Abstract
Molecularly targeted therapies, such as antibodies and small molecule inhibitors have emerged as an important breakthrough in the treatment of many human cancers. One targeted therapy under development is tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) due to its ability to induce apoptosis in a variety of human cancer cell lines and xenografts, while lacking toxicity in most normal cells. TRAIL and apoptosis-inducing agonistic antibodies to the TRAIL death receptors have been the subject of many preclinical and clinical studies in the past decade. However, the sensitivity of individual cancer cell lines of a particular tumor type to these agents varies from highly sensitive to resistant. Various chemotherapy agents have been shown to enhance the apoptosis-inducing capacity of TRAIL receptor-targeted therapies and induce sensitization of TRAIL-resistant cells. This review provides an overview of the mechanisms associated with chemotherapy enhancement of TRAIL receptor-targeted therapies including modulation of the apoptotic (death receptor expression, FLIP, and Bcl-2 or inhibitors of apoptosis (IAP) families) as well as cell signaling (NFκB, Akt, p53) pathways. These mechanisms will be important in establishing effective combinations to pursue clinically and in determining relevant targets for future cancer therapies.
Introduction
Cancer occurs in various tissues of the body with uncontrolled growth and metastasis formation. Depending on the site and type of cancer, treatment will consist of surgical resection, chemotherapy and radiation therapy. The development of molecularly targeted therapies consisting of antibodies and small molecule inhibitors has revolutionized cancer therapy with selective agents that provide favorable and non-overlapping toxicity profiles. Since its discovery in 1995, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) or Apo2 ligand has been investigated as a cancer therapeutic agent. TRAIL induces apoptosis in many human tumor cell lines and tumor xenografts, but not in normal cells.Citation1–Citation4 It has been widely reported that tumor cell killing is increased by combination treatment with drugs. Different classes of drugs sensitize cancer cells to TRAIL and TRAIL receptor agonists by a variety of cellular mechanisms. This review will provide an update on optimizing TRAIL or TRAIL antibody agonists as cancer therapeutics alone and in combination with current clinically used drugs and discuss the cellular mechanisms of enhanced efficacy.
TRAIL and Receptors
TRAIL is a member of the tumor necrosis factor (TNF) superfamily, which currently comprises nineteen type II transmembrane proteins with an intracellular N-terminus. TRAIL contains a conserved TNF homology domain at its C-terminus and is associated with immune system function and homeostasis, similar to many other family members.Citation5 TRAIL exists naturally on the surface of immune cells capable of inducing apoptosis (i.e., natural killer cells) or may be proteolytically cleaved to release the extracellular domain.Citation3,Citation4,Citation6 Cellular and soluble TRAIL form a homotrimer stabilized by a zinc atom and bind to receptors, inducing stable receptor trimers.Citation3,Citation4,Citation7 Six members of the TNF receptor (TNFR) superfamily form a subset known as death receptors (DR), which are characterized by an intracellular death domain.Citation8 TNFR1, which binds to TNF, and Fas/CD95, which binds to Fas ligand, have been examined for their role in immune system function and induction of apoptosis.Citation8,Citation9 Death receptor 4 (DR4, TRAIL-R1) and death receptor 5 (DR5, TRAIL-R2) have been identified to bind with TRAIL. DR4 and DR5 have the capacity to induce apoptotic signaling after TRAIL ligand binding and are the targets of developing cancer therapies. Three additional members of the TNFR superfamily have been identified that bind to TRAIL ().Citation10 Decoy receptor 1 (DcR1, TRAIL-R3) and decoy receptor 2 (DcR2, TRAIL-R4) bind TRAIL but fail to elicit an apoptotic response. A fifth soluble receptor, osteoprotegerin (OPG), also fails to mediate apoptosis.
DR4 was first identifiedCitation11 via sequence homology to the TNFR-1 death domain (DD), a characteristic motif amongst the apoptotic-inducing members of the TNFR superfamily. DR5 was identified by a similar approach.Citation11–Citation15 These receptors are type I transmembrane proteins with two cysteine-rich domains extracellularly and an intracellular death domain, which acts as a site for protein–protein interactions involved in the apoptotic signaling cascade. Overexpression of apoptosis-inducing death receptors, DR4 and DR5, can induce ligand-independent apoptosis via receptor homo- or hetero-oligomerization.Citation12–Citation16 The first decoy receptor, DcR1, has two cysteine-rich extracellular domains and a putative hydrophobic region, but lacks an intracellular domain and instead has a glycosyl-phosphoinositol membrane anchor.Citation12–Citation14,Citation16 This is consistent with the lack of apoptotic signaling and TRAIL induced cytotoxicity in cells overexpressing DcR1. The second decoy receptor, DcR2, has two cysteine-rich extracellular domains and a hydrophobic transmembrane region, but only a partial intracellular DD.Citation17,Citation18 The truncated intracellular domain lacks the ability to induce apoptosis, but has been shown to induce nuclear factor-kappaB (NFκB) activation when the receptor is overexpressed in some systems,Citation17 but not in others.Citation18,Citation19 DcR2 may also produce anti-apoptotic signaling by activation of NFκB.Citation17 The binding of TRAIL to DcR1 and DcR2 may decrease the amount bound to death receptors.Citation20 The fifth receptor, OPG, is a soluble protein first identified by binding to RANKL/TRANCE, but later found to also bind TRAIL.Citation21 Unlike the other receptors, OPG has four cysteine-rich domains but is a soluble receptor lacking transmembrane and cytoplasmic regions. The C-terminal region of OPG has two homologous DD and a heparin binding domain.Citation22 OPG displays the weakest affinity for TRAIL of the five receptors at physiologic temperatures,Citation23 and its relevance is unclear.
The physiologic role of TRAIL has not been fully elucidated; however some insight has been gained. TRAIL may be important in natural killer cell function, virus and tumor cell immune surveillance, autoimmune disease development and airway remodeling and inflammation. TRAIL expression has been shown to be induced by interferon (IFN) in neutrophils,Citation24 natural killer cellsCitation25 and monocytes,Citation26 which may be important in TRAIL-mediated modulation of the immune system.
Mechanisms of Apoptosis by TRAIL Binding to DR4/DR5
TRAIL-induced apoptosis commences with the activation of DR4 or DR5 by ligand binding and receptor trimerization to stimulate the extrinsic and intrinsic apoptosis pathways (). The apoptotic cascade is initiated by the assembly of a death-inducing signaling complex (DISC) with the recruitment of Fas-associating protein with death domain (FADD), an adaptor protein between the death receptor and initiator caspases-8 or 10. The DD of trimerized receptors interacts with a homologous domain within FADD, by which caspase-8 is then recruited via interactions between death effector domains (DED).Citation15,Citation27–Citation32 Caspase-8 is cleaved through autocatalytic processing to produce active subunits.Citation33 The p55 and p52 pro-caspases are cleaved into p43, p41 and p12 fragments. Active p18 and p10 are formed in a second cleavage stage.Citation34 The activity of caspase-8 may also be positively or negatively regulated by ubiquitinated as summarized by Gonzalvez and Ashkenazi.Citation8 In the extrinsic apoptotic pathway, the active caspase-8 subunits interact directly with downstream effector caspases, such as capase-3 or 7, to cleave and activate them. Caspase-3 is then able to cleave many downstream substrates, such as poly (ADP-ribose) polymerase (PARP) and DNA fragmentation factor (DFF), to initiate apoptosis.Citation34
In some tumor cell lines, TRAIL activates the intrinsic apoptotic pathway, which occurs when active caspase-8 cleaves Bid (Bcl-2 inhibitory BH3-domain-containing protein), a Bcl-2 family member. Truncated Bid migrates to the mitochondrial membrane where it stimulates the oligomerization of Bak and Bax. Upon activation, Bax undergoes a conformational change and translocates to the mitochondrial membrane where homooligomers form. Bak exists as an outer mitochondrial membrane protein and forms homo-dimers, trimers and tetramers following activation.Citation35 Next, permeabilization of the outer mitochondrial membrane occurs, allowing release of mitochondrial proteins, including cytochrome c and Smac/DIABLO (second mitochondria-derived activator of caspase/direct inhibitor of apoptosis binding protein with low pI). In the cytosol, Smac/DIABLO interacts with X-linked inhibitor of apoptosis (XIAP) to release caspase-9 and caspase-3 from XIAP inhibition.Citation34 Cytochrome c binds with Apaf-1, dATP and caspase-9 to form the apoptosome where caspase-9 is activated. Active caspase-9 cleaves caspase-3, which then cleaves a variety of substrates to initiate apoptosis.Citation34,Citation36 Crosstalk has been shown to exist between the extrinsic and intrinsic apoptotic pathways, suggesting TRAIL may activate both pathways.
TRAIL and Agonistic Antibodies to TRAIL Receptors as Cancer Therapeutics
TRAIL is promising as a cancer therapeutic agent showing efficacy against tumor cells without the toxicities to normal cells associated with other TNF family members. TNF and Fas ligand both induce cytotoxicity against tumor cells, but in murine models TNF induces a lethal inflammatory response and Fas ligand results in severe hepatotoxicity.Citation37 Early reports indicated certain preparations of recombinant TRAIL also produced hepatotoxicity in vitro.Citation38 A different recombinant form of TRAIL lacking sequence modifications to amino acids 114–281 and with the addition of a modified leucine zipper produced tumor cell apoptotic activity in vitro and tumor growth inhibition in vivo without hepatotoxicity.Citation1,Citation39 Nonhuman primate studies did not reveal any organ or systemic toxicities despite binding to primate receptors with an affinity similar to the human receptor. High doses of TRAIL have been administered and well tolerated in nude mice, rats, cynomolgus monkeys and chimpanzees, but display rapid whole body clearance and short plasma half-lives (3–5 minutes in rodents and 24–31 minutes in non-human primates).Citation1 The relevance of the short half-life to efficacy is still to be determined in clinical trials, which are currently underway. In Phase I studies, no dose-limiting toxicities have been reported, and out of 32 patients, 17 had stable disease and there was one patient with a partial response.Citation40
TRAIL has shown variable cytotoxic activity against a broad spectrum of human tumor cell lines, including breast, colon, lung, pancreatic, prostate, renal and thyroid carcinoma, glioma, multiple myeloma and leukemia.Citation41 However, certain cell lines or tumor types exhibit TRAIL resistance. Many TRAIL and chemotherapy combinations act synergistically against a variety of tumor cell lines and can reverse resistance to either agent ().Citation37 Most of the current clinically used chemotherapy agents have been shown to enhance TRAIL-mediated apoptosis, including cisplatin, doxorubicin, 5-fluorouracil (5-FU) and camptothecin (CPT-11).Citation42 To demonstrate different classes of drugs are capable of producing increased cytotoxicity against non-small cell lung carcinoma cells in combination with TRAIL receptor-targeted therapies, we evaluated TRA-8 cytotoxicity in combination with various chemotherapy agents. shows the activity of doxorubicin, bortezomib and docetaxel in combination with TRA-8 against the A549 lung cancer cell line. These results indicate that each of these chemotherapy agents is capable of sensitizing cells to TRA-8 in a synergistic manner. All three drugs interacted with TRA-8 in a significantly synergistic manner (combination index <1). Doxorubicin is classified as a topoisomerase II inhibitor, docetaxel as a microtubule stabilizer and bortezomib as a proteasome inhibitor, yet each interacts with TRA-8 in the A549 lung cancer cells. As will be described later in greater detail, this may occur through modulation of the intracellular regulatory components of the apoptotic cascade and other cell signaling pathways. provides a summary of chemotherapy agents reported to enhance TRAIL or death receptor antibody efficacy and the apoptotic regulatory proteins the combinations modulate.
