Abstract
Previously, based on high ALDH activity, we showed that cancer stem cells (CSCs) could be identified as ALDHbr cells from an aggressive human osteosarcoma OS99-1 cell line. In this study, we evaluate the impact of BMP-2 on CSCs.Three types of BMP receptors were expressed in freshly sorted ALDHbr cells. In vitro, growth of the sorted ALDHbr cells was inhibited by BMP-2. Using RT-PCR analysis, BMP-2 was found to down-regulate the expression of embryonic stem cell markers Oct3/4, Nanog, and Sox-2, and up-regulate the transcription of osteogenic markers Runx-2 and Collagen Type I. In vivo, all animals receiving ALDHbr cells treated with BMP-2 did not form significant tumors, while untreated ALDHbr cells developed large tumor masses in NOD/SCID mice. Immunostaining confirmed few Ki-67 positive cells were present in the sections of tumor containing ALDHbr cells treated with BMP-2. These results suggest that BMP-2 suppresses tumor growth by reducing the gene expression of tumorigenic factors and inducing the differentiation of CSCs in osteosarcoma. BMP-2 or BMP-2-mimetic drugs, if properly delivered to tumor and combined with traditional therapies, may therefore provide a new therapeutic option for treatment of osteosarcoma.
Introduction
Osteosarcoma is the most common primary, nonhematologic malignant tumor of bone in children and young adolescents, accounting for about 5% of pediatric tumors overall.Citation1,Citation2 Over preceding decades, the use of chemotherapeutics in combination with aggressive surgery has improved long-term survival in these patients to around 65%.Citation3 Approximately 10–20% of patients have metastases at initial diagnosis. Metastasis to the lung is common, and unless the pulmonary metastasis can be eradicated completely, nearly all patients succumb to death.Citation4 Therefore, to more effectively control this disease and improve patient survival rate, there is a pressing need to elucidate the molecular mechanism of human sarcomagenesis and to develop new treatment options for osteosarcomas.
Emerging evidence supports the notion that the capacity of a tumor to grow and propagate is dependent on a small population of cells termed cancer stem cells (CSCs) or tumor-initiating cells.Citation5 The discovery of CSCs has changed the view of how carcinogenesis propagates and the role of chemotherapy. CSCs have been shown to be more resistant to standard chemotherapeutic agents and radiotherapy. Traditional treatment may result in tumor shrinkage, but most tumors recur after treatment, likely because CSCs survive and regenerate tumor growth.Citation6,Citation7 Treatment specifically targeting the CSC population has the potential to be a more effective therapy.
Recently, the existence of CSCs has also been reported in human osteosarcomas.Citation8–Citation10 In addition, our recent studyCitation11 showed that CSCs could be identified in the established human osteosarcoma OS99-1 cell line based on high aldehyde dehydrogenase (ALDH) activity. Cells with high ALDH activity (ALDHbr cells) from OS99-1 xenografts had high tumorigenicity compared to their counterparts with low ALDH activity (ALDHlo cells), generating new tumors with as few as 100 cells in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice through serial transplantations. Eliminating the ALDHbr cells capable of initiating and maintaining osteosarcoma might be an alternative method of osteosarcoma treatment.
Bone morphogenetic proteins (BMPs), members of the transforming growth factor (TGF)-β superfamily, were originally identified as molecules that induce bone and cartilage formation and are now considered as multifunctional cytokines.Citation12 More than 20 BMPs have been shown to play important roles in cell differentiation, proliferation, morphogenesis, cellular survival and apoptosis, in which BMP-2 exhibits potent activity for inducing the formation of both cartilage and bone in vivo and in vitro through specific Type I and Type II receptors. In addition to bone formation, BMP-2 plays pivotal roles in cell proliferation, apoptosis and differentiation.Citation13,Citation14 More recently, there have been a number of studies on the inhibitory effects of BMP-2 in tumorigenesis;Citation15–Citation18 however, the role of BMP-2 in human osteosarcoma remains incompletely understood. This study explores the putative effects of BMP-2 on tumorigenic ALDHbr cells of the OS99-1 cell line.
Results
Expression of BMP receptors in freshly sorted ALDHbr cells.
To determine whether the BMP-2 pathway is perturbed in ALDHbr cells, we first sought to investigate the mRNA expression of BMPR1A, BMPR1B and BMPR2 in freshly sorted ALDHbr cells. PCR analysis revealed that BMPR1A, BMPR1B and BMPR2 were expressed in freshly sorted ALDHbr cells, as shown in . All three types of BMP receptors were further demonstrated to present in ALDHbr cells by immunofluorescent staining (). To evaluate whether the ALDHbr cells might express BMP receptors at different levels compared with the unsorted cells from xenografted tumor, immunofluorescent staining of unsorted cells showed there was no difference on the expressions of those three BMP receptors between the ALDHbr cells and the unsorted cells, as shown in .
