Abstract
A significant issue in drug efficacy studies is animal study design. Here we hypothesize that when evaluating new or existing therapeutics for the treatment of cancer, the location of disease burden will influence drug efficacy. To study this, Female NCr nude mice were inoculated with luciferase-positive human breast cancer cells (LCC6WT-luc) orthotopically (o.t.), intraperitoneally (i.p.) or intracardiacly (i.c.) to create localized, ascites or disseminated disease, respectively. Tumor development was monitored using bioluminescence imaging. Docetaxel (Dt) pharmacokinetics and distribution to sites of tumor growth were determined. Disease progression was followed in animals treated with Dt alone and in combination with QLT0267, an Integrin Linked Kinase inhibitor. Tumor related morbidity was most rapid when cells were inoculated i.c., where disease progression was observed in brain, ovaries, adrenal glands, and lungs. Dt pharmacokinetics were comparable regardless of the model used (mean plasma AUC0-24hrs 482.6 ng/ml*hr), however, Dt levels were lowest in those tissues developing disease following i.c. cell injection. Treatment with low dose Dt (5 mg/kg) increased overall survival and reduced tumor cell growth in all three models but the activity was greatest in mice with orthotopic tumors. Higher doses of Dt (15 mg/kg) was able to prolong survival in animals bearing i.p. tumors but not i.c. tumors. Addition of QLT0267 provided no added benefit above Dt alone in the disseminated model. These studies highlight a need for more comprehensive in vivo efficacy studies designed to assess multiple disease models and multiple endpoints, focusing analysis of drug parameters on the most chemoresistant disease.
See commentary:
Bioluminescence in drug development for cancer: Shedding light on therapeutic efficacy
Introduction
Animal models are a vital tool in the study of cancer and are used to gain a better understanding of cancer biology and to assess the pharmacological behaviour of experimental drugs. Since the late 1950s, xenografts of human tumor cell lines injected subcutaneously into mice have been used to study cancer treatments. These models have been beneficial in the identification of many of the anticancer drugs that are used today, however they are, in general, considered to be poor predictors of therapeutic activity in patients.Citation1,Citation2 Even with the advent of transgenic modelsCitation1,Citation3 or use of orthotopic transplantation of human tumors,Citation4–Citation6 there is little evidence indicating what models are the most predictive for use in the development of drug candidates for cancer indications. This is a serious problem for those investigators wishing to define better treatments for patients with cancers that do not respond well to existing standard of care chemotherapy protocols. Regardless, studies in animal models of cancer must be used to identify drug candidates with therapeutic potential and these models can help to define activity relative to drugs that are already approved for use while also providing insight into mechanism of action, drug distribution, drug metabolism and toxicity.
Retrospective studies have suggested that the best preclinical indicator of partial and complete responses for a drug candidate tested in phase II human clinical trials is demonstrable activity in multiple animal models of cancer.Citation7 As an example, Sathornsumetee et al. completed an elegant retrospective review of the preclinical and clinical trials for vandetanib. In their assessment vandetanib proved efficacious in the treatment of multiple human cancer xenograft models established as subcutaneous, orthotopic or metastatic disease. Subsequent analysis of phase I and II clinical trials demonstrated in patients that vandetanib was a promising new agent.Citation8 Perhaps then, it can be suggested that multiple preclinical models designed to emulate human cancers should be tested in parallel to give the best indication of how an agent will perform in the clinic.
It is widely acknowledged that the effectiveness of anticancer drugs will be influenced by the growth behavior and genetics of the cancer cells used as well as the microenvironment where the tumors form. The latter will have an effect on tumor cell behavior as well as accessibility of drugs to regions where the tumor cells localize. For breast cancer treatments this paradigm is recognized clinically, where chemotherapy regimens utilize different drugs when treating local or metastatic disease.Citation9 These observations again highlight the need for testing experimental drugs in multiple models grown in various sites before their use in the clinic. When considering such an approach, investigators have often relied on use of different tumor cell lines, injected by various routes (such as subcutaneous, intravenous or orthotopic routes) to establish tumors prior to initiating treatment. However, comparison of results obtained in tumor models developed with different cell lines is challenging because treatment outcomes will be dependent on the cell lines used as well as the site of tumor progression. To address the issue of comparing different cell lines inoculated into different site we suggest a strategy relying on use of an isogenic human breast cancer cell line capable of establishing tumors in different sites in vivo depending on the route of cell inoculation as outlined in the following studies. Furthermore, it is argued herein that, once identified, the most chemoresistant models should be used to assess methods for enhancing treatment outcomes involving broader dose escalations, use of combinations and assessment of dose scheduling parameters. This approach, where a single cell line is used to establish disease in multiple locations, can illustrate how the site of tumor growth influences treatment outcomes and more importantly may better predict how an experimental therapeutic will fare in clinical trials.
