studied in different cancers including pancreatic,Citation11 nasopharyngeal,Citation12 thyroid,Citation13 lung adenocarcinoma,Citation14 oral,Citation15 ovarian,Citation16 colorectal,Citation17 breast18 and melanoma.Citation19
A limited number of studies have been carried out in ESCC, wherein researchers have identified a small number of differentially expressed proteins.Citation20–Citation24 A brief summary of studies carried out thus far is provided in . In two of these studies, serum samples from ESCC subjects were analyzed using MALDI-TOF/TOF, in which investigators identified an autoantibody response against heat shock 70 kDa protein 4 (HSP70) and peroxiredoxin 5 (PRDX5).Citation22,Citation23 Laser capture microdissection (LCM) was also used in a proteomic study of ESCC in which 28 proteins were identified, of which 14 were upregulated and 14 were downregulated in ESCC vs. adjacent normal cells.Citation25
In a screening MALDI-TOF-MS study of different cell lines derived from esophageal cancers, including two ESCC cell lines (KYSE-30 and OE-21), 33 differentially expressed proteins were identified.Citation26 In another recent report, Xu et al. performed serum profiling of ESCC with sex- and age-matched controls using SELDI-TOF-MS. They identified 281 protein peaks, including six that followed a diagnostic pattern.Citation27 However, both MALDI-TOF and SELDI-TOF-MS suffer from high false-positive identification rates, because data is based only on a signature pattern that requires downstream high-resolution tandem mass spectrometry for definitive protein identification.Citation28 Thus, in each of the above-cited studies, only a small number of differentially expressed proteins were identified.
In the current study, we employed a SILAC-based quantitative proteomic approach based on accurate mass qTOF to study differential protein expression in the secretomes of ESCC cells vs. non-neoplastic esophageal squamous epithelial cells. To our knowledge, our study is the first of its kind to investigate the ESCC secretome. Moreover, our study demonstrates the potential utility of applying SILAC for biomarker discovery from the ESCC secretome.
Results and Discussion
Secreted proteins can serve as excellent biomarkers for the early detection, prognostication and management of ESCC. To identify potential biomarkers from the ESCC secretome, a quantitative proteomic analysis based on a SILAC strategy was carried out. A non-neoplastic esophageal epithelial cell line (Het-1A) was labeled with ‘heavy’ arginine plus lysine, while 7 ESCC cell lines representing various stages of tumor differentiation were labeled with ‘light’ arginine plus lysine. The conditioned medium containing secreted proteins was processed according to the work flow displayed in . The protein sample was resolved in duplicate by SDS-PAGE and stained with colloidal coomassie. Twenty-four bands from both lanes were excised and in-gel trypsin digestion was then carried out.
Quantitative mass spectrometric analysis of the ESCC secretome.
From quantitative proteomic analyses of the ESCC secretome using high-resolution tandem mass spectrometry coupled with liquid chromatography (LC-MS/MS), 17,310 MS/MS spectra were acquired. Using an FDR cutoff of 1%, 950 unique peptide spectrum matches (PSMs) were obtained, leading to the identification of 441 proteins (Mascot and Spectrum Mill). Proteins identified are summarized in Supplemental Table 1. In , the Venn diagram shows the distribution of proteins identified by either the Mascot or Spectrum Mill search engines. Among the identified proteins, 45% were common to both search engines, while 32 and 23% were unique to the Mascot or Spectrum Mill searches, respectively. and C summarize the distribution of these proteins based on their fold-changes.
From Spectrum Mill searches, 35 proteins were not detected in the normal cell secretome and 5 were not detected in the ESCC secretome. 100 proteins were upregulated ≥ 2-fold in the ESCC vs. the normal cell secretome. 106 proteins exhibited values between <2 and 0.5; these were considered essentially unchanged between the two secretomes. 52 proteins were downregulated < 0.5-fold in the ESCC vs. normal secretome.
Quantitative analysis using Mascot Distiller identified 340 proteins. 10 proteins were not detected in the normal cell secretome, while 1 was not detected in the ESCC secretome. 144 proteins fell between < 2 and 0.5-fold change i.e., their expression was essentially unchanged between the normal and ESCC secretomes. 101 proteins were upregulated ≥ 2-fold in the ESCC secretome vs. that of normal cells, while 84 proteins were downregulated < 0.5-fold in the ESCC secretome.
Details of peptide quantitation corresponding to the identified proteins using Spectrum Mill and Mascot distiller are summarized in Supplemental Tables 2 and 3 respectively. Included in this Table are fold-changes for differential expression between the ESCC and normal cell secretomes, peptide sequence, parent charge, modifications, parent m/z, parent mass and delta parent mass. Representative MS and MS/MS spectra of the selected peptides for known, novel and upregulated proteins are shown in .
Biological function, pathway analysis and cellular localization analysis. We carried out a bioinformatics analysis to classify proteins based on subcellular localization and biological function. Classification was carried out based on annotations in the Human Protein Reference Database (HPRD; www.hprd.org),Citation39 in compliance with Gene Ontology (GO) standards. This summary includes fold-changes for protein expression between the secretomes of ESCC and non-neoplastic cells, along with biological domains and motifs obtained from HPRD. We also searched for previous reports describing detection of these proteins in any biological fluids, using HPRD and the Human proteinpedia (HUPA; www.humanproteinpedia.org).Citation40
Of the 441 proteins identified, 72 contained signal peptides (SP), 11 contained a transmembrane (TM) domain and 15 contained both a TM domain and an SP motif. The MS and MS/MS spectra of representative known and novel proteins are displayed in . In the current study, 343/441 (77.8%) of proteins had been previously reported in biological fluids, including urine, semen, plasma, serum, tear, saliva, synovial fluid, cerebrospinal fluid, bronchoalveolar fluid, blood, milk, colostrum, pancreatic fluid, cerebrospinal fluid, aqueous humor or vitreous humor. Further analysis revealed that 75% of upregulated proteins had been previously reported in one or more biological fluids in normal or diseased conditions.
Known and overexpressed proteins in ESCC. Among overexpressed proteins in ESCC cell lines, we found a number of proteins that have been previously described in the context of ESCC, confirming the validity of our quantitative proteomic approach. A partial list of known and upregulated proteins is shown in . Proteins previously reported as overexpressed in ESCC include matrix metallopeptidase 1 (MMP1),Citation38 enolase 1 (ENO1) and isocitrate dehydrogenase 1 (IDH1). In our earlier mRNA profiling study of ESCC tissues, MMP1 was 11-fold more abundant in cancer vs. adjacent normal tissues.Citation41 In the current study, at the protein level, MMP1 was also ∼24-fold upregulated in the ESCC secretome.
Enolase 1 (ENO1) is widely expressed in varying types of tissues. In our study, ENO1 was 2.3-fold upregulated in the ESCC secretome. ENO1 has also been previously reported as 1.6-fold upregulated in ESCCs vs. adjacent normal epithelia in a proteomic study using 2-dimensional gel electrophoresis (2-DE).Citation21 We identified HSP70 as 2.4-fold upregulated in the ESCC secretome. In another previous study of ESCC patients, an autoantibody against HSP70 was identified by MALDI-TOF/TOF-MS in sera.Citation23 HSP90B1, also known as tumor rejection antigen (gp96), was 3.8-fold upregulated in the current study and was found to be 5-fold upregulated in an earlier study on ESCC. Keratin 1 (KRT1) is another protein that was ∼11-fold upregulated in our study; KRT1 was also 12.1-fold upregulated in a previously published report.Citation25
Finally, our study provides validation at the protein level for several biomarkers previously reported only at the mRNA level in ESCC including transferrin receptor (TFRC) and transforming growth factor, beta-induced, 68 kDa (TGFBI). Transferrin receptor has been described as an independent prognostic factor based on mRNA expression analysis in ESCC.Citation42 In the current study, TFRC and TF were 4.3- and 14.9-fold upregulated in the ESCC secretome.