Effects of BMP-2 on ALDHbr cell growth.
To test whether BMP-2 might inhibit tumorigenicity, we evaluated its effect on sorted tumorigenic ALDHbr cells. As shown in , growth of the ALDHbr cells was significantly inhibited by the addition of 300 ng/mL of BMP-2 in the presence of 0.5% FBS for 48 h (p < 0.05) and ALDHbr cells with 300 ng/ml of BMP-2 treatment for 72 h showed growth inhibition although the significant was not obtained, whereas no growth inhibition was observed at the 300 ng/mL of BMP-2 for 24 h. Treatment of ALDHbr cells with 10 ng/ml at the three time points induced a stimulation of cell growth, to a lesser but significant extent, while no significant growth inhibition was observed at the 100 ng/mL of BMP-2 at the three time points.
BMP-2 downregulates the expression of embryonic stem cell markers in human osteosarcoma tumorigenic cells.
Oct3/4A, Nanog and Sox-2 are important embryonic stem cell markers and have also been implicated in the tumorigenesis of many cancers.Citation19,Citation20 We reported previously that the mRNA expression of Oct3/4A, Nanog and Sox-2 were all consistently higher in ALDHbr cells.Citation11 We therefore examined whether or not BMP-2 might inhibit the gene expression of Oct3/4A, Nanog and Sox-2 in tumorigenic ALDHbr cells. To test this, we chose 300 ng/mL of BMP-2 to treat ALDHbr cells for 48 h, since this dose consistently produced an inhibitory effect in cell growth. ALDHbr cells treated with the same volume of vehicle were used as a control. As shown in , Oct3/4A, Nanog and Sox-2 in ALDHbr cells were all consistently lower than those in control cells (p < 0.05; p < 0.001).
BMP-2 upregulates expression of osteogenic markers in human osteosarcoma tumorigenic cells.
Blockage of stem cell differentiation may lead to tumorigenesis.Citation21 BMP-2 has been shown to act as a potent inducer of osteogenic differentiation.Citation22 We therefore sought to determine whether BMP-2 upregulates the transcription of osteogenic marker Runx-2 and Collagen Type I. Tumorigenic ALDHbr cells treated with BMP-2 at 300 ng/mL for 48 h were assayed for Runx-2 and Collagen Type I mRNA level by qRT-PCR. ALDHbr cells treated with the same volume of vehicle were used as a control. As shown in , Runx-2 and Collagen Type I in ALDHbr cells were all consistently higher than those in control cells (p < 0.05).
BMP-2 inhibits tumor formation of human osteosarcoma tumorigenic cells in vivo.
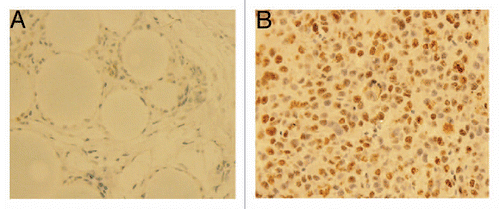
Based on previous findings, we reasoned that if BMP-2 inhibits embryonic stem cell marker expression, it might prevent tumor formation and growth in vivo. Therefore, we next injected freshly sorted tumorigenic ALDHbr cells with different concentrations of BMP-2 treatment or vehicle (control) subcutaneously into NOD/SCID mice. Experiments were performed in triplicate, as previously described in reference Citation23. Tumor growth of the ALDHbr cells was inhibited by the addition of 30 µg BMP-2 per animal ( and B) in all four animals. Hematoxylin and eosin staining of the tumor sections confirmed that tumors formed by ALDHbr cells without BMP-2 treatment contained malignant cells showing characteristic osteosarcoma features, including nuclear atypia, extensive neovascularization and high mitotic activity (), whereas lesions in BMP-2-treated sites contained only residual large vesicle-like Affi-Gel blue beads, foreign-body giant cells and fibrocytes (). No consistent growth inhibition was observed at the lower concentrations of BMP-2 at 1 and 10 µg/animal. To further confirm the tumor growth inhibitory effect of BMP-2 on ALDHbr cells, we performed the nuclear Ki-67 staining by immunohistochemistry. As shown in , few Ki-67 positive cells were present in the sections of tumor from ALDHbr cells treated with BMP-2 (), while most cells were Ki-67 positive in the sections of tumor from ALDHbr cells without BMP-2 treatment ().