Tumor cell resistance to TRAIL-induced apoptosis may be due to the expression of decoy receptors on the cell surface. For this reason, agonistic antibodies may have greater therapeutic potential due to specific targeting of the death receptors without decoy receptor binding, in addition to a longer plasma half-life.Citation42 There has been an immense effort both in academia and the pharmaceutical industry to develop antibodies to TRAIL death receptors. Notable examples currently in clinical trial include: Humanized TRA-8 anti-DR5 (Tigatuzumab, CS-1008) from Daiichi-Sankyo;Citation43–Citation45 fully human antibodies against DR4 (Mapatumumab, HGS-ETR1, TRM-1) or DR5 (Lexatumumab, HGS-ETR2) from Human Genome Sciences; human anti-DR5 (Conatumumab, AMG 655) from Amgen;Citation45,Citation46 and human anti-DR5 antibody (4H6, Apomab) from Genentech Inc.Citation42
TRA-8, a murine antibody to DR5, produced significant tumor growth inhibition of 2LMP breast cancer xenografts and TRA-8 combined with doxorubicin or paclitaxel produced greater tumor inhibition than any agent alone.Citation47 The interaction between doxorubicin and TRA-8 was shown to be synergistic in vivo and was further enhanced by the addition of 60Co radiation therapy. TRA-8 was shown to activate apoptotic pathways and its efficacy was enhanced by doxorubicin similar to what has been observed with TRAIL (). Combination treatment of breast cancer cells with TRAIL or TRA-8 and doxorubicin resulted in activation of caspases, cleavage of Bid and PARP (). Also, there was a reduction in XIAP levels to a varying degree in different cell lines.Citation48 Efficacy of TRA-8 has been observed against breast, cervical, ovarian, pancreatic, glioma and colon cancer cell lines in vitro and in vivo in tumor xenograft models, which was enhanced by combination treatment with chemotherapy drugs.Citation42,Citation47,Citation49–Citation54 In an ex vivo assay of primary ovarian cancer, 79% (15 of 19) of patient tumor specimens demonstrated sensitivity to TRA-8 treatment in a dose-dependent manner associated with the induction of apoptosis.Citation50 A Phase I trial with a humanized version of TRA-8 (Tigatuzumab, CS-1008) has been completed without any dose limiting toxicity and 7 of 17 patients had stable disease.Citation44
Apomab, an additional agonistic DR5 antibody in development, was shown in combination with chemotherapy (paclitaxel and carboplatin) to significantly inhibit tumor growth and prolong survival in mice with orthotopic NCI-H460 lung tumor xenografts.Citation55 In preclinical studies, treatment with mapatumumab, an agonistic antibody to DR4, inhibited the growth of colon, non-small cell lung and renal tumor xenografts in vivo and was shown to induce activation of caspases-3, 8 and 9 in vitro. When combined with 5-FU, CPT-11 or topotecan, mapatumumab produced greater anti-tumor efficacy against colon carcinoma xenografts than any agent alone.Citation56 Mapatumumab has been shown to have a terminal plasma half-life of 6.9–8.7 days in mice. Mapatumumab and lexatumumab, an antibody against DR5, were shown individually to inhibit COLO205 colon cancer xenograft growth in vivo, whereas lexatumumab demonstrated greater growth inhibition with more tumor regressions.Citation57 Mapatumumab and lexatumumab also showed apoptotic activity against 67 and 70% of 27 primary lymphoma samples, respectively.Citation58
Phase I clinical trials have shown mapatumumab and lexatumumab antibodies to be well tolerated with grade 3 toxicity in a small number of patients.Citation59,Citation60 Mapatumumab Phase I clinical trials established that the antibody can be administered safely with no significant hematologic toxicity. Two out of eleven patients had grade 3 elevations of liver function tests, although each had elevated transaminases at baseline. Antibody plasma concentrations comparable to efficacious concentrations in preclinical mouse models were attainable with 10 mg/kg dosing in humans with trough concentrations greater than 1 µg/mL.Citation60 A Phase II trial of mapatumumab in advanced non-small cell lung cancer patients who had received prior chemotherapy demonstrated 10 mg/kg was well tolerated, but no patients responded. Nine of 32 patients had stable disease for a minimum of 4 weeks.Citation61 However, a recent Phase II trial reported no improvement in response rate or progression-free survival with the addition of mapatumumab to paclitaxel and carboplatin in non-small cell lung cancer patients.Citation62 Another Phase II trial in patients with non-Hodgkin's lymphoma reported one complete response, two partial responses and 12 patients had stable disease. Two serious adverse events (shingles or fever) were reported and may have been related to treatment.Citation63 The investigators concluded that higher doses of mapatumumab and future trials with combination chemotherapy are warranted.Citation61 In Phase I trials, lexatumumab was also well tolerated and 12 of 37 patients had stable disease. A maximum tolerated dose of 10 mg/kg was determined as dose limiting toxicities occurred in 3 of 7 patients treated with 20 mg/kg.Citation59 Additional Phase I trials have been reported and Phase II trials are planned. Important to note is that the majority of the patients in the Phase I trials have previously failed treatment and had disease progression on chemotherapy regimens. Therefore, stable disease and a small percentage of patients with partial and complete responses is promising.
The potential of TRAIL targeted therapies lies in their ability to enhance the tumor cytotoxicity of existing chemotherapy or antibody regimens. TRAIL has been combined with rituximab for the treatment of non-Hodgkin's lymphoma;Citation63,Citation64 mapatumumab was used in combination with gemcitabine and cisplatin;Citation65 and lexatumumab was used in combination with gemcitabine, pemetrexed, doxorubicin or FOLFIRI (a combination of leucovorin, 5-FU and irinotecan).Citation66 In each of these trials, preliminary reports suggest that each agent can be safely administered to patients in combination with chemotherapy or antibody regimens. Each of these examples mapatumumab and lexatumumab demonstrated the clinical applicability and promise of TRAIL receptor agonistic antibodies in the treatment of human cancer. As Phase II clinical trials of these targeted therapies combined with chemotherapy continue and are reported, the clinical utility of these therapies will become more apparent.
Determinants of Sensitivity
As described above, TRAIL and agonistic antibodies to the TRAIL death receptors have apoptosis-inducing activity against a variety of human cancer cell types both in vitro and in vivo. However, approximately one-third of human tumor cells are resistant to TRAIL treatment and an additional one-third have only a moderate response.Citation42 Resistance can occur at various points in the apoptotic pathway or in other cellular signaling pathways. A variety of apoptosis regulatory molecules, including death and decoy receptors, FLIP, XIAP and Bcl-XL and signaling pathways, including NFκB and Akt, have been associated with modulating resistance ().Citation67 The mechanism of resistance may be a delicate balance between levels of pro- and anti-apoptotic molecules within the cells. It is likely that synergistic effects between drugs and TRAIL or death receptor antibody agonists are achieved by modulation of one or more of these apoptotic regulatory proteins or signaling pathways. A better understanding of these mechanisms will aid in the development of cancer therapeutics with combination therapies to tip the balance towards apoptosis.
Receptor expression.
TRAIL and its receptors are expressed in a variety of tissues, unlike other TNF superfamily members that show more specific expression patterns. For example, Fas ligand is primarily found in stimulated T cells.Citation11 TRAIL is expressed throughout various parts of the adult human body, including spleen, prostate, thymus, ovary, small intestine, colon, peripheral blood leukocytes, heart, lung, skeletal muscle and kidney.Citation3 The wide expression of TRAIL suggests it is nontoxic to normal cells. Researchers originally hypothesized that relative expression of death and decoy receptors would predict sensitivity of cells to TRAIL.Citation12,Citation17 However, results showed that in many instances basal receptor expression and TRAIL sensitivity did not correlate. No relation of sensitivity and DR4, DR5 and DcR1 expression was shown amongst eleven breast cancer linesCitation68 or in Jurkat leukemia cells.Citation69 A lack of correlation was also reported in multiple pancreatic cancer cell linesCitation70,Citation71 and six hepatocellular carcinoma cell lines.Citation72
However, some chemotherapy agents and radiation have been shown to increase the expression of DR4 and/or DR5, and along with other factors may contribute to TRAIL sensitization. For example, doxorubicin and etoposide have been shown to upregulate levels of DR4 and DR5 and synergize with TRAIL.Citation73,Citation74 DNA damaging chemotherapy agents, including doxorubicin and etoposide, and radiation induce DR5 gene expression via a p53-dependent mechanism.Citation75–Citation79 Takimoto and El-DeiryCitation80 and Liu et al.Citation81 identified intronic p53 binding sites within the DR5 and DR4 genes, respectively. In addition, NFκB has been shown to have binding sites within the DR4 promoter region and intron 1 of the DR5 gene.Citation82,Citation83 Regulation of NFκB by overexpression of active NFκB subunits (cRel, p65) or by etoposide have been shown to increase expression of both receptors.Citation74,Citation83,Citation84 Along with activation of the DR5 promoter, DR5 expression may be subject to transcriptional repression by Yin Yang 1 (YY1) for which a binding site has been suggested within the DR5 promoter.Citation85 Baritaki et al.Citation85 reported that treatment of PC-3 prostate cancer cells with cisplatin, etoposide, doxorubicin or vincristine increased DR5 expression, decreased YY1 expression and sensitized cells to TRAIL-induced apoptosis. A reduction in YY1 levels by siRNA also increased DR5 expression and TRAIL-induced apoptosis. The reduction in YY1 and subsequent increases in DR5 by etoposide were correlated to a decrease in NFκB activity. Later studies showed that a proteasome inhibitor NPI-0052 and a nitric oxide donor DETANONOate sensitized tumor cells to TRAIL-induced with a similar reduction in NFκB activity, decreased YY1 and increased DR5 expression.Citation86,Citation87 Another molecule proposed to regulate the transcription of DR5 is Sp1. A putative binding site within the DR5 promoter for transcription factor Sp1 was identified by Yoshida et al.Citation82,Citation88 Histone deacetylase inhibitors (sodium butyrate in HCT116 colon cancer cells and sodium butyrate and trichostatin A in 786-O renal cell carcinoma cells) were shown to increase the mRNA and protein levels of DR5, which correlated with an increase in apoptosis and caspase activity. Further analysis using mutations within the Sp1 binding sites demonstrated Sp-1 was involved in the increased DR5 expression.Citation89,Citation90 These studies demonstrate the variety of mechanisms and chemotherapeutic agents that can modulate death receptor expression and subsequently sensitize cells to death receptor-modulated apoptosis.
Another means of modulating DR5 expression on the surface of tumor cells by chemotherapy agents is by upregulating ceramide to form ceramide-rich membrane rafts to cluster DR5 and enhance DISC formation.Citation91 Therefore, basal death receptor expression may not predict sensitivity to TRAIL-targeted therapies, but increased death receptor expression on cancer cells by chemotherapy may play a role in sensitization. Another important concept in TRAIL death receptor function is internalization following ligand binding.Citation8 DR4 and DR5 have been shown to undergo dynamin-dependent clathrin-mediated endocytosis upon TRAIL binding, but blockade of internalization by dominant negative dynamin enhanced TRAIL-induced apoptosis.Citation92 Other mechanisms of receptor internalization also exist and the overall impact on TRAIL activity remains unknown. Post-translational modifications of the death receptors have also been associated with TRAIL sensitivity.Citation8,Citation93 Ashkenazi and colleagues found that expression of O-glycosyltransferase GALNT14 mRNA correlated with TRAIL sensitivity of 119 human cancer cell lines using genome-wide profiling. O-glycosylation of DR4 and DR5 promoted clustering of death receptors and DISC formation. When O-glycosylation was inhibited, death receptor complexing and caspase-8 association within the DISC were reduced.Citation93 This post-translational modification of death receptors and correlation to sensitivity may provide a useful biomarker for response in future clinical trials.
Another point to consider in regards to death receptor-targeted therapy is the relative contribution of each receptor to the induction of apoptosis. In certain types of cancer it has been reported either DR4 or DR5 is predominantly responsible for the apoptotic response. Kelley et al.Citation94 used TRAIL variants, which preferentially bind to either DR4 or DR5, to show a greater contribution of DR5 to induction of apoptosis in Colo205 and Colo320 colon cancer cells and MDA-MB-231 breast cancer cells. In a panel of 12 glioma cell lines, DR5 binding antibodies produced cytotoxicity against 8 cell lines, while all were resistant to a DR4 antibody.Citation95 Conversely, mapatumumab (a DR4 antibody) produced greater cytotoxicity than lexatumumab (a DR5 antibody) in 9 of 13 pancreatic cancer cell lines.Citation96 In these studies, mapatumumab produced synergistic cytotoxicity in combination with XIAP inhibitors, while less combination effect was seen with lexatumumab. Primary pancreatic carcinoma cells also were more sensitive to maptumumab.Citation96 Additional studies have highlighted the dominance of DR4-mediated apoptosis in pancreatic cancer and chronic lymphocytic leukemia.Citation97,Citation98 The relevance of these studies to the clinical application of TRAIL receptor-targeted therapies remains to be determined.
FLIP.
FLIP is structurally related to caspase-8 and multiple splice mRNA variants are produced, but the cellular short form (cFLIPS) and long form (cFLIPL) are most commonly detected with each having two DED domains similar to those within FADD and caspase-8.Citation99 cFLIPL also contains a pseudo-caspase domain, which lacks critical cysteine residues required for caspase activation. FLIP can be recruited during DISC formation () to inhibit the apoptotic cascade by binding to FADD or caspase-8 by DED-DED interactions.Citation67 FLIP has been indicated as important in the progression of cancer. For example, Ryu et al.Citation100 showed overexpression of cFLIPL in colonic adenocarcinomas compared to matched normal tissues. FLIP has also been identified in the development of drug and TRAIL resistance in human cancers. FLIP levels were higher in three TRAIL-resistant melanoma cell lines compared to five sensitive lines and actinomycin D treatment of one resistant cell line reduced FLIP levels and significantly sensitized cells to TRAIL.Citation101 A variety of chemotherapy agents have been shown to reduce FLIP levels and enhance susceptibility to TRAIL-induced apoptosis in diverse types of human cancers. For instance, combination treatment with doxorubicin and TRAIL produced tumor growth inhibition of PC3 prostate cancer xenografts and reduced tumoral FLIP levels.Citation102 PPAR-γ ligandsCitation103 and synthetic triterpenoidsCitation104,Citation105 have also been shown to reduce FLIP and sensitize tumor cells to TRAIL-induced apoptosis. In human multiple myeloma cells, an increased FLIP to procaspase-8 ratio was present in TRAIL resistant cells. Treatment with cyclohexamide, bisindolymalemide (a protein kinase C inhibitor) or FLIP oligonucleotides resulted in the reversal of resistance.Citation106 Therefore, FLIP may be an important modulator of TRAIL-resistance in a variety of human tumors, and many agents that reduce FLIP levels enhance TRAIL efficacy. However, other investigators have failed to show any correlation between FLIP levels and TRAIL-resistance and attribute it to other intracellular factors. For example, no relationship between TRAIL susceptibility and FLIP expression was detected in a panel of 28 melanoma cell lines,Citation107 6 lung cancer linesCitation108 or 13 glioma cell lines.Citation109
Bcl-2 family.