Ezrin (EZR) is a cytoplasmic peripheral membrane protein that functions as a protein-tyrosine kinase substrate in microvilli. EZR serves as an intermediate between the plasma membrane and the actin cytoskeleton. EZR plays an important role in cell surface structure adhesion, migration and organization.Citation43 In earlier studies of ESCC, the expression of EZR protein was studied by western blotting, IHC labeling or RT-PCR.Citation44–Citation46 In the current study, EZR was 2.5-fold upregulated in the ESCC secretome. Zeng et al. studied and reported an association of EZR overexpression with poor survival in ESCC using IHC labeling.Citation46 Heat shock protein HSP90 was earlier studied in the context of ESCC, but no significant differences were reported in its expression levels between normal and ESCC subjects.Citation47,Citation48 In our study, it was 3.9-fold upregulated in the ESCC vs. normal cell secretomes.
Neutrophil gelatinase-associated lipocalin (NGAL) or lipocalin 2 (LCN2), is a member of the lipocalin family, which is involved in transport of small lipophilic substances. LCN2 may play an important role in breast cancer in vivo by protecting MMP9 from degradation, thereby enhancing its enzymatic activity and facilitating angiogenesis and tumor growth. Clinically, these published data suggest that the detection of LCN2/MMP9 in urine may be useful in non-invasively predicting the disease status of breast cancer patients.Citation49 Enzymatic levels of the LCN2/MMP9 complex in ESCC have been reported to correlate significantly with depth of tumor invasion.Citation50 Moreover, hypomethylation of LCN2 was reported in ESCC tissues and cell lines, giving rise to the hypothesis that NGAL may play an important role in ESCC.Citation51 In the same study, LCN2 overexpression was found to be positively correlated with cell differentiation in ESCC.Citation51 In our study, LCN2 was not detected in the normal cell secretome, in contrast to the ESCC secretome. We verified the overexpression of LCN2 using western blotting, finding that overexpression of LCN2 was observed in the ESCC secretome but not detectable in the normal secretome (), consistent with the aforementioned earlier study on ESCC.Citation50
Cathepsin D (CTSD) is an aspartic protease involved in tumor progression and other biological processes including cell proliferation, angiogenesis and apoptosis. CTSD overexpression has been reported in cholangiocarcinoma,Citation52 and it has been shown as an independent indicator of poor prognosis in breast cancer.Citation53 In our study, CTSD was 4.8-fold upregulated in the ESCC secretome. CTSD has been tested for its prognostic value in ESCC, but there has been no reported association with clinical factors.Citation54
Downregulated proteins in ESCC. 120 proteins were downregulated ≥ 2-fold in the ESCC secretome vs. the esophageal epithelial cell line secretome. Profilin 2 (PFN2), an actin monomer binding protein, has been shown to be downregulated in nasopharyngeal carcinoma,Citation12 hepatocellular carcinoma,Citation55 pancreatic adenocarcinoma,Citation11 and breast cancer.Citation56 In our study of the ESCC secretome, PFN2 was 0.3-fold downregulated. PFN2 regulates the structure of the cytoskeleton, but its role in ESCC has not yet been explored.
H2A histone family member X (H2AX) is involved in the DNA damage response and mediates DNA repair; this protein was downregulated in the ESCC vs. normal cell secretome, with a fold-change of 0.2. H2AX was also downregulated at the mRNA level in our earlier ESCC transcriptomic study.Citation41 In ESCC, phosphorylation of H2AX has been observed in response to bortezomib drug treatment in an organotypic culture and an in vivo model of ESCC.Citation57 In our study, we did not identify the phosphotyrosine site for H2AX. H2AX is required for checkpoint-mediated arrest of cell cycle progression in response to low doses of ionizing radiation, UV-light or radiometric agents, as well as for efficient repair of DNA double-strand breaks (DSBs), specifically when modified by C-terminal phosphorylation at Ser139 by ATM.Citation58 However, the complete biological importance and role of H2AX are still unclear and there is a need to study its functional relevance in ESCC.
Novel and overexpressed proteins in ESCC. A number of proteins that were identified as overexpressed in ESCC have not been described previously in the context of ESCC. A partial list of those proteins is shown in . Among the novel candidates, PDIA3, YHWAZ, LGALS3BP and GDI2 were overexpressed in the ESCC secretome as compared to the normal cell derived secretome. Protein kinase c inhibitor which is also called YWHAZ or tyrosine 3/tryptophan 5-monooxygenase activation protein, zeta polypeptide was reported to be overexpressed in oral squamous cell carcinoma and amplification of 14-3-3 zeta was also observed in head and neck squamous cell carcinoma,Citation59,Citation60 and urothelial carcinoma.Citation61
Karyopherin beta1 (KPNB1) is also called importin subunit beta-1. Co-operation of KPNB1 and KPNB2 is essential for the nuclear import of proteins containing nuclear localizing signal (NLS).Citation62 Recently, karyopherins have been shown to be responsible for uncontrolled growth and considered as a therapeutic target for cancer.Citation63 KPNB1 was overexpressed in ESCC derived secretome with 2.2-fold. In ESCC, the biological importance of KPNB1 still needs to be explored. Dermicidin (DCD) expression has been associated with cancer cell survival and growth in breast cancer. DCD gene has also been reported in relation to cancer cachexia.Citation64 DCD acts as an oncogene in invasive breast,Citation65 and hepatic cancer cells.Citation66 In our study, DCD was 28-fold upregulated in ESCC derived secretome.
Validation by immunohistochemical staining and western blot analysis. Proteins of interest identified in the current study were further validated by immunohistochemical labeling and western blot analysis to determine their utility as potential biomarkers for ESCC. We selected TGFBI, PDIA3, LGALS3BP and GDI2 based on biological importance, fold-change (≥ 2-fold change upregulation) and reports in other cancers, for further validation in formalin fixed paraffin embedded tissue sections.
The selected proteins for immunohistochemical labeling were also validated by using western blotting. Western blot validation was also done for one downregulated, upregulated and unchanged protein in the secretome derived from ESCC as compared to normal epithelial cells. Western blot-based validation of selected proteins is shown in with SILAC ratios in the secretome derived from normal and ESCC cell lines (pooled). Among the molecules which were validated only by the western blot were thrombospondin-1 (THBS1), Hypoxanthine-guanine phosphoribosyltransferase (HPRT), glutathione S-transferase mu 3 (GSTM3) and lipocalin 2 (LCN2). The IHC scoring for all ESCC patients for TGFBI, PDIA3, LGALS3BP and GDI2 are summarized in the and also provided for individual patient in the Supplemental Table 4.
THBS1 is an extracellular matrix and secreted protein. In an earlier study on transcriptomics of ESCC, we observed 7.9-fold upregulation of THBS1 in ESCC as compared to normal epithelium.Citation41 In ESCC, association of THBS1 was associated with short survival rate.Citation67 In ESCC secretome analysis, it was 2.3-fold overexpressed in secretome of ESCC cell lines as compared to normal. Further, western blot data was in agreement with the findings obtained from SILAC based mass spectrometry data on ESCC secretome.
HPRT protein encoded by HPRT gene was used as control because it was unchanged between secretome derived from ESCC and normal epithelial cells and consistent with earlier studies where it has been used as a reference.Citation68 Another molecule, GSTM3 belongs to the category of antioxidant defense proteins. It was reported to play an important role in breast cancer by protecting normal breast epithelial cells against breast carcinogenesis.Citation69GSTM3 was downregulated in the ESCC-derived secretome with 0.2-fold. These results were confirmed by using western blot analysis and are in agreement with the SILAC ratio that we observed. GSTM3 downregulation indicates the possibility of combat between antioxidants and free radicals in ESCC, but further studies are needed to explore this aspect of ESCC tumorigenesis.
Validation of known biomarker: Transforming growth factor beta induced 68 kDa (TGFBI).