Discussion
Many studiesCitation5,Citation9,Citation11,Citation24–Citation26 have demonstrated that most cancers originate from and are propagated by CSCs. The CSC theory postulates that most conventional cancer therapies are directed at the fast-growing tumor mass rather than the slow-dividing CSCs that lead to tumor recurrence or resistance to treatment.Citation27 Developing novel therapies is greatly needed to specifically target CSCs, which presumably will result in more effective cancer treatment.
BMPs are well-known members of the TGF-β superfamily that play an important role in cell activity in various tissues. There are currently three characterized BMP receptors: BMPR1A, BMPR1B and BMPR2. Activation of the BMP receptor complex initiates intracellular signaling transduction. Recent studies suggest that some BMPs and BMP signaling networks may be involved in tumorigenesis.Citation28 Treatment of human glioblastoma-derived brain tumor-initiating cells with BMPs, such as BMP-4, has been shown to result in inhibition of cell proliferation, induction of differentiation and importantly, a reduction in the ability to form tumors in immunodeficient mice.Citation18 CSCs have been identified as ALDHbr cells in human osteosarcoma;Citation11 however, the effect of BMPs on these putative CSCs has not yet been well-elucidated.
In the present study, we sought to examine the effect of BMP-2 on CSCs in the human osteosarcoma OS99-1 cell line. BMP-2 is approved by the United States Food and Drug Administration and is commercially available for clinical application. It has been reported that BMP signaling for the growth and differentiation of normal or neoplastic cells is dependent on its receptors.Citation29 Using regular PCR and immunostaining, we demonstrated that BMP receptors are expressed in tumorigenic ALDHbr cells, suggesting that BMP-2 could bind to its receptors and activate cell signaling to affect cell activities. Furthermore, we observed that BMP-2 had a significant inhibitory effect on tumorigenic ALDHbr cell proliferation at a concentration of 300 ng/mL as compared with much lower concentrations. This result is consistent with previous studies that have shown an inhibitory effect of BMP-2 on cancer cell growth, including prostate, breast, myeloma, gastric and colon cancers.Citation15,Citation17,Citation30,Citation31 The response to BMP-2, however, is not uniform among all cancers. BMP-2 has been shown to stimulate the growth of pancreatic carcinoma cells.Citation32 In addition, Ide et al.Citation33 showed that BMP-2 inhibited the growth of prostate carcinoma cells in the presence of androgen, but stimulated cell growth in the absence of androgen. Thus, the biologic response of cancer cells to BMP-2 may depend not only on the particular cell type or concentration of BMP used, but may also depend on the presence of other factors that are not yet defined.
Oct3/4, Nanog and Sox-2 are essential transcription factors that regulate self-renewal and pluripotency of embryonic stem cells and play a critical role in the maintenance of self-renewal and pluripotency of embryonic stem cells. Recent studies showed that Oct3/4A, Nanog and Sox-2 have also been implicated in tumorigenesis.Citation19,Citation20 Oct3/4A has been reported as a marker for adult stem cells and could be used to identify cancer stem cells.Citation34 Our previous investigation demonstrated that significantly elevated expression of embryonic stem cell marker genes Oct3/4A, Nanog and Sox-2 was shown in tumorigenic ALDHbr cells as compared to the non-tumorigenic ALDHlo counterpart.Citation11 In this study using real-time PCR, we found BMP-2 decreased the expression of stem cell marker genes Oct3/4A, Nanog and Sox-2 in tumorigenic ALDHbr cells treated with BMP-2 at a concentration of 300 ng/mL for 48 h compared to the control group without BMP-2. These results suggest that BMP-2 may reduce the gene expression of proteins associated with tumorigenesis.
Osteosarcoma may be caused by the disruption of osteogenic differentiation.Citation35 The induction of cell differentiation by BMP-2 has been reported for several cell types including myoblast cells, malignant fibrous histiocytoma cells and teratocarcinoma stem cells.Citation36–Citation38 Analysis of the osteogenic marker Runx-2 and collagen type I in ALDHbr cells showed that BMP-2 increased the expression of the osteogenic marker gene Runx-2 and collagen type I in tumorigenic ALDHbr cells treated with BMP-2 at a concentration of 300 ng/mL for 48 h when compared with untreated controls. These findings suggest that BMP-2 might be able to activate osteogenic differentiation in human osteosarcoma CSCs and thus suppress their tumorigenicity.