The balance between pro- and anti-apoptotic activities of the Bcl-2 family of proteins also regulates sensitivity to TRAIL and other therapies (). This family consists of at least 20 proteins, all of which contain one or more conserved Bcl-2 homology (BH) domains.Citation110,Citation111 Many anti-apoptotic members have been identified, including: Bcl-2, Bcl-XL, Bcl-w, Bfl-1 and Mcl-1. These proteins contain a hydrophobic groove containing residues of their BH1, BH2 and BH3 regions and a hydrophobic C-terminal domain that allows them to target intracellular membranes. The Bax family and the BH3-only family comprise two pro-apoptotic groups. Bax family members have BH1, BH2 and BH3 protein domains similar to the anti-apoptotic proteins, but their C-terminal domain occludes the hydrophobic groove until a conformation change occurs with apoptotic signals. The BH3-only proteins have a short BH3 region and act as internal sensors for damage and antagonize the anti-apoptotic Bcl-2 members. Both Bax and BH3-only pro-apoptotic molecules must be present to produce apoptosis.Citation110
Bcl-2, Bcl-XL, Bcl-w and Mcl-1 strongly inhibit apoptosis in response to many cytotoxic agents in a variety of cell types and overexpression of Bcl-2 or Bcl-XL has been reported to confer resistance to TRAIL in a variety of tumor cells.Citation110,Citation112 For example, Fulda et al.Citation112 reported Bcl-2 overexpression protected neuroblastoma, glioblastoma and breast cancer cells from TRAIL-induced apoptosis. Cleavage of caspase-3, 7, 8 and 9 was reduced, as well as decreased processing of their substrates PARP, DFF45 and XIAP. Protection against TRAIL cytotoxicity was also demonstrated by Bcl-2 overexpression in lungCitation113 and colon cancer cells.Citation114 The expression of Bcl-XL in three pancreatic cancer cell lines was associated with TRAIL resistance.Citation115 Expression of Mcl-1, a more recently described Bcl-2 family member, has also been correlated to TRAIL resistance in cancer cells and knock-down of Mcl-1 levels by various methods, such as small-interfering RNA, sensitized cancer cells to TRAIL-induced apoptosis.Citation116–Citation119
Decreased expression of pro-apoptotic Bax family proteins has also been implicated in TRAIL resistance. TRAIL induced cytochrome c release and apoptosis in Bax or Bak knockout murine embryonic fibroblasts, but not in the double knockout cells, suggesting that in these cells Bax and Bak may provide some compensation for each other.Citation36 In HCT116 colon carcinoma cells, Bax-deficient cells were TRAIL resistant and lacked cleavage of caspase-9, -7 and PARP; however TRAIL sensitivity was restored with camptothecin and etoposide pretreatment which produced an increase in Bak and DR5 expression.Citation120 TRAIL in combination with 5-FUCitation121 or ionizing irradiationCitation122 synergistically induced apoptosis in Bax expressing prostate cancer cells, while cells without Bax were resistant to TRAIL-induced apoptosis in combination with either agent. Han et al.Citation123 reported that resistance to TRAIL cytotoxicity in Bax and Bak deficient Jurkat leukemia cells could be reversed with adenoviral transduction of the Bax gene, but not Bak. These reports indicate that the loss of pro-apoptotic proteins, especially Bax, may be important in the resistance of cancer cells to TRAIL-induced apoptosis.
TRAIL has been combined with a variety of other agents to overcome resistance by modification of the Bcl-2 family of proteins.Citation124 Ray and AlmasanCitation124 reported that TRAIL combined with CPT-11 increased Bax and reduced Bcl-XL expression in prostate cancer cells in vitro; whereas in vivo, they induced increased intratumoral Bak and Bcl-XS expression and decreased Bcl-w and Bcl-XL. Bortezomib, a proteasome inhibitor, was shown to decrease Bcl-2 and Bcl-XL in glioblastoma multiforme cells in vitro and enhance TRAIL-induced cytotoxicity.Citation125 Two TRAIL-resistant colon cancer cell lines produced by Zhu et al.Citation126 were sensitized by bortezomib or MG-132, another proteasome inhibitor, which resulted in increased expression of DR5 and Bik a BH-3 only pro-apoptotic protein. Promising new agents under investigation for combination treatment with TRAIL are small molecule Bcl-2 inhibitors. HA14-1, a Bcl-2 inhibitor, combined with TRAIL resulted in increased apoptosis in Bcl-2 overexpressing TRAIL-resistant SW480 colon carcinoma cells.Citation114 CEM leukemia cells were sensitized to TRAIL by low concentrations of HA14-1 and BH3I-2′ another Bcl-2 inhibitor.Citation127 Bcl-2 siRNA treatment enhanced TRAIL-induced apoptosis in A375 melanoma cancer cells.Citation128 Gossypol, a cottonseed oil extract, has also shown BH3-mimetic properties and sensitized lung and esophageal cancer cells to TRAIL with an increase in apoptosis.Citation129 Another Bcl-2 small molecule inhibitor, ABT-737, was combined with TRAIL to increase cytotoxicity against certain renal, lung and prostate cancer cell lines.Citation130 ABT-737 was also shown to be effective in enhancing TRAIL cytotoxicity against the human pancreatic cell lines PANC-1 and BxPC-3. Mechanistic studies revealed the combination produced greater activation of apoptosis via disassociations of the pro-apoptotic Bcl-2 family members from the anti-apoptotic members to favor apoptosis.Citation131 These strategies highlight the importance of the Bcl-2 family of proteins in TRAIL-induced apoptosis.
IAPs and Smac/DIABLO.
Inhibitors of apoptosis (IAP) proteins are a highly effective cellular means of blocking the apoptotic cascade through interactions with caspases or Smac/DIABLO. Each member of the IAP family is characterized by one to three tandem repeats of a baculoviral IAP repeat (BIR)-binding domain which allow for binding to specific caspases or pro-apoptotic molecules. Many family members have been identified, including cIAP1, cIAP2, XIAP, survivin, BRUCE and NAIP.Citation67 Certain members also have RING domains that allow them to act as ubiquitin E3 ligases to initiate the degradation of target proteins following attachment of ubiquitin molecules.Citation132 XIAP blocks the activity of effector caspase-3 and 7 and prevents the activation of caspase-9 by direct interactions.Citation133,Citation134 Other IAPs function by binding to pro-apoptotic molecules such as Smac/DIABLO, which is a mitochondrial protein released along with cytochrome c following mitochondrial membrane depolarization by certain apoptotic stimuli (). Smac/DIABLO associates with IAPs to inhibit their anti-caspase activity and progression of the apoptotic cascade may be related to the balance of pro- and anti-apoptotic molecules. Many IAPs have been associated with chemotherapy and TRAIL resistance.Citation135–Citation140 XIAP and survivin have been most extensively described to play a major role in TRAIL resistance.Citation67
XIAP appears to be the most potent caspase inhibitor in the family and functions by direct binding to caspases and by serving as ubiquitin-protein ligase for active caspase-3 to promote its degradation.Citation141 Disruption of the XIAP gene in human colon cancer cells was shown to enhance their sensitivity to TRAIL suggesting that XIAP is an important modulator of TRAIL-induced apoptosis.Citation136 Various methods have been used to reduce XIAP protein or messenger RNA levels to reverse TRAIL resistance. In our own studies, doxorubicin decreased XIAP protein levels to a varying extent in breast cancer cell lines.Citation48 Flavopiridol, a cyclin-dependent kinase inhibitor and TRAIL synergistically increased apoptosis in human leukemia cells with reductions in XIAP.Citation142 RNA interference targeting XIAP was used in combination with TRAIL to induce apoptosis in pancreatic cells in vitro and in vivo where the combination induced the regression of PancTu1 tumor xenografts.Citation143 A small molecule Smac/DIABLO mimetic, which binds to XIAP with strong affinity, was shown to synergize with TRAIL or the anti-DR5 antibody HGS-ETR2 (Lexatumumab) against ovarian cancer cells and with TRAIL against breast cancer cell lines.Citation144,Citation145 The modulation of Smac/DIABLO and XIAP may provide future clinical benefit as development of other mimetics continues.
Survivin has a dual function; it inhibits caspase-9 activation within the apoptosome and it has a role in microtubule stability during mitosis that functions in cell cycle progression.Citation146 Li et al.Citation137 showed lower survivin expression in four TRAIL sensitive lines compared to seven more TRAIL resistant uveal melanoma cell lines. Topotecan produced a decrease in survivin and an increase in DR4 and DR5 levels in prostate cancer cells while also increasing susceptibility to TRAIL.Citation147 Decreased survivin expression and TRAIL sensitization of breast cancer cells was also noted with PPAR-γ agonists.Citation148 Survivin antisense RNA has been shown to reverse TRAIL resistance in two uveal melanoma cell lines.Citation137 siRNA-mediated downregulation of XIAP and survivin also have been used to sensitize melanoma and renal cell carcinoma cells to TRAIL.Citation128
Nuclear factor-kappaB signaling.
The nuclear factor-kappa B (NFκB) family members are transcription factors, including cRel, RelA (p65), RelB, p50 (NFκB1) and p52 (NFκB2).Citation149 Each has a conserved Rel homology domain and together form more than ten homo- and heterodimer complexes. Most NFκB dimers interact with the majority of κB DNA binding sites with high affinity; however some interact preferentially with other promoters and can elicit transcription with varied efficiencies.Citation149 NFκB proteins are ubiquitously expressed in cells and their activity is regulated by the inhibitor of κB (IκB) family of proteins (). IκB proteins block nuclear localization signals of functional NFκB dimers by binding to dimerization domains and sequestering the dimers in the cytoplasm. Upon exposure to a NFκB-inducing stimulus, IκB kinase (IKK) complexes are activated and IκB proteins are phosphorylated at serine residues. Following phosphorylation, IκB is ubiquitinated at lysine residues and degraded by the proteasome, which releases active NFκB to translocate to the nucleus. Once active NFκB dimers are located in the nucleus, they can induce transcription of a variety of target genes. NFκB complexes may have a pro- or anti-apoptotic function.Citation41,Citation149 Anti-apoptotic targets include cIAP1/2, XIAP, TRAF1/2, Bfl-1, Bcl-XL, DcR3 and FLIP. Some investigators report pro-apoptotic NFκB activity with the induction of gene transcription and protein expression of DR4, DR5 and TRAIL. However, the balance between pro- and anti-apoptotic signaling requires further study.Citation41,Citation149
TRAIL activation of NFκB signaling activity is complex and may occur through DR4, DR5 and DcR2 signaling (). TRAIL induces NFκB signaling via recruitment of receptor-interacting protein (RIP), a serine threonine kinase, by FADD within the DISC.Citation19 RIP, along with TNF receptor associated factor 2 (TRAF2), stimulates members of the IκB kinase (IKK) complex, NFκB-inducing kinase (NIK) and IKKα/β (IKK1/2),Citation150 which lead to IκB degradation and release of active NFκB dimers. Recruitment of RIP is enhanced when cells are pretreated with a caspase inhibitor.Citation19 Proteolytically active caspase-8 cleaves RIP to form a dominant negative fragment, which blocks the NFκB pathway. Therefore when the apoptotic cascade is activated, NFκB activity is diminished in a caspase-sensitive manner.Citation149
The pro-survival or pro-apoptotic function of NFκB signaling within cells may be dependent on the relative abundance of the various NFκB proteins. Researchers report differences in transcriptional activity of the cRel and RelA proteins. Ravi et al.Citation84 reported that wild-type and RelA double knockout mouse fibroblasts were sensitive to TRAIL-induced apoptosis, but cRel knockout cells were resistant. Forced expression of cRel was shown to enhance sensitivity to TRAIL and increase levels of DR4 and DR5, which could be blocked by IκB expression. RelA expression reduced TRAIL cytotoxicity and increased Bcl-XL levels. Chen and colleaguesCitation151 found that RelA overexpression in MDA-MB-231 breast cancer cells reduced expression of caspase-8, DR4 and DR5 expression, while an increase in cIAP1/2 protected cells from TRAIL-mediated apoptosis. Overexpression of cRel amplified TRAIL-induced apoptosis with an increase in DR4, DR5 and Bcl-XS and reduced cIAP1/2 and survivin. Therefore, NFκB may enhance or hinder apoptosis depending on the permutations of subunits and dimers present in cells.
In many types of human cancer cells, reductions in NFκB anti-apoptotic activity enhance the cytotoxic response to TRAIL. NFκB was shown to be induced by TRAIL treatment in hepatoma cells with activation of IKK and degradation of IκB, while NFκB inhibition increased TRAIL-induced cytotoxicity.Citation152 Proteasome inhibitors are promising modulators of the NFκB pathway, mainly by reducing IκB degradation. Mitsiades et al.Citation153 used bortezomib (PS-341/Velcade), a proteasome inhibitor, to enhance TRAIL-mediated apoptosis in multiple myeloma cells. Bortezomib and geldanamycin, a heat shock protein 90 inhibitor, were shown to synergistically block NFκB activity in TRAIL resistant pancreatic cancer cells. The combination also reduced expression of Bcl-XL, Bcl-2, cIAP1 and cyclin D and reversed resistance to TRAIL.Citation154 Interferon-αCitation155 and curcumin, a plant extract,Citation156 are additional agents that restore cancer cell sensitivity to TRAIL by inhibiting NFκB activity. In TRA-8 resistant BT-474 cells, 24 or 48 h exposure to doxorubicin produced a dramatic decrease in expression of IκBα, compared to untreated control cells, while cells treated with a combination of TRA-8 and doxorubicin had a greater reduction in IκBα protein levels (). A reduction in IκBα generally indicates activation of NFκB signaling. The expression of the active subunits of the NFκB complex determines whether its function is primarily pro- or anti-apoptotic. The NFκB subunit, p65, showed a modest reduction following 3 h of TRA-8 and 24 h of doxorubicin treatment. However, combination treatment substantially reduced p65 levels after 24 h TRA-8 and 48 h doxorubicin exposure. These results indicate that despite a decrease in IκBα, NFκB signaling may be reduced by doxorubicin treatment in breast cancer cell lines. However, blockade of NFκB signaling via inhibition of translocation of NFκB subunits into the nucleus by SN50 or knockdown of p65 by siRNA failed to sensitize BT-474 cells to TRA-8 (). These results show that blockade of only NFκB signaling may not be sufficient to enhance sensitivity to TRAIL receptor-targeted therapies.