The TGFBI protein also called as keratoepithelin, is a 68 kDa extracellular matrix protein with four evolutionarily conserved fasciclin-1 domains and a carboxy-terminal Arg-Gly-Asp (RGD) sequence. TGFBI is a secreted protein which has the ability to bind to fibronectin, collagen as well as integrins. TGFBI was earlier reported to be associated with lung adenocarcinoma.Citation70 Methylation screening has been carried out for TGFBI promoter in human lung and prostate cancers by methylation-specific PCR.Citation71 TGFBI was reported as more abundant in ESCC as compared to the adjacent normal epithelium in our earlier study on whole genome scale gene expression profiling of ESCC,Citation41 and also in gene expression and protein-protein interaction network study on ESCC.Citation41–Citation72 In ESCC secretome, TGFBI was 9.3-fold upregulated as compared to the normal cell derived secretome. TGFBI was positive in 57/137 (42%) ESCC cases. The pattern of staining in the majority of cases was stromal. The staining pattern of TGFBI in ESCC versus normal sections is shown in .
Validation of novel and upregulated biomarkers. Three novel and overexpressed proteins were selected for validation using western blot and immunohistochemical labeling and will be described below.
Protein disulfide isomerase family A, member 3 (PDIA3).
Protein disulfide isomerase family A, member 3 (PDIA3) also known as glucose-regulated protein, 58 kDa (GRP58) is an isomerase enzyme. This gene encodes a protein of the endoplasmic reticulum that interacts with lectin chaperones calreticulin and calnexin to modulate folding of newly synthesized glycoproteins. This protein was once considered to be a phospholipase; however, it has now been demonstrated that the protein actually has protein disulfide isomerase activity. It is believed that complexes of lectins and PDIA3 mediate protein folding by promoting formation of disulfide bonds in their glycoprotein substrates. This protein is 57 kDa with a signal peptide but without a transmembrane domain. Overexpression of PDIA3 has been reported in hepatocellular carcinoma (HCC).Citation73 In our study, PDIA3 was 2.2-fold upregulated in the ESCC derived secretome. In IHC labeling for PDIA3, overexpression of PDIA3 was observed in 127/137 (93%) ESCC cases and the expression in majority of the cases was cytoplasmic and membranous. The staining pattern of PDIA3 in ESCC versus normal sections is shown in .
Galectin-3-binding protein (LGAL S3BP).
Galectin-3-binding protein (LGALS3BP) is a secreted glycoprotein that belongs to the galectin family of beta-galactoside-binding proteins implicated in modulating cell-cell and cell-matrix interactions. It is also known as Mac-2 binding protein. LGALS3BP binds to galectins, beta1-integrins, collagens and fibronectin and has some relevance in cell-cell and cell-extracellular matrix adhesion. LGALS3BP was originally identified as a tumor-associated antigen.Citation74 LGALS3BP was found to be elevated in the sera of cancer and HIV-infected patients.Citation75 In our study, LGALS3BP was 9.3-fold upregulated in ESCC secretome. Expression of LGALS3BP was significantly associated with the distant metastasis in lung and breast cancer patients.Citation76,Citation77 LGALS3BP was earlier studied to explore its value as a biomarker in the serum samples of breast,Citation76 colon,Citation78 ovarian cancer,Citation79 lymphoma,Citation80 and gastric cancer.Citation81 In gastric cancer patients sera, LGALS3BP was significantly correlated with distant metastasis.Citation81 In our study, LGALS3BP was validated using western blot in secretome derived from normal and ESCC cells and results were in correlation with the SILAC ratio. In IHC labeling for LGALS3BP, overexpression of LGALS3BP was observed in 119/137 (87%) ESCC cases and the expression in majority of the cases was cytoplasmic and membranous. The staining pattern of LGALS3BP in normal and ESCC sections is shown in .
GDP dissociation inhibitor 2 (GDI 2).
Another novel and upregulated protein was GDI2. GDI2 binds and solubilizes several membrane-associated Rab proteins in a GDP/GTP-dependent manner.Citation82 GDP dissociation inhibitors are proteins that regulate the GDP-GTP exchange reaction of members of the rab family, small GTP-binding proteins of the ras superfamily that are involved in vesicular trafficking of molecules between cellular organelles. GDIs slow the rate of dissociation of GDP from rab proteins and release GDP from membranebound rabs. The GDI2 gene contains many repetitive elements indicating that it may be prone to inversion/deletion rearrangements. Amplification of chromosome 10p has been reported in head and neck cancers.Citation83 In a study on anaplastic thyroid cancer, upregulation and overexpression of GDI2 was observed suggesting its potential role in thyroid carcinogenesis.Citation1 Increased levels of serum GDI2 has been found in pancreatic adenocarcinoma.Citation84 In our study, GDI2 was 3.6-fold upregulated in ESCC derived secretome. Immunohistochemical labeling for GDI2 showed overexpression of GDI2 in 127/137 ESCC cases and the expression in the majority of the cases was cytoplasmic and membranous. The staining pattern of GDI2 in ESCC versus normal sections is shown in .
To make our observations publicly available and accessible to other researchers, we have submitted our data on the immunohistochemical analysis of transforming growth factor beta induced 68 kDa, protein disulfide isomerase associated 3, galectin 3 binding protein and GDP dissociation inhibitor 2 and summary of protein and peptide list to Human Proteinpedia (HUPA, www.humanproteinpedia.org).Citation40
The ESCC secretome dataset generated using qTOF is freely available for use in its entirety from ProteomeCommons.org. On-line versions of the data may be found at www.proteomecommons.org/member-data.jsp?i = 657 or by searching on the keyword “ESCC Secretome” from the proteomecommons.org main page or alternatively it can be located in the Tranche data repository and can be downloaded from Tranche data repository using “S/BvVTpUE09k0gNOjAP6lbQKxiAzpsd/f92Lu0KfU61Pzoxnb-GPiS9Hn5EqL3BhAl9HD2 + LReVGAGnoI9QSTFDkaJfAA AAAAABGKwg==” and “Do7AlAhIJBy + TBAJeGbOQ9 MmFprHZQU0cdK1wTzpLcFExjQ/L5xM6CanLJfgQB + Prdx jbetyxvSdy8ijsXCC + Jyk4HEAAAAAAAEdmA==” hash.
In summary, we were able to identify a large number of molecules in the ESCC secretome that could be a potential biomarker for ESCC. The immunohistochemical labeling expression pattern for TGFBI, PDIA3, LGALS3BP and GDI2 further support the potential of these molecules to be studied as biomarkers and their presence in secretome support the view that these can be detected in other body fluids.
Materials and Methods
Cell culture and reagents.
Het-1A,Citation29 a non-neoplastic epithelial squamous cell line was obtained from the American Type Culture Collection (catalog # CRL-2692, ATCC, Manassas, VA, USA) and the SILAC labeling was carried out as described earlier.Citation30 The cells were grown in 0.1% gelatin-coated dishes in keratinocyte serum-free medium (KSFM, catalog # RR070016, Invitrogen, Carlsbad, CA) containing 13C615N2 lysine and 13C615N4 arginine with growth supplements containing bovine pituitary extract (BPE), insulin, hydrocortisone, retinoic acid, transferrin, triodothyronine, epinephrine and human epidermal growth factor (EGF), 100 U/ml penicillin, 100 µg/ml streptomycin and 2 mM L-glutamine (Gibco BRL, Grand Island, NY) at 37°C in an atmosphere containing 5% CO2. The TE-1,Citation31 TE-2,Citation32 TE-5,Citation33 TE-8,Citation31,Citation33–Citation34 TE-10,Citation33 TE-11Citation34 and TE-15,Citation31 were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) containing light amino acids (). The details of the cell lines used in this study are listed in .
Preparation of the secretome.
Approximately 4 × 106 cells from ESCCs or Het-1A cells were grown to 80% confluence. The serum starvation was carried out for 12 hours after complete removal of traces of serum and added growth factors by rinsing the cells three times with 20 ml of Dulbecco's phosphate buffered saline (catalog # 14190, Invitrogen, Carlsbad, CA). The ESCC TE series of cell lines as well as Het-1A cells were serum starved for 12 hours in serum-free DMEM containing the appropriate amino acids. The conditioned medium containing the secretome was collected and filtered through 0.22 µm filter (Millipore Corporation, Billerica, MA). The filtrate was subsequently concentrated using a 3,000 Da molecular mass cutoff spin column, Centriprep (Millipore Corporation, Billerica, MA). Protein concentration was measured using Lowry's method. The samples from pooled ESCC and Het-1A derived secretome were resolved by SDS-PAGE. The gel was fixed and stained using colloidal Coomassie stain (catalog # LC6025, Invitrogen, Carlsbad, CA). The gels were excised into twenty four slices and in-gel trypsin digestion was performed as previously described.Citation35 Similarly, a technical replicate of the pooled secretome was prepared.