The specific aims of this study were: (1) to develop and characterize in vitro LCC6 breast cancer cells transfected with a luciferase gene; (2) to determine if LCC6 cells will establish localized (mammary fat pad), ascites and metastatic disease; (3) to explore the use of in vivo bioluminescence imaging (BLI) to visualize and monitor tumor growth following injection of the cells into the mammary fat pad, the peritoneal cavity or intracardiacally; (4) to determine whether the site of tumor cell inoculation and the associated site(s) of disease influence docetaxel (Dt) efficacy and (5) to determine if the more treatment resistant model could be sensitized to docetaxel by combining it with the ILK inhibitor, QLT0267, a targeted agent that has previously been shown to sensitize orthotopic LCC6WTluc breast tumors to docetaxel.Citation10 The results suggest that the experimental metastatic model is most treatment resistant while tumors established following injection into the mammary fat pad appeared most sensitive to treatment with Dt.
Results
Development and in vitro characterization of LCC6 breast cancer cells transfected with a luciferase gene.
The use of BLI as a non-invasive procedure in evaluating tumor growth requires transfection of cell lines with luciferase. Before luciferase transfected LCC6 cells were used in vivo, it was important to compare growth rates and response to Dt treatments of transfected cell lines with parent cell lines in vitro. LCC6WT, LCC6WT-luc and LCC6vector cells were studied in vitro to determine if the transfected cell lines exhibited cell growth attributes and drug sensitivity comparable to the parental cell lines. These data have been summarized in . Tumor cell growth rates, summarized in , indicate that the luciferase transfected cells grow comparably to the parental or vector transfected cell lines, exhibiting doubling times of approximately 24 hrs when the cells are growing exponentially (after day 6). Sensitivity of the luciferase transfected cells to Dt (72 hrs exposure time) was determined using the Alamar blue assay and the results () indicated that the IC50 for Dt when used against the parental cell line LCC6WT or LCC6WT-luc was approximately 0.7 nM. The LCC6vector cell line exhibited a Dt IC50 of 1.0 nM. The results indicate that luciferase transfected cells behave in a manner similar to parental cell lines with respect to in vitro growth and sensitivity to Dt.
Monitoring tumor growth with bioluminescence imaging.
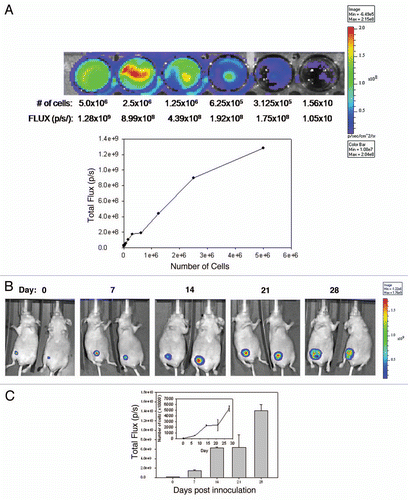
BLI uses light measurements (FLUX) to monitor tumor growth. FLUX data represents the number of luciferase positive cells in a given region of interest. This relationship between cell number and FLUX measurement can be used to estimate the number of cells that are localized to a disease site in vivo. In order to correlate FLUX values determined in vivo to the estimated number of viable cells, dilutions of cells derived from culture were prepared (0–5.0 × 106 cells) and placed in wells of a 48-well plate. The plate was then imaged using IVIS (). FLUX measurements (p/s) were determined and then plotted against the number of cells plated per well () to generate a curve relating FLUX to cell number. These values were used in the remaining studies to determine cell number at disease sites.