Determining the effect of BMP-2 on putative osteosarcoma CSCs in vivo is essential for determining the potential use of BMP-2 clinically. In this study, we found that all animals receiving ALDHbr cells treated with 30 µg BMP-2 per animal did not form significant tumors. Conversely, untreated ALDHbr cells developed large tumor masses in NOD/SCID mice. Ki-67 immunostaining further confirmed the inhibitory effect of BMP-2 on ALDHbr cells. Thus, exposure to BMP-2 may deplete the CSC population and produce a significant decrease in the in vivo tumor-initiating ability of osteosarcoma cells. In contrast to the findings in our study, BMP-2 had previously been reported to promote tumor growth of human osteosarcomas in vivo.Citation22,Citation35 In those studies, BMP-2 was secreted by transduced osteosarcoma cells with adenoviral vectors expressing BMP-2, but not exogenously as was done in our study. The different origin of BMP-2 may explain the contrasting results.
Although our report is the first to provide data indicating that BMP-2 has an inhibitory effect on CSCs in human osteosarcoma, the use of CSCs from one cell line provides limited evidence. Further research using more cell lines and primary tumor is therefore necessary to confirm the findings of this study.
In conclusion, our investigation revealed that BMP-2 inhibited the tumorigenic potential of CSCs in the human osteosarcoma cell line OS99-1. The inhibition may be due to decreased gene expression of proteins associated with tumorigenesis and/or an increased level of differentiation of osteosarcoma CSCs in response to BMP-2. The use of BMP-2 may therefore provide a new approach to human osteosarcoma treatment.
Materials and Methods
Cell culture.
Human osteosarcoma OS99-1 cell line originally derived from a highly aggressive primary human osteosarcomaCitation39 was a generous gift from Dr. Sheila M. Nielsen-Preiss (Montana State University, Bozeman, MT). Cells were routinely cultured in Dulbecco's Modified Eagle Medium (DMEM)/F12 medium (Gibco, Carlsbad, CA) supplemented with 10% FBS (Gibco) in a humidified atmosphere of 5% CO2 in air at 37°C and used when in the log phase of growth.
Transplantation of human osteosarcoma OS99-1 cells into NOD/SCID mice.
To obtain human osteosarcoma tumorigenic cells, subcutaneous injections of OS99-1 cells were performed in NOD/SCID mice (Harlan Laboratories Inc., Indianapolis, IN) to establish xenograft tumors as described previously in reference Citation11. Tumors formed were harvested for preparation of single-cell suspensions and histology. All animal studies were performed according to protocol approved by the Institutional Animal Care and Use Committee of the University of Michigan.
Preparation of single-cell suspensions of xenograft tumor cells.
Xenografted tumors formed by injection of OS99-1 cells were minced with scissors, mixed with 1 mg/mL Collagenase Type II (Sigma-Aldrich Co., St. Louis, MO), incubated for 3–4 h, passed through a 70 µm cell strainer and then washed twice with DMEM/F12/10% FBS medium for cell sorting.
ALDEFLUOR assay and isolation of tumorigenic cells from OS99-1 xenograft tumors by flow cytometry cell sorting.
The ALDEFLUOR kit (Stemcell Technologies, Vancouver, BC, Canada) was used to isolate tumorigenic ALDHbr cells from OS99-1 xenografts, as described previously in reference Citation23. Incubation of cells from xenografts with ALDEFLUOR in the presence of the specific ALDH-inhibitor, dimethylaminobenzaldehyde (DEAB), which results in decreased fluorescence, was used as a negative staining control for the assay. During flow cytometry, mouse H-2Kb antibody (BD Biosciences Pharmingen, San Jose, CA) was used to eliminate cells of mouse origin from the xenograft tumors.
Cell proliferation assay.
To investigate the effects of BMP-2 on cell growth, freshly sorted ALDHbr cells from OS99-1 xenografts were washed and cultured in 10% DMEM/F12 medium for expansion and then inoculated, at 1 × 104 cells per well, with 0.1 mL of the culture medium in a 96-well culture plate (Corning, New York, NY) and for 24 h. Cells were then cultured in 0.5% serum-containing medium for another 24 h after washing in phosphate-buffered saline. Cells were treated with 10, 100 or 300 ng/mL BMP-2 (GenScript Corporation, Piscataway, NJ) diluted in 0.5% serum-containing medium or vehicle control for 24, 48 and 72 h. Cell growth was evaluated using a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay according to the manufacturer's instructions (Promega, Madison, WI). Briefly, the culture medium was removed at different time points and 20 uL of MTS reagent mixed with 100 uL of Dulbecco phosphate-buffered saline was added to each well. Then, the cells were incubated at 37°C in a humidified, 5% CO2 atmosphere for 2 h and its absorbance at 490 nm was measured using Bio-TEK ELx 800 Plate Reader (Bio-Tek Instruments Inc., Wilrijk, Belgium).