LC-MS/MS.
The peptides extracted from in-gel trypsin digestion were dried and reconstituted in 0.1% formic acid and analyzed using the 6520 qTOF mass spectrometer (Agilent Technologies, Santa Clara, California, USA) interfaced with a HPLC Chip Cube System (catalog # G4240-62001, Agilent Technologies, Santa Clara, California, USA). The HPLC-Chip contained a 40 nL enrichment column and a 43 mm × 75 µm analytical column, both made up of a reversed-phase material Zorbax 300SB-C18, particle size of 5 µm. The samples were loaded on the enrichment column using Agilent 1200 series capillary liquid chromatography system equipped with a micro-well plate autosampler at a flow rate of 3 µl/min using solvent A as a loading solvent. An injection flush volume of 4 µl was applied during enrichment step. The peptides were eluted at the flow rate of 400 nl/min using a gradient of 3–40% of 90% acetonitrile containing 0.1% formic acid over 30 minutes.
Data dependent acquisition was carried out using MassHunter workstation data acquisition software (Agilent Technologies Version B.01.03). The qTOF was operated at a capillary voltage of 1,950 V, fragmenter voltage of 175 V, medium isolation width of m/z 4 and collision energy slope of 3 V plus offset of 2 V. In each duty cycle, MS spectra were acquired in the range of m/z 350–1,800 followed by three MS/MS analyses based on preference to charge state in the order of 2+, 3+ and > 3+ ions and a second level preference to abundance.
Mass spectrometry data analysis and protein quantitation.
The mass spectrometry data was searched using Spectrum Mill (Agilent Technologies, Rev. A.03.03) and Mascot (Matrix Science Inc., Version 2.2.0). MS/MS spectral data was processed to generate mascot generic format (mgf) files. The data was searched against human RefSeq Build 35 protein sequence database (34,906 sequences) using both Mascot and Spectrum Mill. Under the search criteria, oxidation of methionine, 13C6 15N4 arginine and 13C6 15N2 lysine were selected as variable modifications and carbamidomethylation of cysteine as fixed modification. In both searches, MS tolerance was set to 100 ppm and MS/MS mass tolerance of 0.1 Da and two missed cleavages were allowed. False discovery rate (FDR) was calculated by searching the data against the decoy database. Peptide spectrum matches (PSMs) at 1% FDR were used for protein identifications. The peptides with single peptide identification from Spectrum Mill or Mascot Distiller were further confirmed by manual inspection of MS/MS spectrum. Relative protein quantitation was carried out using Spectrum Mill and Mascotstiller (Version 2.3.1.0).Citation36
Gene ontology analysis.
We carried out a bioinformatics analysis to classify proteins based on cellular localization and biological function. Classification was carried out based on annotations in human protein reference database (HPRD; www.hprd.org), which is in compliance with gene ontology (GO).Citation37
Tissue specimens.
This study was approved by the Institutional Review Board of the Kidwai Memorial Institute of Oncology, Bangalore. Commercially available tissue microarrays (TMAs) containing formalin fixed ESCC from different sources were used. The TMAs Creative™ Biolabs (catalog # CBL-TMA-046) consisted of 64 ESCCs, three esophageal adenocarcinomas and three normal esophageal tissues. The ESCCs were categorized into well to poorly differentiated from patients aged 41–75 years. The TMAs from Pantomics (catalog # ESC96101) consisted of 34 cases in duplicate, ranging from differentiation grades I–III from patient's ages 36–71 years. The TMAs from FolioBio (catalog # ARY-HH0091) consisted of 40 ESCCs with matching adjacent normal esophageal squamous epithelium. All patients were staged T3N1M0 and with histopathological grades ranged from well to poorly differentiated.
Antibodies.
Immunohistochemical staining for TGFBI, PDIA3, LGALS3BP and GDI2 was performed on paraffinembedded sections. Anti-PDIA3 (dilution 1:1,000, catalog # HPA002645) and anti-TGFBI (dilution 1:500, catalog # HPA008612) were purchased from the Human Protein Atlas (HPA), Stockholm, Sweden. The anti-GDI2 (dilution 1:75, catalog # 10116-1-AP) and anti-LGALS3BP (dilution 1:75, catalog # 10281-1-AP) antibodies were purchased from Proteintech Group, Inc., Chicago, IL, USA. The anti-NGAL (catalog # MAB1757) and anti-THBS1 (catalog # MAB3074) were purchased from the R&D Systems, Minneapolis, MN, USA. The anti-HPRT1 (catalog # WH0003251M1) was purchased from Sigma-Aldrich, St. Louis, MO, USA.
Western blotting.
Twenty micrograms of the conditioned media from normal and ESCC cell lines was resolved by 10% SDS-PAGE, the proteins were transferred onto nitrocellulose membrane, blocked in 5% milk and incubated with the protein specific antibodies overnight at 4°C. After primary antibody treatment, the membranes were washed three times with PBS-tween and subsequently incubated with horseradish peroxidase (HRP) conjugated secondary antibodies at room temperature. After secondary antibody treatment, the blots were washed three times with PBS-tween and detection was carried out using enhanced chemiluminescence detection reagent (Amersham Biosciences). The antibodies for western blotting were used at a concentration of 1 µg/ml.
Immunohistochemical labeling of tissue microarrays.
The immunohistochemical staining was carried out as described previously.Citation38 The TMAs were deparaffinized by incubating the slides at 58°C for two hours. One negative control was used where instead of the primary antibody; antibody diluent was used to check the specificity of the primary antibody. The scoring for immunohistochemical staining was carried out as described previously.Citation41
Conclusions
Using a SILAC-based quantitative proteomic approach, we identified 441 secreted proteins from ESCC cell lines. To our knowledge, ours is the first study of the secretome in ESCC. Although cell culture-based strategies are attractive for the discovery of biomarkers, validation of potential candidates in cancer specimens is still necessary, since cell culture systems do not accurately reflect the complexity of cancer tissue in its microenvironment. In the current study, we identified several candidates that had not previously been reported to be associated with ESCC. Among them were three proteins, PDIA3, GDI2 and LGALS3BP, which were further validated in ESCC patients using tissue microarrays. Secreted proteins PDIA3, GDI2 and LGALS3BP were positive in 127, 127 and 119 of 137 cases, respectively and should be further studied using ELISA or MRM assays in sera of ESCC patients to validate their utility as potential early detection biomarkers.
Secreted candidates have the potential to serve as valuable markers for monitoring the disease and also to stratify patients for various therapeutic options. There is accumulating evidence on the importance of patient selection in order to determine relevant therapy. Further, identification of large number of differentially expressed proteins like PDIA3 in the ESCC secretome can be helpful in developing therapeutic targets and prognostic markers for ESCC.
Abbreviations
| GDI2 | = | GDP dissociation inhibitor 2 |
| H2AX | = | H2A histone family member X |
| KPNB1 | = | karyopherin beta1 |
| LCN2 | = | lipocalin 2 |
| LGALS3BP | = | lectin galactoside binding soluble 3 binding protein |
| MALDI-TOF-MS | = | matrix-assisted laser desorption/ionisation-time-of-flight mass spectrometry |
| PDIA3 | = | protein disulfide isomerase family a member 3 |
| SELDI-TOF-MS | = | surface enhanced laser desorption/ionization time-of-flight mass spectrometry |
| SILAC | = | stable isotope labeling with amino acids in cell culture |
| TGFBI | = | transforming growth factor beta induced 68 kDa |
Figures and Tables
Figure 1 The work flow for discovery and initial validation of biomarkers for esophageal squamous cell carcinoma. For SILAC labeling, Het-1A cells were grown in ‘heavy’ medium and the TE-series ESCC cells were grown in ‘light’ medium as indicated. The secretome was normalized, pooled and resolved by SDS-PAGE. Gel bands were excised and in-gel trypsin digested followed by liquid chromatography tandem mass spectrometry (LC-MS/MS) on a qTOF mass spectrometer. The data was searched using Mascot and Spectrum Mill search engines. Some of the overexpressed proteins that were not previously described (e.g., PDIA3) were validated using western blot and IHC labeling using tissue microarrays.