PI3K and Akt.
Phosphatidylinositol-3 kinase (PI3K) is a major regulator of receptor tyrosine kinase and G protein-coupled receptor activity. Upon stimulus with growth factors of these various receptors, PI3K phosphorylates the plasma membrane phospholipid, phosphatidylinositol-4,5 bisphosphate (PIP2) to phosphatidylinositol-3,4,5 trisphosphate (PIP3).Citation157 One critical downstream effector of PI3K is the serine/threonine kinase Akt. Negative regulation of the PI3K/Akt pathway is primarily by PTEN (phosphatase and tensin homologue deleted on chromosome 10) activity. PTEN dephosphorylates PIP3 to PIP2, which reduces PI3K and Akt activity.Citation158 Akt exists in mammalian cells as three isoforms (Akt1, Akt2 and Akt3). Akt is recruited to the plasma membrane where PIP3 binding induces a conformational change uncovering phosphorylation sites within Akt. Next, 3′-phosphoinositide-dependent kinase 1 (PDK1) phosphorylates Akt and stabilizes its active conformation. Akt has many downstream targets, especially mediators of cell proliferation and cell survival.Citation158 Akt activation promotes cell proliferation through inhibition of glycogen synthase kinase-3, which leads to increased cyclin D expression and cell cycle progression. Akt also phosphorylates p21/Waf1 and p27/Kip2 to prevent their nuclear translocation and anti-proliferative effects.Citation158 Anti-apoptotic effects of Akt include phosphorylation of Bad, which prevents it from inactivating Bcl-XL and blocks cytochrome c release. Akt may also phosphorylate caspase-9 to prohibit its activation. The forkhead transcription factor family is also inactivated by means of phosphorylation by Akt to inhibit its transcription of pro-apoptotic genes. Akt phosphorylates murine double minute-2 protein (MDM2) to enhance p53 degradation and inhibit apoptosis. Akt stimulates the NFκB pathway by activation of IKK to increase IκB degradation, allowing NFκB to induce the expression of a variety of anti-apoptotic proteins.
PI3K, Akt and PTEN have important roles in cancer cell survival and resistance to cell death by many agents, including TRAIL. PTEN is one of the more frequently mutated or deleted tumor suppressors in human tumors.Citation158 Loss of PTEN expression leads to an increase in PIP3 levels resulting in constitutively activated Akt. This has been reported in thyroid, breast, colon, prostate and other tumors. LNCaP prostate cancer cells are reported to be TRAIL resistant due to lack of active PTEN and presence of constitutively active Akt, which may be overcome by PI3K inhibitorsCitation159,Citation160 or dominant negative Akt.Citation159 Restoration of active PTEN expression in LNCaP cells by an adenoviral vector sensitized cells to TNF and TRAIL-induced apoptosis in a FADD-dependent manner.Citation161 Amongst six human gastric cancer cell lines, the most TRAIL-resistant line, SNU-216, exhibited the highest level of Akt activity and FLIPS expression. LY294002, a PI3K inhibitor, was able to decrease both Akt and FLIP and sensitize cells to TRAIL-mediated apoptosis. Furthermore, sensitive cells could be made resistant by overexpression of constitutively active Akt.Citation162,Citation163 In five non-small cell lung cancer cell lines, expression of phospho-Akt inversely correlated with TRAIL sensitivity.Citation164 Akt blocked Bid cleavage and the intrinsic pathway of apoptosis in TRAIL-resistant cells; additionally, PI3K inhibitors, dominant negative Akt expression or PTEN transfection sensitized resistant H1155 lung cancer cells to TRAIL. Conventional chemotherapy agents, including paclitaxel and cisplatin, enhanced TRAIL-mediated apoptosis in SKRC-49 renal cell carcinoma cells by ceramide formation, which produced Akt inactivation.Citation165 Measurements of basal phospho-Akt levels, the active form, in 2LMP and BT-474 breast cancer cells revealed phospho-Akt activity in BT-474 cells with no detection of phospho-Akt (Ser473) in 2LMP cells (). In BT-474 cells, phospho-Akt was reduced by treatment with a combination of TRA-8 and doxorubicin. These results suggest that Akt may contribute to the resistance of BT-474 cells. To further determine the importance of Akt signaling, chemical inhibitors of the pathway were used to interrupt Akt signaling by a variety of mechanisms. BT-474 cells were pretreated with a PI3K inhibitor, LY294002 or an Akt inhibitor, 1L-6-hydroxymethyl-chiro-inositol 2(R)-2-O-methyl-3-O-octadecylcarbonate, for 24 h prior to the addition TRA-8 antibody for an additional 24 h. Neither agent combined with TRA-8 increased cytotoxicity (). These results indicate that doxorubicin in combination with TRA-8 modulated Akt expression in BT-474 cells, but this modulation alone was not the mechanism responsible for increased cytotoxicity after combination treatment. Therefore, the PI3K/Akt pathway may be important in some human tumor cell lines, but not all. Nonetheless, Akt may play a role in cellular resistance to TRAIL-therapy in certain human cancer cell types and modifying the PI3K/Akt pathway in cancer cells may identify new targets to reverse TRAIL resistance.
Growth factors may affect TRAIL response via modulation of the PI3K/Akt pathway. Insulin-like growth factor-1 (IGF-1) decreased TRAIL-induced cytotoxicity in multiple myeloma cellsCitation166 and thyroid carcinoma cellsCitation167 while enhancing Akt and NFκB activity with upregulation of FLIP, survivin, cIAP1 and XIAP levels. Epidermal growth factor (EGF) protected MDA-MB-231 breast cancer cells and human embryonic kidney cells HEK 293 from TRAIL-mediated apoptosis via activation of Akt and blockade of cytochrome c release.Citation168 This activation of the Akt pathway by EGF may be exploited for combination therapy with TRAIL. Shrader et al.Citation169 reported that gefitinib, an EGFR inhibitor, in combination with TRAIL induced increased apoptosis by decreasing active Akt and XIAP levels. Thus, some growth factors modulate TRAIL sensitivity through anti-apoptotic signaling and inhibition of growth factor signaling may increase sensitivity.
p53.
p53 and other members of the p53 pathway can have a profound effect on cancer cells by regulating apoptosis and the cell cycle, while playing an important role in chemotherapy-induced sensitization to the TRAIL ligand and agonistic antibodies. For example, antiproliferative effects of doxorubicin and SN-38, the CPT-11 active metabolite, have been related to the association of p21, a p53 target, with DNA leading to the degradation of c-myc and cdc25A and inducing cell cycle arrest.Citation170 However, p53 deficiency or mutations are very common amongst human cancers and are frequently associated with resistance to chemotherapy.Citation171 Full-length p53 homologous protein, p73, contains a transactivation domain similar to p53 and may induce p53 targets, such as p21 and GADD45, resulting in apoptosis.Citation172,Citation173 Expression of certain isoforms of these proteins may compensate for mutated p53, as p73 activation may induce apoptosis and cell cycle arrest in response to DNA damage.Citation172,Citation173 Pharmacological agents that activate the p53 pathway and induce apoptosis in p53 null or mutant tumor cells are currently in development. A small molecule library was screened in cells lacking p53 to show induction of p53 activity, such as increased p21 and DR5 expression. In vivo tumor xenograft models showed a reduction in tumor weight and increased p53 transcriptional activity with three different p53 stimulating small-molecules.Citation171 Weinmann et al.Citation174 identified a “p53 rescue compound P53R3” by screening a compound library for p53 binding. P53R3 was reported to induce some p53 target genes, including the upregulation of DR5 mRNA, protein and surface expression. P53R3 sensitized nine of twelve glioma cells lines to TRAIL-induced apoptosis. With the large number of pro- and anti-apoptotic proteins regulated by p53, these and similar agents may have importance in the reversal of resistance to TRAIL-based therapies and chemotherapy as well.
Autophagy.
Several studies have the shown that TRAIL induces autophagy in certain cell lines.Citation175–Citation178 Autophagy is a cellular process of recycling macromolecules activated by cellular stress that can either result in lysosome-mediated cell death or cytoprotection.Citation175,Citation179 Han et al.Citation175 reported that HCT116 colon cancer cells overexpressing FLIP did not undergo apoptosis upon treatment with TRAIL, but rather an autophagic response with an increase in Beclin-1 and the presence of autophagosomes (double membrane-bound vesicles that fuse with lysosomes and are characteristic of autophagy).Citation180 Knock-down of Beclin-1 and UVRAG (another autophagy related protein) sensitized these cells to TRAIL-induced apoptosis. In the wild-type HCT116 cells, ∼40% of cells did not undergo apoptosis with TRAIL treatment alone, but were sensitized by Beclin-1 knockdown. Similar studies in TRAIL-resistant Bax-/- HCT116 cells, RKO colon cancer cells, cisplatin-resistant MCF7 and etoposide-resistant MDA-MB-231 breast cancer cells, and U251 and LN229 glioma cell lines showed sensitization to TRAIL-induced apoptosis with Beclin-1 siRNA treatment.Citation175,Citation176,Citation181 The cellular switch between apoptosis and autophagy has been related to the activity of caspase-8 and the activation of the mitochondrial apoptotic pathway.Citation175,Citation177 These studies suggest that novel and existing therapeutic agents which induce autophagy may be useful in sensitizing apoptosis-deficient cancer cells to TRAIL-induced apoptosis.Citation182
Therapeutic Potential of TRAIL and Agonistic Death Receptor Antibodies in Combination Therapy
Resistance to chemotherapy or radiation is a common problem for many cancer patients, and some tumor cells are resistant to TRAIL-induced apoptosis. TRAIL or antibodies targeted to TRAIL death receptors have been shown to interact with various chemotherapeutic agents to sensitize cells in an additive to synergistic manner. The mechanisms of sensitization include induction of increased cell surface death receptor expression or increased activation of the intrinsic or extrinsic apoptotic pathways via modulation of apoptotic regulatory proteins. As previously described, many therapeutic agents sensitize cancer cells to TRAIL-induced apoptosis by modulation of the various apoptotic regulatory proteins. Many classes of chemotherapy agents are used for the treatment of cancer () and have been shown to enhance the efficacy of TRAIL and death receptor agonistic antibodies. With such a large variety of drugs sensitizing cancer cells to TRAIL receptor-targeted therapies, further study is needed to determine if sensitization occurs via similar mechanisms for drugs with very different primary mechanisms of action.
Murine in vivo tumor xenograft models have been used to investigate the efficacy of TRAIL and drug or radiation combination treatment on tumor growth inhibition. TRAIL with either 5-FU or CPT-11 produced greater anti-tumor effects than either agent alone against primary human colon cancer samples implanted into SCID mice. TRAIL and CPT-11 combination treatment achieved complete tumor regression in 50% of animals.Citation183 In an orthotopic NCI-H460 lung cancer model, TRAIL combined with paclitaxel and carboplatin significantly inhibited tumor growth and increased 90 day survivial.Citation184 These examples encompass only a small fraction of studies describing the in vivo effects of TRAIL or death receptor agonistic antibodies in combination with chemotherapy in a variety of tumor types.Citation1,Citation63 A recently published review by Ashkenazi and HerbstCitation63 provides a summary of chemotherapy agents used in combination with TRAIL in multiple preclinical in vivo models of human carcinomas.
In addition to chemotherapy, radiation has also been shown to increase the efficacy of TRAIL. Breast, lung, colorectal and head and neck cancer cell lines were treated in vitro with TRAIL plus irradiation resulting in synergistic induction of apoptosis in five of six tumor cell lines and increased DR5 expression in four cell lines.Citation185 Chinnaiyan et al.Citation78 reported a p53-dependent synergistic effect of TRAIL and radiation against breast cancer cell lines and tumor regression of MCF-7 tumor xenografts. Sequential treatment with radiation followed by TRAIL 24 h later synergistically inhibited PC-3 prostate and MCF-7 breast tumor xenograft growth and increased survival in nude mice with caspase-3 activation detected in both models.Citation79,Citation186 Recently, X-irradiation in combination with TRAIL was shown to synergistically inhibit the growth of MKN45 and MKN28 human gastric cancer xenografts. Caspase-3 activation was shown by combination treatment in normoxic and hypoxic regions of the tumors.Citation187 These studies highlight the potential for TRAIL-based therapies in combination with standard therapeutic agents for cancer treatment.
Newer agents currently at various stages of clinical development also show promise for combination treatment with TRAIL and death receptor antibodies, including proteasome inhibitors (bortezomib),Citation125,Citation153,Citation188–Citation191 histone deacetylase inhibitors (HDAC),Citation192–Citation197 heat shock protein 90 (HSP90),Citation198,Citation199 inhibitors and small molecule apoptotic modulators.Citation131,Citation145,Citation200 TRAIL death receptor therapies may have an impact on a variety of cancer types. Beneficial combinations may include already approved drugs and newer agents currently under development.
Disclosure of Potential Conflicts of Interest
Dr. Buchsbaum has intellectual property interests related to the TRA-8 anti-DR5 antibody.