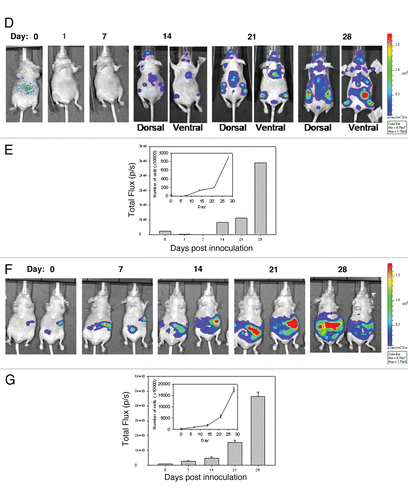
Next, NCr nude mice were inoculated o.t., i.p. and i.c. to determine if the three models, primary mammary fat pad tumor, ascites and experimental metastatic disease respectively could be established and monitored using BLI. The results summarized in show the growth of disease using BLI for animals that have been inoculated with LCC6WT-luc cells orthotopically ( and C), in the left ventricle ( and E) and in the peritoneal cavity ( and G). Animals were imaged by whole body scans using BLI once a week for 6 weeks. , E and G show the quantity of light FLUX on the indicated days. In order to generate tumor growth curves (insets to , E and G), FLUX data were related to cell number using the data provided in . The results summarized in lead to several conclusions. First, LCC6WT-luc cell proliferation is faster when the cells are inoculated i.p. For example, if the conversion of flux data to viable cell number is accurate, then 28 days after i.p. tumor cell inoculation the whole body scan would suggest that approximately 150 million luciferase positive cells are present. If the cells are given by i.c. injection, then the tumor cell burden is almost 20-fold lower. Our calculated doubling times are 5 days for the i.p. and o.t. models and 7.5 days for the i.c. model. It needs to be recognized, however, that these differences may be due in part to the fact that there are limitations to quantification and comparison of BLI data, that stem from a differential depth of tumor growth within the mouse body or differences in components of the tissues being analyzed. Furthermore, the luciferin was administered i.p. thus the luciferase-labeled cells in the peritoneal cavity may have far greater access to the substrate when compared to tumor cells within other tissues or organs. There is also the possibility that the different routes of administration result in differential survival of the inoculated cells. Second, orthotopic injection of LCC6WT-luc cells results in well defined localized disease that does not appear to disseminate from the site of injection (). Third, i.c. injection of LCC6WT-luc cells does produce a disseminated disease with growth in many discrete regions. Isolation of various organs and an assessment of bioluminescence indicated established tumors in the brain, lungs, ovary, lymph nodes, bones (femur and rib cage) and occasionally heart. The latter is likely a reflection of the inoculation site and localization of cells in that tissue. Finally, i.p. injection of LCC6WT-luc cells resulted in ascites formation, which is evident by broad and diffuse bioluminescence within the peritoneal cavity. It is notable that on day 28, when animals were terminated, tumor cells were present within the ascites fluid, within tumor nodules that could be found within the peritoneal cavity and within the pancreas. These studies illustrate that o.t., i.p. and i.c. inoculation of LCC6WT-luc cells results in primary mammary fat pad tumor, ascites and experimental metastatic disease respectively that can be monitored via BLI.
Docetaxel pharmacokinetics and tumor drug levels.
Using pharmacokinetic analyses, the plasma clearance and tumor/organ specific accumulation of Dt was determined for each of the three disease models established in the previous experiment. Orthotopic, metastatic and ascites tumors were established as described in the Methods and seven days after cell inoculation, animals were given an i.v. injection of [3H]-labeled Dt (see Materials and Methods). At various time points (0.5, 1, 2, 4 and 24 hrs) blood was collected by cardiac puncture and tumor tissue and/or ascites fluid was harvested. Dt elimination following i.v. injection was comparable in all three tumor bearing models (). The average Dt AUC0–24 h was 482.6 ng/ml*hr and there were no significant differences in drug levels at the specified time points between the different tumor models (p > 0.05).