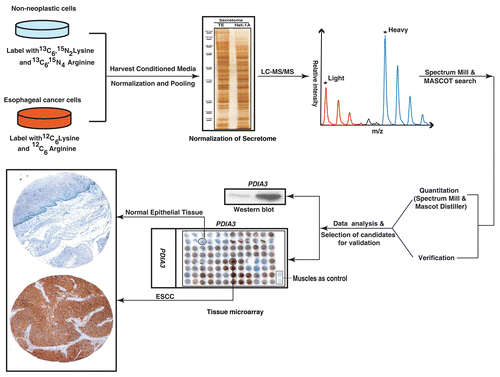
Figure 2 Protein profiled using the SILAC strategy. (A) Venn diagram showing proteins identified by Mascot and Spectrum Mill search algorithms. (B) Distribution of proteins identified by Spectrum Mill plotted against the log2 ratios as indicated. Proteins for which peptides were only observed in cancer cell lines are indicated in red while those for which peptides were identified only in the normal cell line are shown in green. (C) Distribution of proteins identified by Mascot plotted against the log2 ratios as indicated. Proteins for which peptides were only observed in cancer cell lines are indicated in red while those for which peptides were identified only in the normal cell line are shown in green.
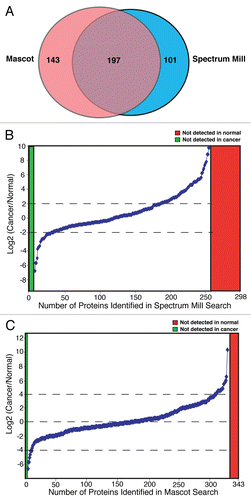
Figure 3 MS and MS/MS spectra of selected differentially expressed proteins. MS and MS/MS spectra of peptide from representative differentially expressed proteins identified in this study. (A) Matrix metalloproteinase 1 (MMP1); (B) TGFbeta induced, 68 KD (TGFBI); (C) GDP dissociation inhibitor 2 (GDI2); (D) Protein disulfide isomerase A3 (PDIA3); (E) Nicotinamide phosphoribosyltransferase (NAMPT) and (F) Lectin, galactoside-binding, soluble, 3 binding protein (LGALS3BP).
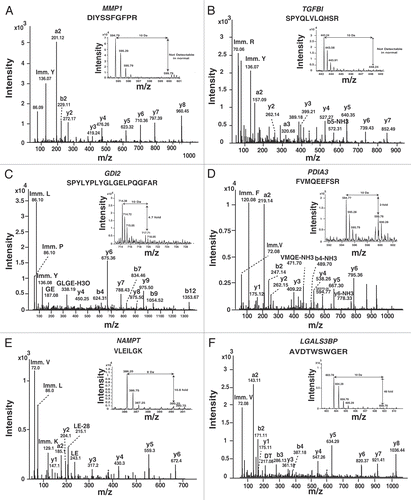
Figure 4 Western blot validation for selected proteins identified in the ESCC secretome. Pooled conditioned media from different ESCC cell lines and normal cell line was tested for expression of the indicated proteins using commercially available antibodies.
Figure 5 Validation of TGFBI using immunohistochemical labeling. Expression of TGFBI in representative normal esophageal squamous mucosa (A). Expression of TGFBI in ESCC is observed in both stromal and epithelial cell compartments (B).
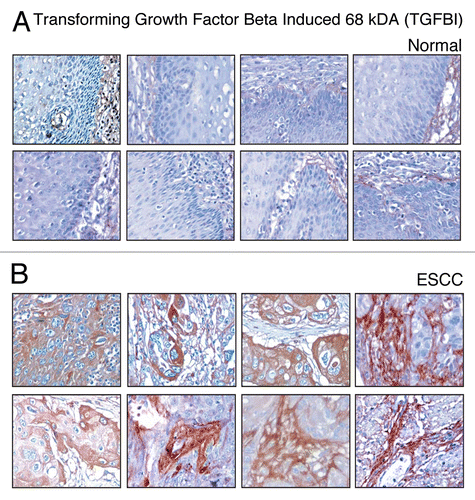
Figure 6 Validation of PDIA3 using immunohistochemical labeling. Expression of PDIA3 in representative normal esophageal squamous mucosa (A). Expression of PDIA3 in ESCC is observed in both stromal and epithelial cell compartments (B).
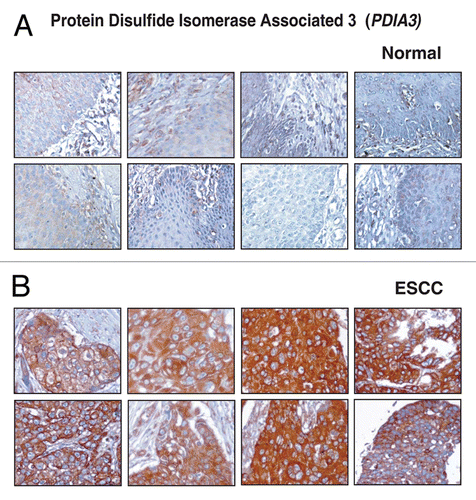
Figure 7 Validation of LGALS3BP using immunohistochemical labeling. Expression of LGALS3BP in representative normal esophageal squamous mucosa (A). Expression of LGALS3BP in ESCC is observed in both stromal and epithelial compartments (B).
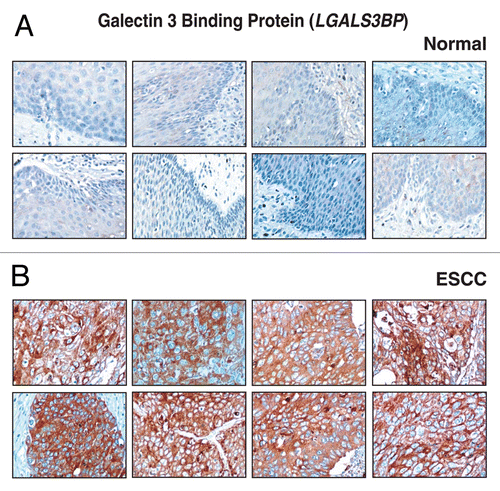
Figure 8 Validation of GDI2 using immunohistochemical labeling. Expression of GDI2 in representative normal esophageal squamous mucosa (A). Expression of GDI2 in ESCC is observed in both stromal and epithelial compartments (B).
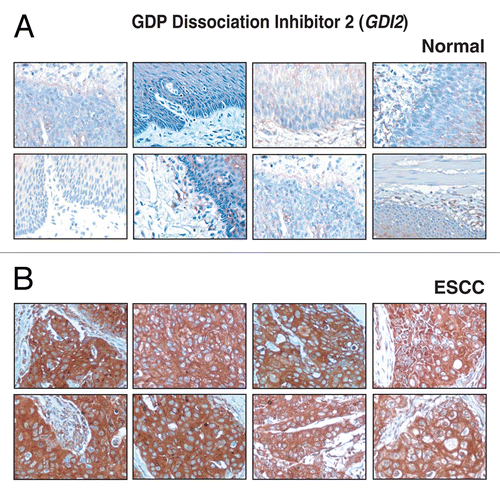
Table 1 Summary of published mass spectrometry-based proteomic studies in ESCC
Table 2 Details of the esophageal cancer cell lines and normal epithelial cell line used in the study
Table 3 Partial list of overexpressed proteins that were previously reported in esophageal squamous cell carcinoma
Table 4 Partial list of novel proteins identified as overexpressed in esophageal squamous cell carcinoma
Table 5 Summary of immunohistochemical labeling of different molecules in ESCC cases
Additional material
Download Zip (183.6 KB)Acknowledgements
We thank the Department of Biotechnology (DBT), Government of India for research support to the Institute of Bioinformatics, Bangalore. M.K.K. is a recipient of an independent Senior Research Fellowship award (IRIS ID# 2006-02010) from the Indian Council of Medical Research (ICMR), New Delhi, India. We thank the Council for Scientific and Industrial Research (CSIR), India for the research support to N.P. and H.P. and the University Grants Commission (UGC), India for the research support to S.R. and Y.S. The work was supported in part by grant CA146799, DK087454 and CA85069 to S.J.M. Also, this work was supported in part by grant NCI P01-CA098101 (Mechanisms of Esophageal Carcinogenesis and its Cell Culture Core) and an American Cancer Society Research Professorship to A.K.R. We thank Agilent Technologies for access to instrumentation.