Figures and Tables
Figure 1 TRAIL receptors. Ligand binds to cysteine-rich domains. The death receptors have death domains that provide interactions with intercellular proteins to induce apoptotic signaling. Figure based on Almodovar et al.Citation10
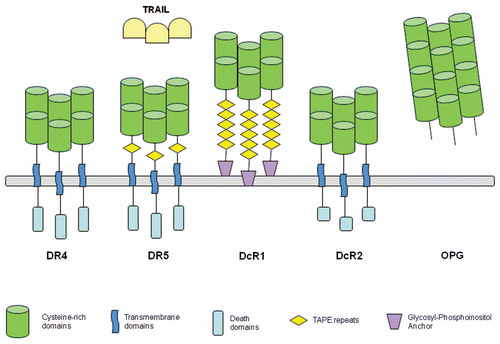
Figure 2 The death receptor induced extrinsic and intrinsic apoptotic pathways. Each pathway begins with caspase-8 activation. Extrinsic pathway proceeds with the direct activation of caspase-3 by activated caspase-8. Intrinsic or mitochondrial pathway activation involves the cleavage of Bid to activate Bcl-2 family members to depolarize the mitochondrial membrane and release cytochrome c and Smac/DIABLO into the cytosol. Once released these molecules interact with Apaf-1 to activate caspase-9, which activates effector caspase-3. In some cells, the extrinsic pathway is reported to be sufficient for TRAIL-induced apoptosis, while in other cells both pathways are activated. Chemotherapy drugs may increase activation of the intrinsic pathway to enhance TRAIL receptor targeted therapies.
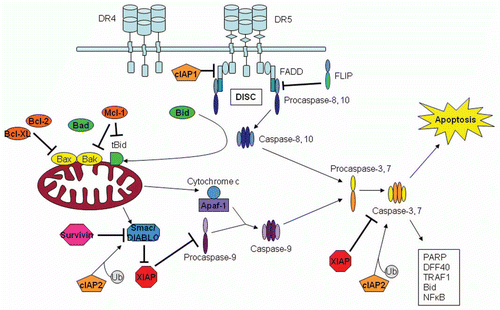
Figure 3 TRA-8 induced cytotoxicity against A549 lung carcinoma cells was enhanced by chemotherapy pretreatment. Cells (1,000 per well) were plated in 96 well plates and incubated at 37°C for 24 h. Then cells were treated with doxorubicin, docetaxel or bortezomib for 24 h prior to the addition of TRA-8. Cell viability was determined via ATPLite assay after 24 h of TRA-8 treatment. ATP levels are reported relative to untreated control cells and represent the mean and SD of quadruplicate samples from three independent experiments.
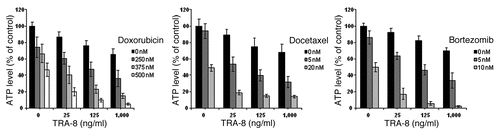
Figure 4 TRA-8 or TRAIL induced cytotoxicity was enhanced by doxorubicin pretreatment. Cells (1,000 per well) were plated in 96 well plates and incubated at 37°C for 24 h. Then cells were treated with DOX for 24 h prior to the addition of TRA-8 or TRAIL. Cell viability was determined via ATPLite assay after 24 h of TRA-8 or TRAIL treatment. ATP levels are reported relative to untreated control cells and represent the mean and SD of quadruplicate samples from three independent experiments.
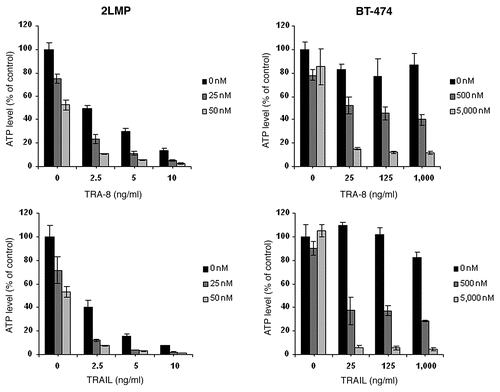
Figure 5 Caspase-3, -8, -9 and PARP cleavage and decreased Bid and XIAP levels were induced by TRA-8 or TRAIL in sensitize cells and with combination treatment in resistant cells. 2LMP and BT-474 cells were pretreated for 24 h with DOX (50 and 5,000 nM, respectively) before the addition of TRA-8 or TRAIL (125 and 1,000 ng/ml; TRA-8, 0.84 and 6.7 nM; TRAIL, 5 and 40 nM) for 3 h. Whole cell lysates were analyzed by western blot analysis using antibodies specific for the identified protein: caspase-3 (Stressgen); caspase-8, PARP (BD PharMingen); caspase-9, Bid (cell signaling) and XIAP (R&D Systems). Actin (Sigma) was used as a loading control.
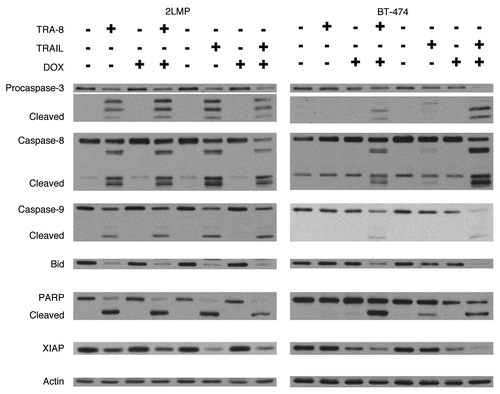
Figure 6 NFκB pathway activation via death receptor signaling. TRAIL has been reported to induce the canonical NFκB signaling pathway, which involves the inactivation of IκB subunits by the IKK complex to release active NFκB homodimers or heterodimers. Generally, these active NFκB dimers translocate to the nucleus and induce gene transcription. NFκB may have different functions depending on the NFκB subunits present and their binding partners. Chen et al.Citation151 reported the p65 subunit to be anti-apoptotic and cRel to be pro-apoptotic.
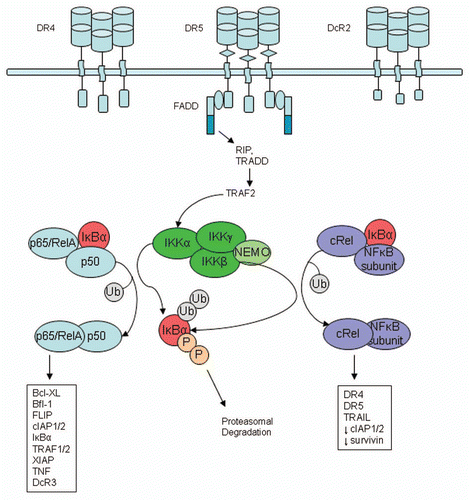
Figure 7 Expression of IκBα or p65 after exposure to TRA-8 and doxorubicin. BT-474 cells were treated with DOX (5 µM for 24 h samples and 3 µM for 48 h samples) and TRA-8 (1,000 ng/ml) prior to western blot analysis with antibodies to IκBα (Cell Signaling) and p65 (Santa Cruz).
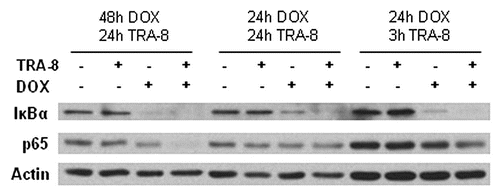
Figure 8 Inhibition of NFκB did not enhance cytotoxicity of TRA-8 against BT-474 breast cancer cells. Cells were pretreated with the NFκB translocation inhibitor SN50 or p65 specific siRNA for 24 h prior to the addition of TRA-8. Cell viability was determined via ATPLite assay after 24 h of TRA-8 treatment.
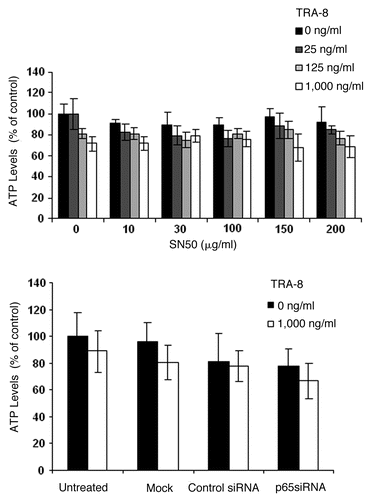
Figure 9 Akt activity in breast cancer cell lines. 2LMP and BT-474 cells were treated with TRA-8 (125 and 1,000 ng/ml) for 3 h following pretreatment with DOX (50 and 5,000 nM) for 24 h. Phospho-Akt (Ser473) and Akt were detected with specific primary antibodies (cell signaling) in whole cell lysates.
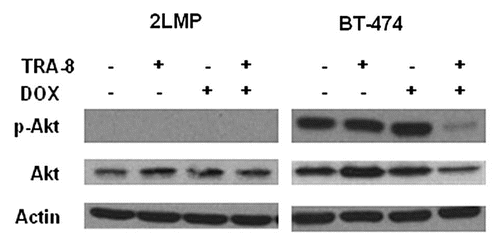
Figure 10 Pretreatment with Akt inhibitors did not enhance cytotoxicity of TRA-8 against BT-474 breast cancer cells. Cells were treated with the PI3K inhibitor, LY294002 or an Akt inhibitor, 1L-6-hydroxymethyl-chiro-inositol 2(R)-2-O-methyl-3-O-octadecylcarbonate, for 24 h prior to the addition of TRA-8. Cell viability was determined via ATPLite assay after 24 h of TRA-8 treatment.
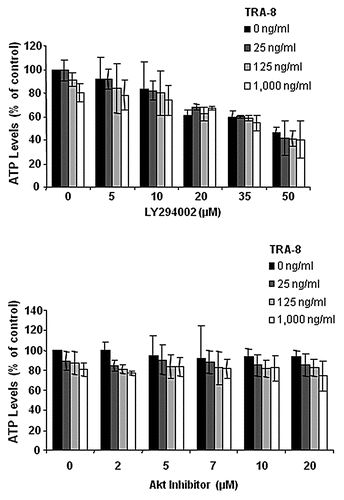
Table 1 Chemotherapeutic agents that interact with TRAIL and mechanisms of sensitization
Table 2 Intracellular proteins and signaling pathways associated with resistance to TRAIL and TRAIL receptor-targeted therapies
Table 3 Bcl-2 proteins
Acknowledgements
This work was supported in part by DOD grant #W81XWH-06-1-0706, DOD grant #BCDAMD17-00-1-0119 and NIH Breast Cancer SPORE 5P50CA089019-08.