Semiquantitative real-time polymerase chain reaction (PCR).
To test the expression of BMP receptors in ALDHbr cells, total RNA was extracted from freshly sorted ALDHbr cells using RNeasy Mini Kit (Qiagen Inc., Valencia, CA). Reverse transcription was made using the SuperScript First-Strand Synthesis Kit (Invitrogen, Carlsbad, CA). For semiquantitative PCR, 1 µL of target cDNA conversion mixture was amplified using Hotstar Taq DNA Polymerase (Qiagen) for 35 cycles at 94°C for 30 s, at 56°C for 30 s and at 72°C for 1 min. The PCR primers included BMPR1A (forward, 5′-AAT GGA GTA ACC TTA GCA CCA GAG-3′; reverse, 5′-AGC TGA GTC CAG GAA CCT GTA C-3′), BMPR1B (forward, 5′-GGT TGC CTG TGG TCA CTT CTG G-3′; reverse, 5′-TAG TCT GTG ATT AGG TAC AAC TGG-3′,), BMPR2 (forward, 5′-TCA GAT ATA TGG CAC CAG AAG TG-3′; reverse, 5′-GTG GAG AGG CTG GTG ACA CTT G-3′,) and β-actin (forward, 5′-GCG GGA AAT CGT GCG TGA CAT T-3′; reverse, 5′-GGC AGA TGG TCG TTT GGC TGA ATA-3′). MCF-7 cell lines were used as positive controls for BMP receptors, as described previously in reference Citation18. PCR products were visualized by electrophoresis in agarose (1%) gels and stained with SYBR gel stain (Invitrogen).
Quantitative real-time polymerase chain reaction (qPCR).
Freshly sorted ALDHbr cells were washed and cultured for expansion and then inoculated, at 1 × 105 cells, with 2 mL of the culture medium in a 6-well culture plate. After 24 h incubation, the medium was replaced with 0.5% serum-containing medium for 24 h and then replaced with 0 and 300 ng/mL BMP-2 diluted in 0.5% serum-containing medium. After 48 h, total RNA was extracted as described above. Quantitative real-time PCR of embryonic stem cell marker Oct3/4A (Genbank accession number: NM_002701.4), Nanog (Genbank accession number: NM_024865.2), Sox-2 (Genbank accession number: NM_003106.2) and osteogenic marker Runx-2 (Genbank accession number: NM_001015051.2) and Collagen Type I (Genbank accession number: NM_000088.3) and β-actin gene expression were run in triplicate using Eppendorf Mastercycler Realplex Detection System (Eppendorf, Germany). Real-time quantitative primers were designed and purchased from Applied Biosystems. A positive standard curve for each primer was obtained using serially-diluted cDNA sample mixture. The quantity of gene expression was calculated using standard samples and normalized with β-actin.
In vivo co-treatment experiments.
To examine the tumor inhibitory effect of BMP-2 on tumorigenic ALDHbr cells, 1 × 104 freshly sorted ALDHbr cells were mixed with Affi-Gel blue beads (Bio-Rad Laboratories, Philadelphia, PA) loaded with 1, 10 or 30 µg of BMP-2 at 37°C for 1 h. Sorted ALDHbr cells treated with the same volume of vehicle were used as a control and then ALDHbr cells with different concentrations of BMP-2 or vehicle mixed with Matrigel (BD Biosciences, San Jose, CA) (1:1 volume) were subcutaneously injected into right and left lower abdominal area of NOD/SCID mice.
Immunocytochemical and immunohistochemical staining.
One million freshly sorted ALDHbr cells and unsorted cells from OS99-1 xenografted tumors were put on a slide using the cytospin cytocentrifuge and spun for 5 min at 2,000 rpm. Immunocytochemistry was performed using three antibodies: BMPR1A, BMPR1B and BMPR2 (1:50; Santa Cruz Biotechnology Inc., Santa Cruz, CA). The cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories Inc., Burlingame, CA). Fluorescent cells were visualized and digital images were captured using an Olympus microscope (Olympus America Inc., Milville, NY). For immunohistochemistry, formalin-fixed paraffin sections of tumor samples were stained with mouse anti-Ki-67 antibody (1:100; Santa Cruz Biotechnology Inc.) using standard procedures.