References
- Oka D, Yamashita S, Tomioka T, Nakanishi Y, Kato H, Kaminishi M, Ushijima T. The presence of aberrant DNA methylation in noncancerous esophageal mucosae in association with smoking history: a target for risk diagnosis and prevention of esophageal cancers. Cancer 2009; 115:3412 - 3426
- Yu C, Chen K, Zheng H, Guo X, Jia W, Li M, Zeng M, Li J, Song L. Overexpression of astrocyte elevated gene-1 (AEG-1) is associated with esophageal squamous cell carcinoma (ESCC) progression and pathogenesis. Carcinogenesis 2009; 30:894 - 901
- Xue H, Lu B, Lai M. The cancer secretome: a reservoir of biomarkers. J Transl Med 2008; 6:52
- Zhang W, Matrisian LM, Holmbeck K, Vick CC, Rosenthal EL. Fibroblast-derived MT1-MMP promotes tumor progression in vitro and in vivo. BMC Cancer 2006; 6:52
- Pellitteri-Hahn MC, Warren MC, Didier DN, Winkler EL, Mirza SP, Greene AS, Olivier M. Improved mass spectrometric proteomic profiling of the secretome of rat vascular endothelial cells. J Proteome Res 2006; 5:2861 - 2864
- Chevallet M, Diemer H, Van Dorssealer A, Villiers C, Rabilloud T. Toward a better analysis of secreted proteins: the example of the myeloid cells secretome. Proteomics 2007; 7:1757 - 1770
- Kratchmarova I, Kalume DE, Blagoev B, Scherer PE, Podtelejnikov AV, Molina H, et al. A proteomic approach for identification of secreted proteins during the differentiation of 3T3-L1 preadipocytes to adipocytes. Mol Cell Proteomics 2002; 1:213 - 222
- Liu J, Hong Z, Ding J, Liu J, Zhang J, Chen S. Predominant release of lysosomal enzymes by newborn rat microglia after LPS treatment revealed by proteomic studies. J Proteome Res 2008; 7:2033 - 2049
- An E, Lu X, Flippin J, Devaney JM, Halligan B, Hoffman EP, et al. Secreted proteome profiling in human RPE cell cultures derived from donors with age related macular degeneration and age matched healthy donors. J Proteome Res 2006; 5:2599 - 2610
- Mathias RA, Wang B, Ji H, Kapp EA, Moritz RL, Zhu HJ, Simpson RJ. Secretome-based proteomic profiling of Ras-transformed MDCK cells reveals extracellular modulators of epithelial-mesenchymal transition. J Proteome Res 2009; 8:2827 - 2837
- Gronborg M, Kristiansen TZ, Iwahori A, Chang R, Reddy R, Sato N, et al. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol Cell Proteomics 2006; 5:157 - 171
- Wu HY, Chang YH, Chang YC, Liao PC. Proteomics analysis of nasopharyngeal carcinoma cell secretome using a hollow fiber culture system and mass spectrometry. J Proteome Res 2009; 8:380 - 389
- Iannetti A, Pacifico F, Acquaviva R, Lavorgna A, Crescenzi E, Vascotto C, et al. The neutrophil gelatinase-associated lipocalin (NGAL), a NFkappaB-regulated gene, is a survival factor for thyroid neoplastic cells. Proc Natl Acad Sci USA 2008; 105:14058 - 14063
- Zhong L, Roybal J, Chaerkady R, Zhang W, Choi K, Alvarez CA, et al. Identification of secreted proteins that mediate cell-cell interactions in an in vitro model of the lung cancer microenvironment. Cancer Res 2008; 68:7237 - 7245
- Weng LP, Wu CC, Hsu BL, Chi LM, Liang Y, Tseng CP, et al. Secretome-based identification of Mac-2 binding protein as a potential oral cancer marker involved in cell growth and motility. J Proteome Res 2008; 7:3765 - 3775
- Kobayashi R, Deavers M, Patenia R, Rice-Stitt T, Halbe J, Gallardo S, Freedman RS. 14-3-3 zeta protein secreted by tumor associated monocytes/macrophages from ascites of epithelial ovarian cancer patients. Cancer Immunol Immunother 2009; 58:247 - 258
- Wu CC, Chen HC, Chen SJ, Liu HP, Hsieh YY, Yu CJ, et al. Identification of collapsin response mediator protein-2 as a potential marker of colorectal carcinoma by comparative analysis of cancer cell secretomes. Proteomics 2008; 8:316 - 332
- Dombkowski AA, Cukovic D, Novak RF. Secretome analysis of microarray data reveals extracellular events associated with proliferative potential in a cell line model of breast disease. Cancer Lett 2006; 241:49 - 58
- Paulitschke V, Kunstfeld R, Mohr T, Slany A, Micksche M, Drach J, et al. Entering a new era of rational biomarker discovery for early detection of melanoma metastases: secretome analysis of associated stroma cells. J Proteome Res 2009; 8:2501 - 2510
- Du XL, Hu H, Lin DC, Xia SH, Shen XM, Zhang Y, et al. Proteomic profiling of proteins dysregulted in Chinese esophageal squamous cell carcinoma. J Mol Med 2007; 85:863 - 875
- Fu L, Qin YR, Xie D, Chow HY, Ngai SM, Kwong DL, et al. Identification of alpha-actinin 4 and 67 kDa laminin receptor as stage-specific markers in esophageal cancer via proteomic approaches. Cancer 2007; 110:2672 - 2681
- Fujita Y, Nakanishi T, Hiramatsu M, Mabuchi H, Miyamoto Y, Miyamoto A, et al. Proteomics-based approach identifying autoantibody against peroxiredoxin VI as a novel serum marker in esophageal squamous cell carcinoma. Clin Cancer Res 2006; 12:6415 - 6420
- Fujita Y, Nakanishi T, Miyamoto Y, Hiramatsu M, Mabuchi H, Miyamoto A, et al. Proteomics-based identification of autoantibody against heat shock protein 70 as a diagnostic marker in esophageal squamous cell carcinoma. Cancer Lett 2008; 263:280 - 290
- Uemura N, Nakanishi Y, Kato H, Saito S, Nagino M, Hirohashi S, Kondo T. Transglutaminase 3 as a prognostic biomarker in esophageal cancer revealed by proteomics. Int J Cancer 2009; 124:2106 - 2115
- Zhou G, Li H, Gong Y, Zhao Y, Cheng J, Lee P, Zhao Y. Proteomic analysis of global alteration of protein expression in squamous cell carcinoma of the esophagus. Proteomics 2005; 5:3814 - 3821
- Breton J, Gage MC, Hay AW, Keen JN, Wild CP, Donnellan C, et al. Proteomic screening of a cell line model of esophageal carcinogenesis identifies cathepsin D and aldo-keto reductase 1C2 and 1B10 dysregulation in Barrett's esophagus and esophageal adenocarcinoma. J Proteome Res 2008; 7:1953 - 1962
- Xu SY, Liu Z, Ma WJ, Sheyhidin I, Zheng ST, Lu XM. New potential biomarkers in the diagnosis of esophageal squamous cell carcinoma. Biomarkers 2009; 14:340 - 346
- Engwegen JY, Gast MC, Schellens JH, Beijnen JH. Clinical proteomics: searching for better tumour markers with SELDI-TOF mass spectrometry. Trends Pharmacol Sci 2006; 27:251 - 259
- Ando T, Ishiguro H, Kimura M, Mitsui A, Mori Y, Sugito N, et al. The overexpression of caveolin-1 and caveolin-2 correlates with a poor prognosis and tumor progression in esophageal squamous cell carcinoma. Oncol Rep 2007; 18:601 - 609
- Harsha HC, Molina H, Pandey A. Quantitative proteomics using stable isotope labeling with amino acids in cell culture. Nat Protoc 2008; 3:505 - 516