References
- Ashkenazi A, Pai R, Fong S, Leung S, Lawrence D, Marsters S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 1999; 104:155 - 162
- Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 1999; 5:157 - 163
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995; 3:673 - 682
- Pitti R, Marsters S, Ruppert S, Donahue C, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem 1996; 271:12687 - 12690
- Bouralexis S, Findlay DM, Evdokiou A. Death to the bad guys: targeting cancer via Apo2L/TRAIL. Apoptosis 2005; 10:35 - 51
- Boehrer S, Nowak D, Hoelzer D, Mitrou P, Chow K. The molecular biology of TRAIL-mediated signaling and its potential therapeutic exploitation in hematopoietic malignancies. Curr Med Chem 2006; 13:2091 - 2100
- Wassenaar T, Quax W, Mark A. The conformation of the extracellular binding domain of Death Receptor 5 in the presence and absence of the activating ligand TRAIL: a molecular dynamics study. Proteins 2008; 70:333 - 343
- Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene 2010; 29:4752 - 4765
- Ashkenazi A, Dixit V. Death receptors: signaling and modulation. Science 1998; 281:1305 - 1308
- Ruiz de Almodóvar C, López-Rivas A, Redondo J, Rodríguez A. Transcriptional regulation of the TRAIL-R3 gene. Vitam Horm 2004; 67:51 - 63
- Pan G, O'Rourke K, Chinnaiyan A, Gentz R, Ebner R, Ni J, et al. The receptor for the cytotoxic ligand TRAIL. Science 1997; 276:111 - 113
- MacFarlane M, Ahmad M, Srinivasula SM, Fernandes-Alnemri T, Cohen GM, Alnemri ES. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J Biol Chem 1997; 272:25417 - 25420
- Sheridan J, Marsters S, Pitti R, Gurney A, Skubatch M, Baldwin D, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 1997; 277:818 - 821
- Schneider P, Bodmer J, Thome M, Hofmann K, Holler N, Tschopp J. Characterization of two receptors for TRAIL. FEBS Lett 1997; 416:329 - 334
- Walczak H, Degli-Esposti M, Johnson R, Smolak P, Waugh J, Boiani N, et al. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J 1997; 16:5386 - 5397
- Pan G, Ni J, Wei Y, Yu G, Gentz R, Dixit V. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 1997; 277:815 - 818
- Degli-Esposti M, Dougall W, Smolak P, Waugh J, Smith C, Goodwin R. The novel receptor TRAIL-R4 induces NFkappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity 1997; 7:813 - 820
- Marsters S, Sheridan J, Pitti R, Huang A, Skubatch M, Baldwin D, et al. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol 1997; 7:1003 - 1006
- Harper N, Farrow S, Kaptein A, Cohen G, MacFarlane M. Modulation of tumor necrosis factor apoptosis-inducing ligand-induced NFkappaB activation by inhibition of apical caspases. J Biol Chem 2001; 276:34743 - 34752
- Cha S, Song Y, Oh B. Specificity of molecular recognition learned from the crystal structures of TRAIL and the TRAIL:sDR5 complex. Vitam Horm 2004; 67:1 - 17
- Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem 1998; 273:14363 - 14367
- Yamaguchi K, Kinosaki M, Goto M, Kobayashi F, Tsuda E, Morinaga T, et al. Characterization of structural domains of human osteoclastogenesis inhibitory factor. J Biol Chem 1998; 273:5117 - 5123
- Truneh A, Sharma S, Silverman C, Khandekar S, Reddy M, Deen K, et al. Temperature-sensitive differential affinity of TRAIL for its receptors. DR5 is the highest affinity receptor. J Biol Chem 2000; 275:23319 - 23325
- Cassatella M, Huber V, Calzetti F, Margotto D, Tamassia N, Peri G, et al. Interferon-activated neutrophils store a TNF-related apoptosis-inducing ligand (TRAIL/Apo-2 ligand) intracellular pool that is readily mobilizable following exposure to proinflammatory mediators. J Leukoc Biol 2006; 79:123 - 132
- Sato K, Hida S, Takayanagi H, Yokochi T, Kayagaki N, Takeda K, et al. Antiviral response by natural killer cells through TRAIL gene induction by IFNalpha/beta. Eur J Immunol 2001; 31:3138 - 3146
- Griffith T, Wiley S, Kubin M, Sedger L, Maliszewski C, Fanger N. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med 1999; 189:1343 - 1354
- Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NFκB pathway. Immunity 1997; 7:821 - 830
- Kuang AA, Diehl GE, Zhang J, Winoto A. FADD is required for DR4- and DR5-mediated apoptosis: Lack of TRAIL-induced apoptosis in FADD-deficient mouse embryonic fibroblasts. J Biol Chem 2000; 275:25065 - 25068
- Kischkel FC, Lawrence DA, Tinel A, LeBlanc H, Virmani A, Schow P, et al. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J Biol Chem 2001; 276:46639 - 46646
- Suliman A, Lam A, Datta R, Srivastava RK. Intracellular mechanisms of TRAIL: apoptosis through mitochondrial-dependent and -independent pathways. Oncogene 2001; 20:2122 - 2133
- Bodmer J, Holler N, Reynard S, Vinciguerra P, Schneider P, Juo P, et al. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell Biol 2000; 2:241 - 243
- Thomas L, Henson A, Reed J, Salsbury F, Thorburn A. Direct binding of Fas-associated death domain (FADD) to the tumor necrosis factor-related apoptosis-inducing ligand receptor DR5 is regulated by the death effector domain of FADD. J Biol Chem 2004; 279:32780 - 32785
- Muzio M, Stockwell B, Stennicke H, Salvesen G, Dixit V. An induced proximity model for caspase-8 activation. J Biol Chem 1998; 273:2926 - 2930
- Hao C, Song J, Vilimanovich U, Kneteman N. Modulation of TRAIL signaling complex. Vitam Horm 2004; 67:81 - 99
- Scorrano L, Korsmeyer S. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun 2003; 304:437 - 444
- Kandasamy K, Srinivasula S, Alnemri E, Thompson C, Korsmeyer S, Bryant J, et al. Involvement of proapoptotic molecules Bax and Bak in tumor necrosis factorrelated apoptosis-inducing ligand (TRAIL)-induced mitochondrial disruption and apoptosis: differential regulation of cytochrome c and Smac/DIABLO release. Cancer Res 2003; 63:1712 - 1721
- Wu X, Ogawa O, Kakehi Y. TRAIL and chemotherapeutic drugs in cancer therapy. Vitam Horm 2004; 67:365 - 383
- Jo M, Kim T, Seol D, Esplen J, Dorko K, Billiar T, et al. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med 2000; 6:564 - 567
- Kelley S, Harris L, Xie D, Deforge L, Totpal K, Bussiere J, et al. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics and safety. J Pharmacol Exp Ther 2001; 299:31 - 38
- Herbst RS, Mendolson DS, Ebbinghaus S, Gordon MS, O'Dwyer P, Lieberman G, et al. A phase I safety and pharmacokinetic (PK) study of recombinant Apo2L/TRAIL, an apoptosis-inducing protein in patients with advanced cancer. J Clin Oncol 2006; 24:124
- Fulda S, Debatin K. Modulation of TRAIL signaling for cancer therapy. Vitam Horm 2004; 67:275 - 290
- Buchsbaum D, Zhou T, Lobuglio A. TRAIL receptor-targeted therapy. Future Oncol 2006; 2:493 - 508
- Yada A, Yazawa M, Ishida S, Yoshida H, Ichikawa K, Kurakata S, et al. A novel humanized anti-human death receptor 5 antibody CS-1008 induces apoptosis in tumor cells without toxicity in hepatocytes. Ann Oncol 2008; 19:1060 - 1067
- Saleh MN, Percent I, Wood TE, Posey J III, Shah J, Carlisle R, et al. A phase I study of CS-1008 (humanized monoclonal antibody targeting death receptor 5 or DR5), administered weekly to patients with advanced solid tumors or lymphomas. J Clin Oncol 2008; 26:3537
- Wiezorek J, holland P, Graes J. death receptor agonists as a targeted therapy for cancer. Clin Cancer Res 2010; 16:1701 - 1708
- LoRusso P, Hong D, Heath E, Kurzrock R, Wang D, Hsu M, et al. First-in-human study of AMG 655, a pro-apoptotic TRAIL receptor-2 agonist, in adult patients with advanced solid tumors. J Clin Oncol 2007; 25:3534
- Buchsbaum D, Zhou T, Grizzle W, Oliver P, Hammond C, Zhang S, et al. Antitumor efficacy of TRA-8 anti-DR5 monoclonal antibody alone or in combination with chemotherapy and/or radiation therapy in a human breast cancer model. Clin Cancer Res 2003; 9:3731 - 3741
- Amm HM, Oliver PG, Buchsbaum DJ. XIAP siRNA or chemotherapy increase apoptosis of breast cancer cells treated with TRA-8 anti-DR5 antibody. AACR Annual Meeting 2006; Washington, DC Proc Am Assoc Cancer Res 41
- Straughn J Jr, Oliver PG, Zhou T, Wang W, Alvarez RD, Grizzle WE, et al. Anti-tumor activity of TRA-8 anti-death receptor 5 (DR5) monoclonal antibody in combination with chemotherapy and radiation therapy in a cervical cancer model. Gynecol Oncol 2006; 101:46 - 54
- Estes J, Oliver P, Straughn JJ, Zhou T, Wang W, Grizzle W, et al. Efficacy of anti-death receptor 5 (DR5) antibody (TRA-8) against primary human ovarian carcinoma using a novel ex vivo tissue slice model. Gynecol Oncol 2007; 105:291 - 298
- DeRosier L, Buchsbaum D, Oliver P, Huang Z, Sellers J, Grizzle W, et al. Combination treatment with TRA-8 anti death receptor 5 antibody and CPT-11 induces tumor regression in an orthotopic model of pancreatic cancer. Clin Cancer Res 2007; 13:5535 - 5543
- Derosier L, Vickers S, Zinn K, Huang Z, Wang W, Grizzle W, et al. TRA-8 anti-DR5 monoclonal antibody and gemcitabine induce apoptosis and inhibit radiologically validated orthotopic pancreatic tumor growth. Mol Cancer Ther 2007; 6:3198 - 3207
- Fiveash JB, Gillespie GY, Oliver PG, Zhou T, Belenky ML, Buchsbaum DJ. Enhancement of glioma radiation therapy and chemotherapy response with targeted antibody therapy against death receptor 5. Int J Radiat Oncol Biol Phys 2008; 71:507 - 516
- Oliver P, LoBuglio A, Zinn K, Kim H, Nan L, Zhou T, et al. Treatment of human colon cancer xenografts with TRA-8 anti-death receptor 5 antibody alone or in combination with CPT-11. Clin Cancer Res 2008; 14:2180 - 2189
- Jin H, Yang R, Ross J, Fong S, Carano R, Totpal K, et al. Cooperation of the agonistic DR5 antibody apomab with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Clin Cancer Res 2008; 14:7733 - 7740
- Pukac L, Kanakaraj P, Humphreys R, Alderson R, Bloom M, Sung C, et al. HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody, induces cell death in multiple tumor types in vitro and in vivo. Br J Cancer 2005; 92:1430 - 1441
- Marini P, Denzinger S, Schiller D, Kauder S, Welz S, Humphreys R, et al. Combined treatment of colorectal tumors with agonistic TRAIL receptor antibodies HGS-ETR1 and HGS-ETR2 and radiotherapy: enhanced effects in vitro and dose-dependent growth delay in vivo. Oncogene 2006; 25:5145 - 5154
- Georgakis G, Li Y, Humphreys R, Andreeff M, O'Brien S, Younes M, et al. Activity of selective fully human agonistic antibodies to the TRAIL death receptors TRAIL-R1 and TRAIL-R2 in primary and cultured lymphoma cells: induction of apoptosis and enhancement of doxorubicin- and bortezomib-induced cell death. Br J Haematol 2005; 130:501 - 510
- Plummer R, Attard G, Pacey S, Li L, Razak A, Perrett R, et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res 2007; 13:6187 - 6194
- Tolcher A, Mita M, Meropol N, von Mehren M, Patnaik A, Padavic K, et al. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol 2007; 25:1390 - 1395
- Greco F, Bonomi P, Crawford J, Kelly K, Oh Y, Halpern W, et al. Phase 2 study of mapatumumab, a fully human agonistic monoclonal antibody which targets and activates the TRAIL receptor-1, in patients with advanced non-small cell lung cancer. Lung Cancer 2008; 61:82 - 90
- Von Pawel J, Harvey JH, Spigel DR, Dediu M, Reck M, Cebotaru CL, et al. A randomized phase II trial of mapatumumab, a TRAIL-R1 agonist monoclonal antibody, in combination with carboplatin and paclitaxel in patients with advanced NSCLC. 46th ASCO Annual Meeting 2010; Chicago, IL J Clin Oncol 18
- Ashkenazi A, Herbst R. To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest 2008; 118:1979 - 1990
- Yee L, Fanale M, Dimick K, Calvert S, Robins C, Ing J, et al. A phase Ib safety and pharmacokinetic (PK) study of recombinant human Apo2L/TRAIL in combination with rituximab in patients with low-grade non-Hodgkin lymphoma. 43rd ASCO Annual Meeting: J Clin Oncol 2007; 8078
- Oldenhuis C, Mom C, Sleijfer S, Gietema JA, Fox NL, Corey A, et al. A phase I study with the agonstic TRAIL-R1 antibody, mapatumumab, in combination with gemcitabine and cisplatin. 44th ASCO Annual Meeting 2008; Chicago, IL J Clin Oncol 3540
- Sikic BI, Wakelee HA, Mehren Mv, Lewis N, Calvert AH, Plummer ER, et al. A phase Ib study to assess the safety of lexatumumab, a human monoclonal antibody that activates TRAIL-R2, in combination with gemcitabine, pemetrexed, doxorubicin or FOLFIRI. J Clin Oncol 2007; 25:14006
- Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther 2005; 12:228 - 237
- Keane MM, Ettenberg SA, Nau MM, Russell EK, Lipkowitz S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res 1999; 59:734 - 741
- Jang Y, Park K, Chung H, Kim H. Analysis of the phenotypes of Jurkat clones with different TRAIL-sensitivities. Cancer Lett 2003; 194:107 - 117
- Ozawa F, Friess H, Kleeff J, Xu Z, Zimmermann A, Sheikh M, et al. Effects and expression of TRAIL and its apoptosis-promoting receptors in human pancreatic cancer. Cancer Lett 2001; 163:71 - 81