Peritoneal fluid from mice inoculated i.p. with LCC6WT-luc cells was collected and analyzed for Dt. These data have been summarized in . Following i.v. injection, Dt accumulates in the peritoneum over the first 2 hrs and the concentration in ascites fluid is maintained in the peritoneal fluid collected from the peritoneal cavity over the 24 hrs time course. The AUC0–24 hrs for Dt in the peritoneal cavity was 432.6 ng/ml*hr. Since bioluminesence data suggested that following i.p. inoculation the LCC6WT-luc cells tumors appeared within the pancreas and for this reason the pancreas was assessed for Dt levels (). The pancreatic tissue levels ranged from 0.4–0.1 ng/mg tissue with an AUC0–24 hrs of 3.74 ng/mg*hr. An AUC0–24 hrs of 287.9 ng/mg*hr was calculated for the mammary fat pad of mice inoculated with LCC6WT-luc cells orthotopically (). As already indicated, when the tumor cells were given by an intracardiac injection the cells established tumors in various tissues in the mouse. This is illustrated by the representative BLI data provided in . Disease development, however, was most consistent within the adrenal glands and ovaries and for this reason Dt levels were determined in these tissues following i.v. administration of Dt to animals with established systemic disease. The results summarized in E, indicate that Dt accumulation in the adrenal glands was low, ranging from 0.1–0.007 ng/mg with an AUC0–24 hrs of 0.842 ng/mg*hr while the ovaries exhibit the lowest levels of Dt from 0.08–0.008 ng/mg with an AUC0–24 hrs of 0.476 ng/mg*hr (). Together these data indicate that plasma clearance of Dt is not influenced by tumor inoculation method, however, there is a substantial difference in Dt available to tumor tissues based on site of tumor growth secondary to site of tumor cell inoculation.
Dt treatment of mice bearing LCC6WT-luc tumors established after orthotopic, intracardiac and intraperitoneal injections of LCC6WT-luc cells.
In order to determine whether there is a differential efficacy of Dt depending on tumor inoculation route, animals bearing orthotopic, metastatic and ascites tumors were evaluated for tumor growth using BLI and survival, where control untreated animals were compared to Dt treated animals. Orthotopic, metastatic and ascites tumors were established in NCr nude mice as described above 7 days after cell inoculation the mice were treated with vehicle or 5 mg/kg Dt; a well tolerated Dt dose selected to better differentiate between the drug's activity in the models. In , results obtained when mice with established orthotopic (), metastatic () and ascites () tumors were treated with Dt are compared. BLI analysis indicated that Dt treatment caused a significant reduction in tumor growth as measured on day 21 in all three models when compared to control mice (p < 0.05). Treatment effects were also reflected in the survival curves which highlight two important points. First, when evaluating the controls it is clear that the most aggressive disease develops following i.c. injection of the LCC6WT-luc cells, where the median survival time (MST) is 23 days. This is in contrast to the data provided in , which would suggest that the mice given the i.c. injection of cells has the lowest tumor burden. Control animals inoculated with LCC6WT-luc cells via the o.t. or i.p. routes exhibited MSTs of 28 and 32 days, respectively. Second, when treated with 5 mg/kg Dt the increase in MST was greatest (13 days) when Dt was used to treat disease established after o.t. inoculation of the LCC6WT-luc cells. There was a 1.42-, 1.26- and 1.25-fold increase in the MST in the o.t., i.c. and i.p. models, respectively when compared to vehicle treated animals (Fig. D). Since animals with established orthotopic tumors were most sensitive to treatment with Dt, the remaining studies focused on the more treatment resistant models.
An important aim of this study was to determine whether the resistant disease can be sensitized to treatment. One method to sensitize tumors is dose escalation. From , it was established that the i.p. and i.c. animals were least responsive to low dose Dt, here we show that, when administered at higher doses (), Dt is more effective in prolonging the survival time of animals bearing i.p. tumors (p < 0.001) (), where >80% of the animals survived when treated with Dt at a dose of 15 mg/kg (Q7D × 4). In contrast, by day 76, 100% of the i.c. inoculated animals had to be sacrificed due to tumor growth, even when treated with 15 mg/kg Dt ().
Assessing whether the metastatic model could be sensitized to docetaxel by combining its use with the ILK inhibitor QLT0267.