- Miyazaki T, Kato H, Nakajima M, Sohda M, Fukai Y, Masuda N, et al. FAK overexpression is correlated with tumour invasiveness and lymph node metastasis in oesophageal squamous cell carcinoma. Br J Cancer 2003; 89:140 - 145
- Nishihira T, Hashimoto Y, Katayama M, Mori S, Kuroki T. Molecular and cellular features of esophageal cancer cells. J Cancer Res Clin Oncol 1993; 119:441 - 449
- Terashita Y, Sasaki H, Haruki N, Nishiwaki T, Ishiguro H, Shibata Y, et al. Decreased peroxisome proliferator-activated receptor gamma gene expression is correlated with poor prognosis in patients with esophageal cancer. Jpn J Clin Oncol 2002; 32:238 - 243
- Katsuta M, Miyashita M, Makino H, Nomura T, Shinji S, Yamashita K, et al. Correlation of hypoxia inducible factor-1alpha with lymphatic metastasis via vascular endothelial growth factor-C in human esophageal cancer. Exp Mol Pathol 2005; 78:123 - 130
- Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 1996; 68:850 - 858
- Kandasamy K, Pandey A, Molina H. Evaluation of several MS/MS search algorithms for analysis of spectra derived from electron transfer dissociation experiments. Anal Chem 2009; 81:7170 - 7180
- Prasad TS, Kandasamy K, Pandey A. Human protein reference database and human proteinpedia as discovery tools for systems biology. Methods Mol Biol 2009; 577:67 - 79
- Kashyap MK, Marimuthu A, Peri S, Kumar GSS, Jacob HCK, Prasad TSK, et al. Overexpression of periostin and lumican in esophageal squamous cell carcinoma. Cancers 2010; 2:133 - 142
- Peri S, Navarro JD, Kristiansen TZ, Amanchy R, Surendranath V, Muthusamy B, et al. Human protein reference database as a discovery resource for proteomics. Nucleic Acids Res 2004; 32:497 - 501
- Kandasamy K, Keerthikumar S, Goel R, Mathivanan S, Patankar N, Shafreen B, et al. Human proteinpedia: a unified discovery resource for proteomics research. Nucleic Acids Res 2009; 37:773 - 781
- Kashyap MK, Marimuthu A, Kishore CJ, Peri S, Keerthikumar S, Prasad TS, et al. Genomewide mRNA profiling of esophageal squamous cell carcinoma for identification of cancer biomarkers. Cancer Biol Ther 2009; 8:36 - 46
- Wada S, Noguchi T, Takeno S, Kawahara K. PIK3CA and TFRC located in 3q are new prognostic factors in esophageal squamous cell carcinoma. Ann Surg Oncol 2006; 13:961 - 966
- Tsukita S, Yonemura S. Cortical actin organization: lessons from ERM (ezrin/radixin/moesin) proteins. J Biol Chem 1999; 274:34507 - 34510
- Gao SY, Li EM, Cui L, Lu XF, Meng LY, Yuan HM, et al. Sp1 and AP-1 regulate expression of the human gene VIL2 in esophageal carcinoma cells. J Biol Chem 2009; 284:7995 - 8004
- Xie JJ, Xu LY, Xie YM, Zhang HH, Cai WJ, Zhou F, et al. Roles of ezrin in the growth and invasiveness of esophageal squamous carcinoma cells. Int J Cancer 2009; 124:2549 - 2558
- Zeng H, Xu L, Xiao D, Zhang H, Wu X, Zheng R, et al. Altered expression of ezrin in esophageal squamous cell carcinoma. J Histochem Cytochem 2006; 54:889 - 896
- Chen JH, Chen LM, Xu LY, Wu MY, Shen ZY. Expression and significance of heat shock proteins in esophageal squamous cell carcinoma. Zhonghua Zhong Liu Za Zhi 2006; 28:758 - 761
- Faried A, Sohda M, Nakajima M, Miyazaki T, Kato H, Kuwano H. Expression of heat-shock protein Hsp60 correlated with the apoptotic index and patient prognosis in human oesophageal squamous cell carcinoma. Eur J Cancer 2004; 40:2804 - 2811
- Fernandez CA, Yan L, Louis G, Yang J, Kutok JL, Moses MA. The matrix metalloproteinase-9/neutrophil gelatinase-associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clin Cancer Res 2005; 11:5390 - 5395
- Zhang H, Xu L, Xiao D, Xie J, Zeng H, Wang Z, et al. Upregulation of neutrophil gelatinase-associated lipocalin in oesophageal squamous cell carcinoma: significant correlation with cell differentiation and tumour invasion. J Clin Pathol 2007; 60:555 - 561
- Cui L, Xu LY, Shen ZY, Tao Q, Gao SY, Lv Z, et al. NGALR is overexpressed and regulated by hypomethylation in esophageal squamous cell carcinoma. Clin Cancer Res 2008; 14:7674 - 7681
- Srisomsap C, Sawangareetrakul P, Subhasitanont P, Chokchaichamnankit D, Chiablaem K, Bhudhisawasdi V, et al. Proteomic studies of cholangiocarcinoma and hepatocellular carcinoma cell secretomes. J Biomed Biotechnol 2010; 437143
- Berchem G, Glondu M, Gleizes M, Brouillet JP, Vignon F, Garcia M, Liaudet-Coopman E. Cathepsin-D affects multiple tumor progression steps in vivo: proliferation, angiogenesis and apoptosis. Oncogene 2002; 21:5951 - 5955
- Wang Y, Chen J, Meng L, Liu J, Du D, Zou Y. [A histopathologic and immunohistochemical study of prognostic factors in esophageal squamous cell carcinoma]. Zhonghua Bing Li Xue Za Zhi 2000; 29:267 - 271
- Wu N, Zhang W, Yang Y, Liang YL, Wang LY, Jin JW, et al. Profilin 1 obtained by proteomic analysis in all-trans retinoic acid-treated hepatocarcinoma cell lines is involved in inhibition of cell proliferation and migration. Proteomics 2006; 6:6095 - 6106
- Braun M, Fountoulakis M, Papadopoulou A, Vougas K, Seidel I, Holler T, et al. Downregulation of microfilamental network-associated proteins in leukocytes of breast cancer patients: potential application to predictive diagnosis. Cancer Genomics Proteomics 2009; 6:31 - 40
- Lioni M, Noma K, Snyder A, Klein-Szanto A, Diehl JA, Rustgi AK, et al. Bortezomib induces apoptosis in esophageal squamous cell carcinoma cells through activation of the p38 mitogen-activated protein kinase pathway. Mol Cancer Ther 2008; 7:2866 - 2875
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 2001; 276:42462 - 42467
- Ralhan R, Desouza LV, Matta A, Chandra Tripathi S, Ghanny S, Dattagupta S, et al. iTRAQ-multidimensional liquid chromatography and tandem mass spectrometry-based identification of potential biomarkers of oral epithelial dysplasia and novel networks between inflammation and premalignancy. J Proteome Res 2009; 8:300 - 309
- Lin M, Morrison CD, Jones S, Mohamed N, Bacher J, Plass C. Copy number gain and oncogenic activity of YWHAZ/14-3-3zeta in head and neck squamous cell carcinoma. Int J Cancer 2009; 125:603 - 611
- Heidenblad M, Lindgren D, Jonson T, Liedberg F, Veerla S, Chebil G, et al. Tiling resolution array CGH and high density expression profiling of urothelial carcinomas delineate genomic amplicons and candidate target genes specific for advanced tumors. BMC Med Genomics 2008; 1:3
- van der Watt PJ, Maske CP, Hendricks DT, Parker MI, Denny L, Govender D, et al. The Karyopherin proteins, Crm1 and Karyopherin beta1, are overexpressed in cervical cancer and are critical for cancer cell survival and proliferation. Int J Cancer 2009; 124:1829 - 1840