- Mori T, Doi R, Toyoda E, Koizumi M, Ito D, Kami K, et al. Regulation of the resistance to TRAIL-induced apoptosis as a new strategy for pancreatic cancer. Surgery 2005; 138:71 - 77
- Yamanaka T, Shiraki K, Sugimoto K, Ito T, Fujikawa K, Ito M, et al. Chemotherapeutic agents augment TRAIL-induced apoptosis in human hepatocellular carcinoma cell lines. Hepatology 2000; 32:482 - 490
- Wu X, Kakehi Y, Mizutani Y, Nishiyama H, Kamoto T, Megumi Y, et al. Enhancement of TRAIL/Apo2L-mediated apoptosis by adriamycin through inducing DR4 and DR5 in renal cell carcinoma cells. Int J Cancer 2003; 104:409 - 417
- Gibson SB, Oyer R, Spalding AC, Anderson SM, Johnson GL. Increased expression of death receptors 4 and 5 synergizes the apoptosis response to combined treatment with etoposide and TRAIL. Mol Cell Biol 2000; 20:205 - 212
- Wu G, Burns T, McDonald Er, Jiang W, Meng R, Krantz I, et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet 1997; 17:141 - 143
- Wang S, El-Deiry W. Requirement of p53 targets in chemosensitization of colonic carcinoma to death ligand therapy. Proc Natl Acad Sci USA 2003; 100:15095 - 15100
- Wagner K, King F, Nomoto K, Knee D, Hampton G, Nasoff M, et al. Activation and suppression of the TRAIL death-receptor pathway in chemotherapy sensitive and resistant follicular lymphoma cells. Cancer Biol Ther 2003; 2:534 - 540
- Chinnaiyan AM, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL, et al. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci USA 2000; 97:1754 - 1759
- Shankar S, Singh T, Chen X, Thakkar H, Firnin J, Srivastava R. The sequential treatment with ionizing radiation followed by TRAIL/Apo-2L reduces tumor growth and induces apoptosis of breast tumor xenografts in nude mice. Int J Oncol 2004; 24:1133 - 1140
- Takimoto R, El-Deiry WS. Wild-type p53 transactivates the KILLER/DR5 gene through an intronic sequence-specific DNA-binding site. Oncogene 2000; 19:1735 - 1743
- Liu X, Yue P, Khuri FR, Sun SY. p53 upregulates death receptor 4 expression through an intronic p53 binding site. Cancer Res 2004; 64:5078 - 5083
- Yoshida T, Maeda A, Tani N, Sakai T. Promoter structure and transcription initiation sites of the human death receptor 5/TRAIL-R2 gene. FEBS Lett 2001; 507:381 - 385
- Mendoza FJ, Ishdorj G, Hu X, Gibson SB. Death receptor-4 (DR4) expression is regulated by transcription factor NFkappaB in response to etoposide treatment. Apoptosis 2008; 13:756 - 770
- Ravi R, Bedi G, Engstrom L, Zeng Q, Mookerjee B, Gélinas C, et al. Regulation of death receptor expression and TRAIL/Apo2L-induced apoptosis by NFkappaB. Nat Cell Biol 2001; 3:409 - 416
- Baritaki S, Huerta-Yepez S, Sakai T, Spandidos DA, Bonavida B. Chemotherapeutic drugs sensitize cancer cells to TRAIL-mediated apoptosis: upregulation of DR5 and inhibition of Yin Yang 1. Mol Cancer Ther 2007; 6:1387 - 1399
- Baritaki S, Suzuki E, Umezawa K, Spandidos DA, Berenson J, Daniels TR, et al. Inhibition of Yin Yang 1-dependent repressor activity of DR5 transcription and expression by the novel proteasome inhibitor NPI-0052 contributes to its TRAIL-enhanced apoptosis in cancer cells. J Immunol 2008; 180:6199 - 6210
- Huerta-Yepez S, Vega M, Escoto-Chavez SE, Murdock B, Sakai T, Baritaki S, et al. Nitric oxide sensitizes tumor cells to TRAIL-induced apoptosis via inhibition of the DR5 transcription repressor Yin Yang 1. Nitric Oxide 2009; 20:39 - 52
- Yoshida T, Sakai T. Promoter of TRAIL-R2 gene. Vitam Horm 2004; 67:35 - 49
- Kim YH, Park JW, Lee JY, Kwon TK. Sodium butyrate sensitizes TRAIL-mediated apoptosis by induction of transcription from the DR5 gene promoter through Sp1 sites in colon cancer cells. Carcinogenesis 2004; 25:1813 - 1820
- VanOosten R, Moore J, Karacay B, Griffith T. Histone deacetylase inhibitors modulate renal cell carcinoma sensitivity to TRAIL/Apo-2L-induced apoptosis by enhancing TRAIL-R2 expression. Cancer Biol Ther 2005; 4:1104 - 1112
- Dumitru CA, Carpinteiro A, Trarbach T, Hengge UR, Gulbins E. Doxorubicin enhances TRAIL-induced cell death via ceramide-enriched membrane platforms. Apoptosis 2007; 12:1533 - 1541
- Kohlhaas SL, Craxton A, Sun XM, Pinkoski MJ, Cohen GM. Receptor-mediated endocytosis is not required for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. J Biol Chem 2007; 282:12831 - 12841
- Wagner K, Punnoose E, Januario T, Lawrence D, Pitti R, Lancaster K, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med 2007; 13:1070 - 1077
- Kelley RF, Totpal K, Lindstrom SH, Mathieu M, Billeci K, Deforge L, et al. Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR) 5 than DR4 to apoptosis signaling. J Biol Chem 2005; 280:2205 - 2212
- Nagane M, Shimizu S, Mori E, Kataoka S, Shiokawa Y. Predominant antitumor effects by fully human anti-TRAIL-receptor2 (DR5) monoclonal antibodies in human glioma cells in vitro and in vivo. Neuro Oncol 2010; 12:687 - 700
- Stadel D, Mohr A, Ref C, Macfarlane M, Zhou S, Humphreys R, et al. TRAIL-induced apoptosis is preferentially mediated via TRAIL receptor 1 in pancreatic carcinoma cells and profoundly enhanced by XIAP inhibitors. Clin Cancer Res 2010; 16:5734 - 5749
- MacFarlane M, Inoue S, Kohlhaas SL, Majid A, Harper N, Kennedy DB, et al. Chronic lymphocytic leukemic cells exhibit apoptotic signaling via TRAIL-R1. Cell Death Differ 2005; 12:773 - 782
- Lemke J, Noack A, Adam D, Tchikov V, Bertsch U, Röder C, et al. TRAIL signaling is mediated by DR4 in pancreatic tumor cells despite the expression of functional DR5. J Mol Med 2010; 88:729 - 740
- Roth W, Reed J. FLIP protein and TRAIL-induced apoptosis. Vitam Horm 2004; 67:189 - 206
- Ryu B, Lee M, Chi S, Kim Y, Park J. Increased expression of cFLIP(L) in colonic adenocarcinoma. J Pathol 2001; 194:15 - 19
- Griffith T, Chin W, Jackson G, Lynch D, Kubin M. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol 1998; 161:2833 - 2840
- El-Zawahry A, McKillop J, Voelkel-Johnson C. Doxorubicin increases the effectiveness of Apo2L/TRAIL for tumor growth inhibition of prostate cancer xenografts. BMC Cancer 2005; 5:2
- Kim Y, Suh N, Sporn M, Reed J. An inducible pathway for degradation of FLIP protein sensitizes tumor cells to TRAIL-induced apoptosis. J Biol Chem 2002; 277:22320 - 22329
- Suh W, Kim Y, Schimmer A, Kitada S, Minden M, Andreeff M, et al. Synthetic triterpenoids activate a pathway for apoptosis in AML cells involving downregulation of FLIP and sensitization to TRAIL. Leukemia 2003; 17:2122 - 2129
- Hyer M, Croxton R, Krajewska M, Krajewski S, Kress C, Lu M, et al. Synthetic triterpenoids cooperate with tumor necrosis factor-related apoptosis-inducing ligand to induce apoptosis of breast cancer cells. Cancer Res 2005; 65:4799 - 4808
- Mitsiades N, Mitsiades C, Poulaki V, Anderson K, Treon S. Intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human multiple myeloma cells. Blood 2002; 99:2162 - 2171
- Zhang X, Franco A, Myers K, Gray C, Nguyen T, Hersey P. Relation of TNF-related apoptosis-inducing ligand (TRAIL) receptor and FLICE-inhibitory protein expression to TRAIL-induced apoptosis of melanoma. Cancer Res 1999; 59:2747 - 2753
- Frese S, Brunner T, Gugger M, Uduehi A, Schmid RA. Enhancement of Apo2L/TRAIL (tumor necrosis factor-related apoptosis-inducing ligand)-induced apoptosis in non-small cell lung cancer cell lines by chemotherapeutic agents without correlation to the expression level of cellular protease caspase-8 inhibitory protein. J Thorac Cardiovasc Surg 2002; 123:168 - 174
- Hao C, Beguinot F, Condorelli G, Trencia A, Van Meir E, Yong V, et al. Induction and intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apotosis in human malignant glioma cells. Cancer Res 2001; 61:1162 - 1170
- Cory S, Adams J. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2002; 2:647 - 656
- Walensky L. BCL-2 in the crosshairs: tipping the balance of life and death. Cell Death Differ 2006; 13:1339 - 1350
- Fulda S, Meyer E, Debatin K. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene 2002; 21:2283 - 2294
- Sun S, Yue P, Zhou J, Wang Y, Choi Kim H, Lotan R, et al. Overexpression of BCL2 blocks TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in human lung cancer cells. Biochem Biophys Res Commun 2001; 280:788 - 797
- Sinicrope FA, Penington RC, Tang XM. Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis is inhibited by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA 14-1, in human colon cancer cells. Clin Cancer Res 2004; 10:8284 - 8292
- Hinz S, Trauzold A, Boenicke L, Sandberg C, Beckmann S, Bayer E, et al. Bcl-XL protects pancreatic adenocarcinoma cells against CD95- and TRAIL-receptor-mediated a poptosis. Oncogene 2000; 19:5477 - 5486
- Han J, Goldstein L, Gastman B, Rabinowich H. Interrelated roles for Mcl-1 and BIM in regulation of TRAIL-mediated mitochondrial apoptosis. J Biol Chem 2006; 281:10153 - 10163
- Clohessy J, Zhuang J, de Boer J, Gil-Gómez G, Brady H. Mcl-1 interacts with truncated Bid and inhibits its induction of cytochrome c release and its role in receptor-mediated apoptosis. J Biol Chem 2006; 281:5750 - 5759
- Taniai M, Grambihler A, Higuchi H, Werneburg N, Bronk S, Farrugia D, et al. Mcl-1 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. Cancer Res 2004; 64:3517 - 3524
- Mott J, Kobayashi S, Bronk S, Gores G. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene 2007; 13:6133 - 6140
- LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, et al. Tumor-cell resistance to death receptor-induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med 2002; 8:274 - 281
- von Haefen C, Gillissen B, Hemmati PG, Wendt J, Guner D, Mrozek A, et al. Multidomain Bcl-2 homolog Bax but not Bak mediates synergistic induction of apoptosis by TRAIL and 5-FU through the mitochondrial apoptosis pathway. Oncogene 2004; 23:8320 - 8332
- Wendt J, von Haefen C, Hemmati P, Belka C, Dörken B, Daniel PT. TRAIL sensitizes for ionizing irradiation-induced apoptosis through an entirely Bax-dependent mitochondrial cell death pathway. Oncogene 2005; 24:4052 - 4064
- Han J, Goldstein L, Gastman B, Rabinovitz A, Wang G, Fang B, et al. Differential involvement of Bax and Bak in TRAIL-mediated apoptosis of leukemic T cells. Leukemia 2004; 18:1671 - 1680
- Ray S, Almasan A. Apoptosis induction in prostate cancer cells and xenografts by combined treatment with Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand and CPT-11. Cancer Res 2003; 63:4713 - 4723
- Yin D, Zhou H, Kumagai T, Liu g, Ong J, Black K, et al. Proteasome inhibitors PS-341 causes cell growth arrest and apoptosis in human gliobastoma multiforme (GBM). Oncogene 2005; 24:344 - 354
- Zhu H, Guo W, Zhang L, Wu S, Teraishi F, Davis J, et al. Proteasome inhibitors-mediated TRAIL resensitization and Bik accumulation. Cancer Biol Ther 2005; 4:781 - 786
- Hao J, Yu M, Liu F, Newland A, Jia L. Bcl-2 inhibitors sensitize tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by uncoupling of mitochondrial respiration in human leukemic CEM cells. Cancer Res 2004; 64:3607 - 3616
- Chawla-Sarkar M, Bae S, Reu F, Jacobs B, Lindner D, Borden E. Downregulation of Bcl-2, FLIP or IAPs (XIAP and survivin) by siRNAs sensitizes resistant melanoma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ 2004; 11:915 - 923
- Yeow WS, Baras A, Chua A, Nguyen DM, Sehgal SS, Schrump DS, et al. Gossypol, a phytochemical with BH3-mimetic property, sensitizes cultured thoracic cancer cells to Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. J Thorac Cardiovasc Surg 2006; 132:1356 - 1362
- Song J, Kandasamy K, Kraft A. ABT-737 induces expression of the death receptor 5 and sensitizes human cancer cells to TRAIL-induced apoptosis. J Biol Chem 2008; 283:25003 - 25013
- Huang S, Sinicrope F. BH3 mimetic ABT-737 potentiates TRAIL-mediated apoptotic signaling by unsequestering Bim and Bak in human pancreatic cancer cells. Cancer Res 2008; 68:2944 - 2951
- Vaux D, Silke J. IAPs, RINGs and ubiquitylation. Nat Rev Mol Cell Biol 2005; 6:287 - 297
- Deveraux QL, Reed JC. IAP family proteins—suppressors of apoptosis. Genes Dev 1999; 13:239 - 252
- Eckelman B, Salvesen G, Scott F. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep 2006; 7:988 - 994
- Amantana A, London C, Iversen P, Devi G. X-linked inhibitor of apoptosis protein inhibition induces apoptosis and enhances chemotherapy sensitivity in human prostate cancer cells. Mol Cancer Ther 2004; 3:699 - 707
- Cummins J, Kohli M, Rago C, Kinzler K, Vogelstein B, Bunz F. X-linked inhibitor of apoptosis protein (XIAP) is a nonredundant modulator of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human cancer cells. Cancer Res 2004; 64:3006 - 3008
- Li H, Niederkorn J, Neelam S, Alizadeh H. Resistance and susceptibility of human uveal melanoma cells to TRAIL-induced apoptosis. Arch Ophthalmol 2005; 123:654 - 661
- Ng C, Zisman A, Bonavida B. Synergy is achieved by complementation with Apo2L/TRAIL and actinomycin D in Apo2L/TRAIL-mediated apoptosis of prostate cancer cells: role of XIAP in resistance. Prostate 2002; 53:286 - 299