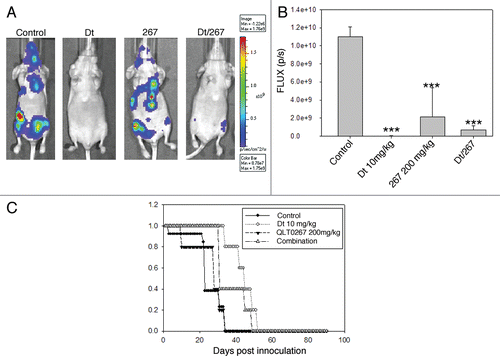
The results presented thus far indicate that the metastatic disease is least sensitive to Dt when used at a dose close to the maximum tolerated dose. This may be explained in part by limited drug delivery to tissues with established disease (). To determine whether treatment of the experimental metastatic model can be improved, Dt was used in combination QLT0267, an agent that targets ILK and can sensitize cells to cytotoxic agents by inhibiting the AKT pathway.Citation10–Citation12 Previously it was shown that QLT0267 improved the efficacy of Dt when used to treat the orthotopic LCC6 model.Citation10 In the present study, QLT0267 and Dt were used alone and in combination to treat animals with established LCC6WT-luc metastatic tumors. Metastatic disease (i.c.) was established and treated as described in the Methods. The results of this in vivo efficacy study have been summarized in . Tumor growth was monitored using BLI ( and B). Survival () was determined based on the time in days before animals were terminated due to poor health status. Tumors in animals treated with Dt, QLT0267 and Dt/QLT0267 showed reduced bioluminescence on day 21 post cell injection when compared to vehicle control treated mice. Quantification of total light flux demonstrated that the tumor burden on day 21 was significantly less in mice that had received treatment as compared to mice treated with the vehicle control (p < 0.0001), however animals treated with the combination of Dt/QLT0267 did not exhibit benefits that were significantly better than that achieved with Dt alone. This is likely because Dt treatment was so effective when assessing therapy at this time point. Interestingly, analysis of survival data () (as defined by animal health) suggested that a reduction in tumor burden, as exemplified by treatment with Dt, has minimal effect on survival. A possible explanation for this may be that 10 mg/kg was able to slow tumor growth so that on day 21 when images were taken minimal disease burden is observed, but later images illustrate that disease continues to develop in Dt treated animals after day 21. Furthermore, for animals treated with QLT0267 (200 mg/kg) the median survival time was 31 days for LCC6WT-luc (n = 5) as compared to 23 days for control animals (n = 13). Animals treated with Dt exhibited a median survival time of 45 days for LCC6WT-luc. Animals treated with the QLT0267/Dt combination exhibited a median survival time that was not significantly better than that achieved with QLT0267 alone and, surprisingly, suggested that the treatment outcomes when using the drug combination was worse than that achieved with Dt alone.
Discussion
Although efficacy data obtained in animal based tumor models are required for the development of new anticancer drugs and to assess drug combination effects, these models are widely criticized because the data obtained is not highly predictive of activity in humans. There are many examples that could be highlighted,Citation16–Citation22 but in general strong evidence of activity in well characterized preclinical models is rarely recapitulated in clinical trials. The poor correlation between preclinical studies and clinical results has been attributed to various limitations in the animal models used, models which do not adequately represent the human disease and/or the stage of disease development. The latter refers to the fact that many new drug candidates are assessed in patients who exhibit late stage disease; disease that has progressed following extensive standard of care treatment regimens that is often systemic (metastatic), and there are very few metastatic models of human cancer that are routinely used. Investigators have suggested approaches to address these limitations, however, the solution to this problem is not simple and to date no one approach has been developed which can accurately predict therapeutic effects in humans. Retrospective studies have clearly shown that under situations where significant therapeutic benefits have been observed in early clinical studies the preclinical data to support development of the drug consisted of strong evidence of therapeutic effects in multiple (five or more) tumor models. The studies described in this paper were designed to validate the use of multiple models of breast cancer where the site of disease development varied (orthotopic, ascites or systemic), but the human breast cancer cell line (LCC6WT-luc) used to generate the different models was the same. Validation was based on use of Dt, an agent that is well recognized as an effective drug in the treatment of breast cancer.Citation13,Citation14 This approach allowed the comparison of a single cell line grown in different microenvironments and its response to Dt.Citation15 Docetaxel was therapeutically active in all three models used; consistent with the fact that Dt has demonstrated positive activity in early (adjuvant) treatment of breast cancer and late stage metastatic disease.Citation13,Citation14 However, when using a well-tolerated low dose of Dt, treatment effects were greatest in animals bearing orthotopic tumors and least effective in animals with systemic disease. Although the site of disease development did not affect Dt elimination from the plasma (), Dt delivery to sites of disease development following i.c. inoculation of cells was remarkably lower than that observed to sites involved when the cells were inoculated i.p. and orthotopically. Thus reduced Dt activity in models with systemic disease could be explained by a more aggressive disease phenotype as well as reduced drug delivery to tissues/organs with progressing tumors. The organs for biodistribution, such as the lungs and liver, are not addressed in this paper, as a future direction it will be important to determine if drug distribution to target organs is influenced by site of tumor inoculation.