- Kau TR, Way JC, Silver PA. Nuclear transport and cancer: from mechanism to intervention. Nat Rev Cancer 2004; 4:106 - 117
- Monitto CL, Dong SM, Jen J, Sidransky D. Characterization of a human homologue of proteolysisinducing factor and its role in cancer cachexia. Clin Cancer Res 2004; 10:5862 - 5869
- Porter D, Weremowicz S, Chin K, Seth P, Keshaviah A, Lahti-Domenici J, et al. A neural survival factor is a candidate oncogene in breast cancer. Proc Natl Acad Sci USA 2003; 100:10931 - 10936
- Stewart GD, Skipworth RJ, Ross JA, Fearon K, Baracos VE. The dermcidin gene in cancer: role in cachexia, carcinogenesis and tumour cell survival. Curr Opin Clin Nutr Metab Care 2008; 11:208 - 213
- Zhou ZQ, Cao WH, Xie JJ, Lin J, Shen ZY, Zhang QY, et al. Expression and prognostic significance of THBS1, Cyr61 and CTGF in esophageal squamous cell carcinoma. BMC Cancer 2009; 9:291
- Gubern C, Hurtado O, Rodriguez R, Morales JR, Romera VG, Moro MA, et al. Validation of housekeeping genes for quantitative real-time PCR in in-vivo and in-vitro models of cerebral ischaemia. BMC Mol Biol 2009; 10:57
- Yu KD, Fan L, Di GH, Yuan WT, Zheng Y, Huang W, et al. Genetic variants in GSTM3 gene within GSTM4-GSTM2-GSTM1-GSTM5-GSTM3 cluster influence breast cancer susceptibility depending on GSTM1. Breast Cancer Res Treat 2010; 121:485 - 496
- Skonier J, Neubauer M, Madisen L, Bennett K, Plowman GD, Purchio AF. cDNA cloning and sequence analysis of beta ig-h3, a novel gene induced in a human adenocarcinoma cell line after treatment with transforming growth factor-beta. DNA Cell Biol 1992; 11:511 - 522
- Shah JN, Shao G, Hei TK, Zhao Y. Methylation screening of the TGFBI promoter in human lung and prostate cancer by methylation-specific PCR. BMC Cancer 2008; 8:284
- Wong FH, Huang CY, Su LJ, Wu YC, Lin YS, Hsia JY, et al. Combination of microarray profiling and proteinprotein interaction databases delineates the minimal discriminators as a metastasis network for esophageal squamous cell carcinoma. Int J Oncol 2009; 34:117 - 128
- Teramoto R, Minagawa H, Honda M, Miyazaki K, Tabuse Y, Kamijo K, et al. Protein expression profile characteristic to hepatocellular carcinoma revealed by 2D-DIGE with supervised learning. Biochim Biophys Acta 2008; 1784:764 - 772
- Ullrich A, Sures I, D'Egidio M, Jallal B, Powell TJ, Herbst R, et al. The secreted tumor-associated antigen 90K is a potent immune stimulator. J Biol Chem 1994; 269:18401 - 18407
- Calabrese G, Sures I, Pompetti F, Natoli G, Palka G, Iacobelli S. The gene (LGALS3BP) encoding the serum protein 90K, associated with cancer and infection by the human immunodeficiency virus, maps at 17q25. Cytogenet Cell Genet 1995; 69:223 - 225
- Iacobelli S, Sismondi P, Giai M, D'Egidio M, Tinari N, Amatetti C, et al. Prognostic value of a novel circulating serum 90K antigen in breast cancer. Br J Cancer 1994; 69:172 - 176
- Marchetti A, Tinari N, Buttitta F, Chella A, Angeletti CA, Sacco R, et al. Expression of 90K (Mac-2 BP) correlates with distant metastasis and predicts survival in stage I non-small cell lung cancer patients. Cancer Res 2002; 62:2535 - 2539
- Ulmer TA, Keeler V, Loh L, Chibbar R, Torlakovic E, Andre S, et al. Tumor-associated antigen 90K/Mac-2-binding protein: possible role in colon cancer. J Cell Biochem 2006; 98:1351 - 1366
- Scambia G, Panici PB, Baiocchi G, Perrone L, Iacobelli S, Mancuso S. Measurement of a monoclonal-antibody-defined antigen (90K) in the sera of patients with ovarian cancer. Anticancer Res 1988; 8:761 - 764
- Zhang DS, Jiang WQ, Li S, Zhang XS, Mao H, Chen XQ, et al. Predictive significance of serum 90K/Mac-2BP on chemotherapy response in non-Hodgkin's lymphoma. Ai Zheng 2003; 22:870 - 873
- Park YP, Choi SC, Kim JH, Song EY, Kim JW, Yoon DY, et al. Upregulation of Mac-2 binding protein by hTERT in gastric cancer. Int J Cancer 2007; 120:813 - 820
- Chinni SR, Brenz M, Shisheva A. Modulation of GDP-dissociation inhibitor protein membrane retention by the cellular redox state in adipocytes. Exp Cell Res 1998; 242:373 - 380
- Speicher MR, Howe C, Crotty P, du Manoir S, Costa J, Ward DC. Comparative genomic hybridization detects novel deletions and amplifications in head and neck squamous cell carcinomas. Cancer Res 1995; 55:1010 - 1013
- Sun ZL, Zhu Y, Wang FQ, Chen R, Peng T, Fan ZN, et al. Serum proteomic-based analysis of pancreatic carcinoma for the identification of potential cancer biomarkers. Biochim Biophys Acta 2007; 1774:764 - 771
- Zhang LY, Ying WT, Mao YS, He HZ, Liu Y, Wang HX, et al. Loss of clusterin both in serum and tissue correlates with the tumorigenesis of esophageal squamous cell carcinoma via proteomics approaches. World J Gastroenterol 2003; 9:650 - 654
- Qi Y, Chiu JF, Wang L, Kwong DL, He QY. Comparative proteomic analysis of esophageal squamous cell carcinoma. Proteomics 2005; 5:2960 - 2971
- Wang SJ, Zhang LW, Yu WF, Yu JK, Zheng S, Li YS, et al. Establishment of a diagnostic model of serum protein fingerprint pattern for esophageal cancer screening in high incidence area and its clinical value. Zhonghua Zhong Liu Za Zhi 2007; 29:441 - 443
- Liu WL, Zhang G, Wang JY, Cao JY, Guo XZ, Xu LH, et al. Proteomics-based identification of autoantibody against CDC25B as a novel serum marker in esophageal squamous cell carcinoma. Biochem Biophys Res Commun 2008; 375:440 - 445
- Shao CCS, Chen L, Cobos E, Wang J, Haab BB, Gao W. Antibody microarray analysis of serum glycans in esophageal squamous cell carcinoma cases and controls. Proteomics Clin Appl 2009; 3:923 - 931
- Hoshino I, Matsubara H, Akutsu Y, Nishimori T, Yoneyama Y, Murakami K, et al. Gene expression profiling induced by histone deacetylase inhibitor, FK228, in human esophageal squamous cancer cells. Oncol Rep 2007; 18:585 - 592
- Gu ZD, Li JY, Li M, Gu J, Shi XT, Ke Y, Chen KN. Matrix metalloproteinases expression correlates with survival in patients with esophageal squamous cell carcinoma. Am J Gastroenterol 2005; 100:1835 - 1843
- Yamamoto S, Tomita Y, Hoshida Y, Iizuka N, Kidogami S, Miyata H, et al. Expression level of valosin-containing protein (p97) is associated with prognosis of esophageal carcinoma. Clin Cancer Res 2004; 10:5558 - 5565
- Wu X, Wanders A, Wardega P, Tinge B, Gedda L, Bergstrom S, et al. Hsp90 is expressed and represents a therapeutic target in human oesophageal cancer using the inhibitor 17-allylamino-17-demethoxygeldanamycin. Br J Cancer 2009; 100:334 - 343