- Roa W, Chen H, Fulton D, Gulavita S, Shaw A, Th'ng J, et al. X-linked inhibitor regulating TRAIL-induced apoptosis in chemoresistant human primary glioblastoma cells. Clin Invest Med 2003; 26:231 - 242
- Yang L, Cao Z, Yan H, Wood W. Coexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: implication for cancer specific therapy. Cancer Res 2003; 63:6815 - 6824
- Aggarwal B, Bhardwaj U, Takada Y. Regulation of TRAIL-induced apoptosis by ectopic expression of antiapoptotic factors. Vitam Horm 2004; 67:453 - 483
- Rosato R, Dai Y, Almenara J, Maggio S, Grant S. Potent antileukemic interactions between flavopiridol and TRAIL/Apo2L involve flavopiridol-mediated XIAP downregulation. Leukemia 2004; 18:1780 - 1788
- Vogler M, Walczak H, Stadel D, Haas T, Genze F, Jovanovic M, et al. Targeting XIAP bypasses Bcl-2-mediated resistance to TRAIL and cooperates with TRAIL to suppress pancreatic cancer growth in vitro and in vivo. Cancer Res 2008; 68:7956 - 7965
- Petrucci E, Pasquini L, Petronelli A, Saulle E, Mariani G, Riccioni R, et al. A small molecule Smac mimic potentiates TRAIL-mediated cell death of ovarian cancer cells. Gynecol Oncol 2007; 105:481 - 492
- Foster F, Owens T, Tanianis-Hughes J, Clarke R, Brennan K, Bundred N, et al. Targeting inhibitor of apoptosis proteins in combination with ErbB antagonists in breast cancer. Breast Cancer Res 2009; 11:41
- Altieri D. Molecular circuits of apoptosis regulation and cell division control: the survivin paradigm. J Cell Biochem 2004; 92:656 - 663
- Griffith T, Kemp T. The topoisomerase I inhibitor topotecan increases the sensitivity of prostate tumor cells to TRAIL/Apo-2L-induced apoptosis. Cancer Chemother Pharmacol 2003; 52:175 - 184
- Lu M, Kwan T, Yu C, Chen F, Freedman B, Schafer J, et al. Peroxisome proliferator-activated receptor gamma agonists promote TRAIL-induced apoptosis by reducing survivin levels via cyclin D3 repression and cell cycle arrest. J Biol Chem 2005; 280:6742 - 6751
- Wajant H. TRAIL and NFkappaB signaling—a complex relationship. Vitam Horm 2004; 67:101 - 132
- Hu W, Johnson H, Shu H. Tumor necrosis factor-related apoptosis-inducing ligand receptors signal NFkappaB and JNK activation and apoptosis through distinct pathways. J Biol Chem 1999; 274:30603 - 30610
- Chen X, Kandasamy K, Srivastava R. Differential roles of RelA (p65) and c-Rel subunits of nuclear factor kappaB in tumor necrosis factor-related apoptosis-inducing ligand signaling. Cancer Res 2003; 63:1059 - 1066
- Kim Y, Schwabe R, Qian T, Lemasters J, Brenner D. TRAIL-mediated apoptosis requires NFkappaB inhibition and the mitochondrial permeability transition in human hepatoma cells. Hepatology 2002; 36:1498 - 1508
- Mitsiades C, Treon S, Mitsiades N, Shima Y, Richardson P, Schlossman R, et al. TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug resistance in multiple myeloma: therapeutic applications. Blood 2001; 98:795 - 804
- Bai J, Sui J, Demirjian A, Vollmer C Jr, Marasco W, Callery MP. Predominant Bcl-XL knockdown disables antiapoptotic mechanisms: tumor necrosis factor-related apoptosis-inducing ligand-based triple chemotherapy overcomes chemoresistance in pancreatic cancer cells in vitro. Cancer Res 2005; 65:2344 - 2352
- Shigeno M, Nakao K, Ichikawa T, Suzuki K, Kawakami A, Abiru S, et al. Interferon-alpha sensitizes human hepatoma cells to TRAIL-induced apoptosis through DR5 upregulation and NFkappaB inactivation. Oncogene 2003; 22:1653 - 1662
- Deeb D, Jiang H, Gao X, Hafner M, Wong H, Divine G, et al. Curcumin sensitizes prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factorkappaB through suppression of IkappaBalpha phosphorylation. Mol Cancer Ther 2004; 3:803 - 812
- Whang Y, Yuan X, Liu Y, Majumder S, Lewis T. Regulation of sensitivity to TRAIL by the PTEN tumor suppressor. Vitam Horm 2004; 67:409 - 426
- Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis 2004; 9:667 - 676
- Chen X, Thakkar H, Tyan F, Gim S, Robinson H, Lee C, et al. Constitutively active Akt is an important regulator of TRAIL sensitivity in prostate cancer. Oncogene 2001; 20:6073 - 6083
- Nesterov A, Lu X, Johnson M, Miller GJ, Ivashchenko Y, Kraft AS. Elevated Akt activity protects the prostate cancer cell line LNCaP from TRAIL-induced apoptosis. J Biol Chem 2001; 276:10767 - 10774
- Yuan X, Whang Y. PTEN sensitizes prostate cancer cells to death receptor-mediated and drug-induced apoptosis through a FADD-dependent pathway. Oncogene 2002; 21:319 - 327
- Nam S, Jung G, Hur G, Chung H, Kim W, Seol D, et al. Upregulation of FLIP(S) by Akt, a possible inhibition mechanism of TRAIL-induced apoptosis in human gastric cancers. Cancer Sci 2003; 94:1066 - 1073
- Bortul R, Tazzari P, Cappellini A, Tabellini G, Billi A, Bareggi R, et al. Constitutively active Akt1 protects HL60 leukemia cells from TRAIL-induced apoptosis through a mechanism involving NFkappaB activation and cFLIP(L) upregulation. Leukemia 2003; 17:379 - 389
- Kandasamy K, Srivastava RK. Role of the phosphatidylinositol 3′-kinase/PTEN/Akt kinase pathway in tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in non-small cell lung cancer cells. Cancer Res 2002; 62:4929 - 4937
- Asakuma J, Sumitomo M, Asano T, Asano T, Hayakawa M. Selective Akt inactivation and tumor necrosis actor-related apoptosis-inducing ligand sensitization of renal cancer cells by low concentrations of paclitaxel. Cancer Res 2003; 63:1365 - 1370
- Mitsiades C, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D, et al. Activation of NFkappaB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene 2002; 21:5673 - 5683
- Poulaki V, Mitsiades C, Kotoula V, Tseleni-Balafouta S, Ashkenazi A, Koutras D, et al. Regulation of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in thyroid carcinoma cells. Am J Pathol 2002; 161:643 - 654
- Gibson E, Henson E, Haney N, Villanueva J, Gibson S. Epidermal growth factor protects epithelial-derived cells from tumor necrosis factor-related apoptosisinducing ligand-induced apoptosis by inhibiting cytochrome c release. Cancer Res 2002; 62:488 - 496
- Shrader M, Pino MS, Lashinger L, Bar-Eli M, Adam L, Dinney CP, et al. Gefitinib reverses TRAIL resistance in human bladder cancer cell lines via inhibition of AKT-mediated X-linked inhibitor of apoptosis protein expression. Cancer Res 2007; 67:1430 - 1435
- Vigneron A, Cherier J, Barré B, Gamelin E, Coqueret O. The cell cycle inhibitor p21waf1 binds to the myc and cdc25A promoters upon DNA damage and induces transcriptional repression. J Biol Chem 2006; 281:34742 - 34750
- Wang W, Kim S, El-Deiry W. Small-molecule modulators of p53 family signaling and antitumor effects in p53-deficient human colon tumor xenografts. Proc Natl Acad Sci USA 2006; 103:11003 - 11008
- Isakoff SJ, Leong C, Vidnovic N, DeYoung MP, Sgroim D, Goss PE, et al. p63/p73 expression mediates cisplatin sensitivity in a subset of triple-negative primary breast cancer: Implications for a new clinical trial. J Clin Oncol 2007; 25:10522
- Vayssade M, Haddada H, Faridoni-Laurens L, Tourpin S, Valent A, Bénard J, et al. p73 functionally replaces p53 in Adriamycin-treated, p53-deficient breast cancer cells. Int J Cancer 2005; 116:860 - 869
- Weinmann L, Wischhusen J, Demma M, Naumann U, Roth P, Dasmahapatra B, et al. A novel p53 rescue compound induces p53-dependent growth arrest and sensitises glioma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ 2008; 15:718 - 729
- Han J, Hou W, Goldstein LA, Lu C, Stolz DB, Yin XM, et al. Involvement of protective autophagy in TRAIL resistance of apoptosis-defective tumor cells. J Biol Chem 2008; 283:19665 - 19677
- Hou W, Han J, Lu C, Goldstein LA, Rabinowich H. Enhancement of tumor-TRAIL susceptibility by modulation of autophagy. Autophagy 2008; 4:940 - 943
- Hou W, Han J, Lu C, Goldstein LA, Rabinowich H. Autophagic degradation of active caspase-8: a crosstalk mechanism between autophagy and apoptosis. Autophagy 2010; 6:891 - 900
- Mills KR, Reginato M, Debnath J, Queenan B, Brugge JS. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is required for induction of autophagy during lumen formation in vitro. Proc Natl Acad Sci USA 2004; 101:3438 - 3443
- Dalby KN, Tekedereli I, Lopez-Berestein G, Ozpolat B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy 2010; 6:322 - 329
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol 2010; 221:3 - 12
- Niu TK, Cheng Y, Ren X, Yang JM. Interaction of Beclin 1 with survivin regulates sensitivity of human glioma cells to TRAIL-induced apoptosis. FEBS Lett 2010; 584:3519 - 3524
- Turcotte S, Giaccia AJ. Targeting cancer cells through autophagy for anticancer therapy. Curr Opin Cell Biol 2010; 22:246 - 251
- Naka T, Sugamura K, Hylander B, Widmer M, Rustum Y, Repasky E. Effects of tumor necrosis factor-related apoptosis-inducing ligand alone and in combination with chemotherapeutic agents on patients' colon tumors grown in SCID mice. Cancer Res 2002; 62:5800 - 5806
- Jin H, Yang R, Fong S, Totpal K, Lawrence D, Zheng Z, et al. Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand cooperates with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Cancer Res 2004; 64:4900 - 4905
- Marini P, Schmid A, Jendrossek V, Faltin H, Daniel PT, Budach W, et al. Irradiation specifically sensitises solid tumor cell lines to TRAIL mediated apoptosis. BMC Cancer 2005; 5:5
- Shankar S, Singh T, Srivastava R. Ionizing radiation enhances the therapeutic potential of TRAIL in prostate cancer in vitro and in vivo: Intracellular mechanisms. Prostate 2004; 61:35 - 49
- Takahashi M, Yasui H, Ogura A, Asanuma T, Kubota N, Tsujitani M, et al. X irradiation combined with TNF alpha-related apoptosis-inducing ligand (TRAIL) reduces hypoxic regions of human gastric adenocarcinoma xenografts in SCID mice. J Radiat Res (Tokyo) 2008; 49:153 - 161
- Shanker A, Brooks AD, Tristan CA, Wine JW, Elliott PJ, Yagita H, et al. Treating metastatic solid tumors with bortezomib and a tumor necrosis factor-related apoptosis-inducing ligand receptor agonist antibody. J Natl Cancer Inst 2008; 100:649 - 662
- Luster T, Carrell J, McCormick K, Sun D, Humphreys R. Mapatumumab and lexatumumab induce apoptosis in TRAIL-R1 and TRAIL-R2 antibody-resistant NSCLC cell lines when treated in combination with bortezomib. Mol Cancer Ther 2009; 8:292 - 302
- Liu X, Yue P, Chen S, Hu L, Lonial S, Khuri F, et al. The proteasome inhibitor PS-341 (bortezomib) upregulates DR5 expression leading to induction of apoptosis and enhancement of TRAIL-induced apoptosis despite upregulation of c-FLIP and survivin expression in human NSCLC cells. Cancer Res 2007; 67:4981 - 4988
- Chen K, Yeh P, Hsu C, Hsu C, Lu Y, Hsieh H, et al. Bortezomib overcomes tumor necrosis factor-related apoptosis-inducing ligand resistance in hepatocellular carcinoma cells in part through the inhibition of the phosphatidylinositol-3-kinase/Akt pathway. J Biol Chem 2009; 284:11121 - 11133
- Fulda S. Modulation of TRAIL-induced apoptosis by HDAC inhibitors. Curr Cancer Drug Targets 2008; 8:132 - 140
- Gillespie S, Borrow J, Zhang X, Hersey P. Bim plays a crucial role in synergistic induction of apoptosis by the histone deacetylase inhibitor SBHA and TRAIL in melanoma cells. Apoptosis 2006; 11:2251 - 2265
- Pathil A, Armeanu S, Venturelli S, Mascagni P, Weiss T, Gregor M, et al. HDAC inhibitor treatment of hepatoma cells induces both TRAIL-independent apoptosis and restoration of sensitivity to TRAIL. Hepatology 2006; 43:425 - 434
- Shankar S, Singh T, Fandy T, Luetrakul T, Ross D, Srivastava R. Interactive effects of histone deacetylase inhibitors and TRAIL on apoptosis in human leukemia cells: involvement of both death receptor and mitochondrial pathways. Int J Mol Med 2005; 16:1125 - 1138
- Vanoosten RL, Moore JM, Ludwig AT, Griffith TS. Depsipeptide (FR901228) enhances the cytotoxic activity of TRAIL by redistributing TRAIL receptor to membrane lipid rafts. Mol Ther 2005; 11:542 - 552
- Inoue S, MacFarlane M, Harper N, Wheat L, Dyer M, Cohen G. Histone deacetylase inhibitors potentiate TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in lymphoid malignancies. Cell Death Differ 2004; 11:193 - 206
- Georgakis G, Li Y, Rassidakis G, Martinez-Valdez H, Medeiros L, Younes A. Inhibition of heat shock protein 90 function by 17-allylamino-17-demethoxy-geldanamycin in Hodgkin's lymphoma cells downregulates Akt kinase, dephosphorylates extracellular signal-regulated kinase, and induces cell cycle arrest and cell death. Clin Cancer Res 2006; 12:584 - 590
- Siegelin M, Habel A, Gaiser T. 17-AAG sensitized malignant glioma cells to death-receptor mediated apoptosis. Neurobiol Dis 2009; 33:243 - 249
- Ray S, Bucur O, Almasan A. Sensitization of prostate carcinoma cells to Apo2L/TRAIL by a Bcl-2 family protein inhibitor. Apoptosis 2005; 10:1411 - 1418