Statistical analysis.
Data were expressed as mean ± SD. Statistically significant differences were determined by two-tailed Student's t-test and SPSS 11 software (SPSS Inc., Chicago, IL) and defined as p < 0.05.
Figures and Tables
Figure 1 Expression of BMP receptors in human osteosarcoma tumorigenic ALDHbr cells. (A) Messenger RNA transcripts for BMP receptors. Lane 1: negative control; Lane 2: freshly sorted ALDHbr cells; Lane 3: MCF7 cells (positive control). Representative immunofluorescent staining of BMPR1A (B), BMPR1B (C) and BMPR2 (D) in freshly sorted ALDHbr cells. Representative immunofluorescent staining of BMPR1A (E), BMPR1B (F) and BMPR2 (G) in unsorted cells from OS99-1 xenografted tumors, and there was no difference on the expressions of those three BMP receptors between the ALDHbr cells and the unsorted cells. The nuclei counterstained with 4′,6-diamidino-2 phenylindole (DAPI) (blue areas).
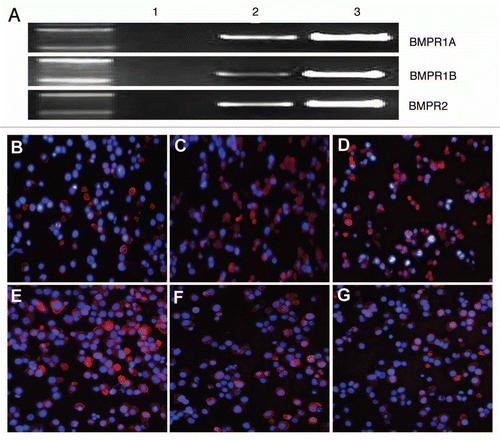
Figure 2 Effects of BMP-2 on ALDHbr cell growth. Freshly sorted ALDHbr cells were cultured for expansion and then inoculated in 96-well plates at a density of 1 × 104 cells/well for 24 h. Cells were then cultured in 0.5% serum-containing medium for another 24 h after washing in phosphate-buffered saline. Cells were treated with 10, 100 or 300 ng/mL BMP-2 diluted in 0.5% serum-containing medium or vehicle control for 24, 48 and 72 h. Growth of the ALDHbr cells was significantly inhibited by the addition of 300 ng/mL of BMP-2 in the presence of 0.5% FBS for 48 h (p < 0.05). each experiment was performed three times; representative examples are shown.
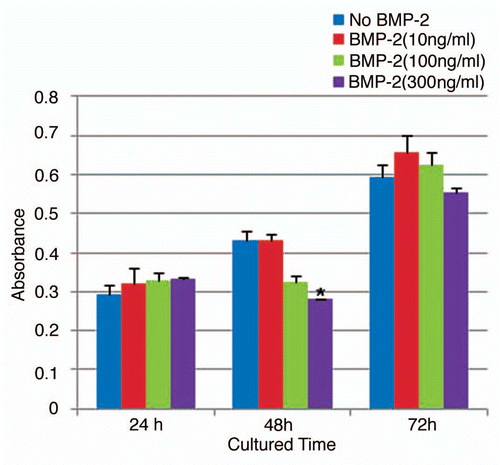
Figure 3 BMP-2 downregulates expression of embryonic stem cell markers in human osteosarcoma tumorigenic ALDHbr cells. Relative quantitative mRNA expression of Oct3/4a, Nanog and Sox-2 genes in ALDHbr cells freshly isolated from OS99-1 xenografts treated with 300 ng/mL of BMP-2 for 48 h. Gene expression levels were normalized to β-actin. Oct3/4, Nanog and Sox-2 in ALDHbr cells were all consistently lower than those in control cells (*p < 0.05; **p < 0.001). each experiment was performed three times; representative examples are shown.
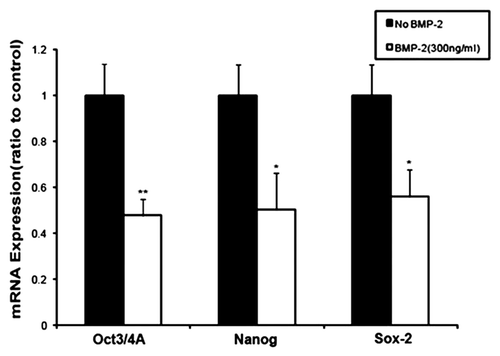
Figure 4 BMP-2 upregulates expression of osteogenic markers in human osteosarcoma tumorigenic ALDHbr cells. Relative quantitative mRNA expression of Runx-2 and collagen type I genes in ALDHbr cells freshly isolated from OS99-1 xenografts treated with 300 ng/mL of BMP-2 for 48 h. Gene expression levels were normalized to β-actin. Runx-2 and collagen type I in ALDHbr cells were all consistently higher than those in control cells (*p < 0.05). Each experiment was performed three times; representative examples are shown.