Dt is used frequently in patients with relapse (treatment refractory/resistant and metastatic) disease, but in this setting it offers improvements in median survival time, not survival.Citation13 This suggests that the therapeutic benefits of Dt will be greatest when used in a combination regimen. An important aim of this study was to determine if the most resistant disease could be sensitized to treatment whether by dose escalation or by combination strategies. We note that by increasing Dt to maximum tolerated dose of 15 mg/ml animals did exhibit modest increases in survival. More importantly, previous studies from our laboratory have demonstrated that in the orthotopic LCC6 breast cancer model, the therapeutic effects of Dt can be enhanced when it is used in combination with QLT0267, an investigative drug candidate that targets ILK and, as a result, inhibits the AKT survival pathway.Citation5 To explore whether the QLT0267/Dt combination would be effective at treating a resistant metastatic disease in the current study we applied the combination to the experimental metastatic model. Interestingly, the activity of Dt in combination with QLT0267 did not prove to be beneficial over Dt alone. As such, these results would predict that combinations of QLT0267 with Dt would not be better than single agent Dt in a relapsed disease setting. This is perhaps also consistent with previous results indicating that QLT0267 interacts with Dt in a manner that prevents invasion and metastasis and thus it would have less impact on treatment outcomes following metastasis. Our preclinical studies predict that clinical benefits in patients with breast cancer would most likely be observed if QLT0267 was combined with Dt to treat patients in the adjuvant or neoadjuvant setting.
Materials and Methods
Chemicals.
Docetaxel (Dt) was obtained from the British Columbia Cancer Agency Pharmacy (Vancouver, BC Canada). Dt was reconstituted in 13% ethanol for a final concentration of 10 mg/ml. Luciferin was obtained from Caliper Life Sciences (Almeda, CA), and reconstituted in sterile PBS for a final concentration of 15 mg/ml. QLT0267 was a generous gift from QLT Inc. (Vancouver, BC Canada). Tritium labeled docetaxel was obtained from American Radiolabeled Chemicals (St. Louis, MO).
Cell-lines and culture.
MDA-MB-435/LCC6 (LCC6) estrogen receptor negative breast cancer cellsCitation16 were a generous gift from Dr. Robert Clarke (Georgetown University, Washington, DC). The breast cancer origin of the LCC6 parental cell line, MDA-MB-435, is controversial. Based on studies of Ross et al. and Rae et al. it has been suggested that the MDA-MB-435 cell line is of a melanoma origin sharing features of the M14 melanoma cell line. However, Sellappan et al. have been able to demonstrate that MDA-MB-435 cells can be induced to express breast differentiation-specific proteins and secrete milk lipids. Furthermore Neve et al. have demonstrated that the MDA-MB-435 cell line shares many molecular features with breast cancer cell lines of breast epithelium origin. In studies from our laboratoryCitation21 using a LCC6 cell line permanently transfected with the Her2/neu gene (LCC6Her2 cell line), we have been able to demonstrate that the Her2/neu positive variant exhibits enhanced survival under stress, overproduction of VEGF, activation of NFκB and in vivo sensitivity to trastuzumab (aka Herceptin); results that are consistent with what is known about Her2/neu positive breast cancer models. Although the M14 melanoma cell line and the MDA-MB-435 cell line show striking similarities with respect to genetic profile leading to the belief that both are in fact the M14 cell line, it has been established that human breast cancers do indeed express melanocytic markersCitation22,Citation23 (and more recently even neuronal markers).Citation24 Finally, most recent karyotype evidence has shown that both cell lines were derived from a female patient consistent with the origin of MDA-MB-435 and not M14 cells.Citation25 Thus, we believe it is justifiable to use these cells as a model breast cancer cell line.
Stock cells lines were maintained in the absence of penicillin and streptomycin and screened for mycoplasma prior to preparing a stock of cells that were frozen for use in experiments. Cells were re-suspended in freezing media (10% DMS0 in FBS) and slowly frozen in Nalgene® 1°C freezing containers (Rochester, NY) containing 100% isopropanol at −80°C for 24 hours before storage in liquid nitrogen. Frozen cells were quickly thawed at 37°C, centrifuged to remove freezing media, plated and passaged twice before use in experiments. LCC6 and LCC6WT-luc cells used for experiments were maintained in DMEM/high glucose supplemented with L-glutamine (2 mmol/L; DMEM and L-glutamine from Stem Cell Technologies, Vancouver, BC Canada), 5 mM penicillin/streptomycin (Stem Cell) and 10% fetal bovine serum (FBS) (Hyclone, Logan, UT). All cells were maintained at 37°C and 5% CO2 in a humidified atmosphere and allowed to undergo no more than 20 passages.