Figure 5 BMP-2 inhibits tumor formation by human osteosarcoma tumorigenic ALDHbr cells in vivo. (A and B) Representative tumor growth without BMP-2 treatment at the injection site in a NOD/SCID mouse. No significant tumor formation is seen at the injection site in BMP-2 treatment counterpart. (C) Representative hematoxylin and eosin staining of tumor generated from ALDHbr cells without BMP-2 treatment reveals presence of malignant cells showing characteristic osteosarcoma features, including nuclear atypia, extensive neovascularization and high mitotic activity (arrows). Large vesicle-like structures were Affi-Gel blue beads. (D) Injection site of ALDHbr cells treated with BMP-2 contained only residual large vesicle-like Affi-Gel blue beads, foreign-body giant cells (arrows) and fibrocytes.
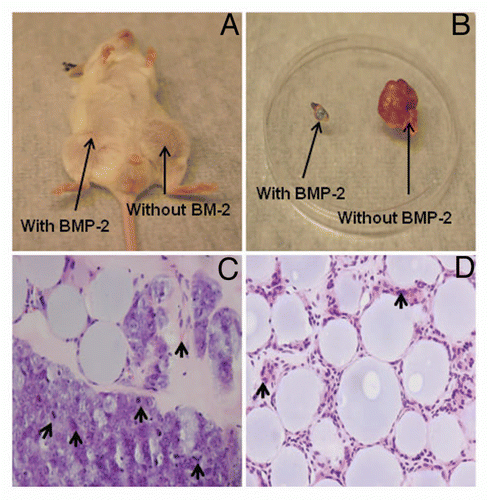
Figure 6 Nuclear Ki-67 staining by immunohistochemistry. (A) Few positive cells were seen in the sections containing ALDHbr cells treated with 30 µg of BMP-2. Large vesicle-like structures were Affi-Gel blue beads. (B) Representative tissue sections of tumor formed by ALDHbr cells without BMP-2 treatment showed most cells were Ki-67 positive.
Acknowledgements
The authors gratefully acknowledge funding support provided by National Institutes of Health grant R01AR056649 and the pilot research fund of the Department of Neurosurgery, University of Michigan. The authors would also like to thank Dr. Sheila M. Nielsen-Preiss from Montana State University for the generous gift of human osteosarcoma cell line OS99-1. The authors also thank Mrs. Holly Wagner for assistance in the preparation of the manuscript and Mr. Martin J. White for flow cytometry.
References
- Kansara M, Thomas DM. Molecular pathogenesis of osteosarcoma. DNA Cell Biol 2007; 26:1 - 18
- Tang N, Song WX, Luo J, Haydon RC, He TC. Osteosarcoma development and stem cell differentiation. Clin Orthop Relat Res 2008; 466:2114 - 2130
- Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival—a report from the Children's Oncology Group. J Clin Oncol 2008; 26:633 - 638
- Kager L, Zoubek A, Potschger U, Kastner U, Flege S, Kempf-Bielack B, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol 2003; 21:2011 - 2018
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res 2007; 67:1030 - 1037
- Costello RT, Mallet F, Gaugler B, Sainty D, Arnoulet C, Gastaut JA, et al. Human acute myeloid leukemia CD34+/CD38− progenitor cells have decreased sensitivity to chemotherapy and Fas-induced apoptosis, reduced immunogenicity and impaired dendritic cell transformation capacities. Cancer Res 2000; 60:4403 - 4411
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer 2005; 5:275 - 284
- Gibbs CP, Kukekov VG, Reith JD, Tchigrinova O, Suslov ON, Scott EW, et al. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia 2005; 7:967 - 976
- Levings PP, McGarry SV, Currie TP, Nickerson DM, McClellan S, Ghivizzani SC, et al. Expression of an exogenous human Oct-4 promoter identifies tumor-initiating cells in osteosarcoma. Cancer Res 2009; 69:5648 - 5655
- Wang L, Park P, Lin CY. Characterization of stem cell attributes in human osteosarcoma cell lines. Cancer Biol Ther 2009; 8:543 - 552
- Wang L, Park P, Zhang H, La Marca F, Lin CY. Prospective identification of tumorigenic osteosarcoma cancer stem cells in OS99-1 cells based on high aldehyde dehydrogenase activity. Int J Cancer 2011; 128:294 - 303
- Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev 1996; 10:1580 - 1594
- Thawani JP, Wang AC, Than KD, Lin CY, La Marca F, Park P. Bone morphogenetic proteins and cancer: review of the literature. Neurosurgery 2010; 66:233 - 246
- Hruska KA, Mathew S, Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res 2005; 97:105 - 114
- Beck SE, Jung BH, Fiorino A, Gomez J, Rosario ED, Cabrera BL, et al. Bone morphogenetic protein signaling and growth suppression in colon cancer. Am J Physiol Gastrointest Liver Physiol 2006; 291:135 - 145
- Kawamura C, Kizaki M, Yamato K, Uchida H, Fukuchi Y, Hattori Y, et al. Bone morphogenetic protein-2 induces apoptosis in human myeloma cells with modulation of STAT3. Blood 2000; 96:2005 - 2011
- Wen XZ, Miyake S, Akiyama Y, Yuasa Y. BMP-2 modulates the proliferation and differentiation of normal and cancerous gastric cells. Biochem Biophys Res Commun 2004; 316:100 - 106
- Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 2006; 444:761 - 765
- Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 2008; 40:499 - 507
- Santagata S, Ligon KL, Hornick JL. Embryonic stem cell transcription factor signatures in the diagnosis of primary and metastatic germ cell tumors. Am J Surg Pathol 2007; 31:836 - 845
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer and cancer stem cells. Nature 2001; 414:105 - 111
- Luo X, Chen J, Song WX, Tang N, Luo J, Deng ZL, et al. Osteogenic BMPs promote tumor growth of human osteosarcomas that harbor differentiation defects. Lab Invest 2008; 88:1264 - 1277
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007; 1:555 - 567
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003; 100:3983 - 3988
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994; 367:645 - 648
- Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, et al. Identification of cells initiating human melanomas. Nature 2008; 451:345 - 349
- Giuffrida D, Rogers I. Targeting cancer stem cell lines as a new treatment of human cancer. Recent Pat Anticancer Drug Discov 2010; 5:205 - 218
- Clement JH, Raida M, Sanger J, Bicknell R, Liu J, Naumann A, et al. Bone morphogenetic protein 2 (BMP-2) induces in vitro invasion and in vivo hormone independent growth of breast carcinoma cells. Int J Oncol 2005; 27:401 - 407
- Orui H, Imaizumi S, Ogino T, Motoyama T. Effects of bone morphogenetic protein-2 on human tumor cell growth and differentiation: a preliminary report. J Orthop Sci 2000; 5:600 - 604
- Brubaker KD, Corey E, Brown LG, Vessella RL. Bone morphogenetic protein signaling in prostate cancer cell lines. J Cell Biochem 2004; 91:151 - 160
- Pouliot F, Blais A, Labrie C. Overexpression of a dominant negative type II bone morphogenetic protein receptor inhibits the growth of human breast cancer cells. Cancer Res 2003; 63:277 - 281
- Kleeff J, Maruyama H, Ishiwata T, Sawhney H, Friess H, Buchler MW, et al. Bone morphogenetic protein 2 exerts diverse effects on cell growth in vitro and is expressed in human pancreatic cancer in vivo. Gastroenterology 1999; 116:1202 - 1216
- Ide H, Yoshida T, Matsumoto N, Aoki K, Osada Y, Sugimura T, et al. Growth regulation of human prostate cancer cells by bone morphogenetic protein-2. Cancer Res 1997; 57:5022 - 5027
- Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis 2005; 26:495 - 502
- Haydon RC, Luu HH, He TC. Osteosarcoma and osteoblastic differentiation: a new perspective on oncogenesis. Clin Orthop Relat Res 2007; 454:237 - 246
- Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol 1994; 127:1755 - 1766
- Pera MF, Herszfeld D. Differentiation of human pluripotent teratocarcinoma stem cells induced by bone morphogenetic protein-2. Reprod Fertil Dev 1998; 10:551 - 555
- Yamate J, Kotera T, Kuwamura M, Kotani T. Potential osteogenic differentiation of cisplatin-resistant rat malignant fibrous histiocytoma-derived cell lines. Exp Toxicol Pathol 2007; 58:299 - 309
- Gillette JM, Gibbs CP, Nielsen-Preiss SM. Establishment and characterization of OS 99-1, a cell line derived from a highly aggressive primary human osteosarcoma. In Vitro Cell Dev Biol Anim 2008; 44:87 - 95