Lentivirus transfections.
Constructs for the lentivirus (LV) vector containing the luciferase (luc) and Green Fluorescent Protein (GFP) genes were obtained from Dr. Alice Mui (Jack Bell Research Center, Vancouver General Hospital) who also assisted in the transfection of the LCC6vector and LCC6WT-luc cells lines. Briefly, the luciferase coding sequence was isolated from the pGL-3 vector (Promega, Madison, WI) and cloned into the lentiviral vector FG9 downstream of the CMV-LTR and UBiC promoter. To generate luciferase-expressing lentivirus (Lentiluc), this vector was cotransfected using calcium phosphate with packaging constructs pRSVREV, pMDLg/pRRE and the VSV-G expression plasmid pHCMVG into HEK-293T cells. Five million 293T HEK cells were plated on poly-L-lysine-coated tissue culture plates allowed to adhere for 24 hrs. The following day, 10 µg of the transducing vector, 7.5 µg of the packaging vector and 2.5 µg of the VSV envelope pMD.G were co-transfected by LipofectAMINE 2000 (Invitrogen, Burlington, ON Canada), according to the manufacturer's instructions. After 24 hrs, fresh media was applied to cells and cells were cultured for another 24 hrs. Conditioned medium was then collected and cleared of debris by low speed centrifugation, filtered and stored at −70°C. Collection of supernatant was done daily for 4 days, pooled and ultracentrifuged. The pellet was re-suspended in 500 µl of media and aliquots were stored at −70°C. A similar method was used to generate GFP-expressing lentivirus.
The LCC6 cells were then infected with Lenti-luc virus (25 µL concentrated viral supernatant/mL medium). Briefly, one million cells were seeded in each well of a 12-well plate in 500 µl of complete media and allowed to adhere for 24 hrs. Subsequently, Lenti-vector or Lenti-Luc and the Lenti-GFP constructs were added to the media at a ratio of 10:1 in the presence of Polybrene (8 µg/mL medium). After approximately 5 hrs of incubation, the cells were washed with PBS, fresh culture medium was added and cells were incubated for up to 6 days. To enrich for luciferase positive cells, cells were first sorted by FACS for GFP expression. Subsequently GFP-positive cells were re-plated in low concentrations into soft agar in the wells of a 96-well plate. Luciferin was added to each well and plates were imaged using IVIS (see below) to identify luciferase positive colonies. Positive colonies were selected expanded and used for the in vitro and in vivo studies described below.
Growth curves and cell viability assay.
Metabolic activity (used as a measure of cell viability) was determined using the Alamar Blue® assay (Medicorp Inc., Montreal, QC, Canada) run according to instructions provided by the manufacturer. For growth curves, 1,000 cells were seeded in triplicate onto 96-well flat bottom tissue culture plates (Techno Plastic Products AG, Switzerland) and allowed to adhere to the substratum for 24 hrs under normal growth conditions (37°C and 5% CO2 in a humidified atmosphere). Thereafter, for 7 days the number of viable cells was estimated by Alamar blue. For cytotoxicity assays, 6,000 cells/well (seeded in triplicate onto 96-well flat bottom tissue culture plates) were allowed to adhere for 24 hrs under normal growth conditions (37°C and 5% CO2 in a humidified atmosphere). Serial dilutions of Dt or vehicle controls diluted in DMEM cell culture medium were added to wells and cells were grown for an additional 72 hrs before measuring cell viability. To assess cell viability, cells were incubated with 10% resazurin solution for 4 hrs at 37°C and fluorescence was measured at 560/590 nm using an Optima fluorescence plate reader (BMG Labtech, Durham, NC). Relative fluorescence determined from drug treated cells was normalized to fluorescence determined from control cells (cells grown in the absence of added drug) and the percent (%) cell viability relative to vehicle control treated cells (100% viability) was determined. Background fluorescence was subtracted from all samples and results of experiments conducted in triplicate at least three times were averaged.
Animal studies.
All animal studies were conducted in accordance with and approved by institutional (University of British Columbia) guidelines for humane animal treatment and according to the current guidelines of the Canadian Council of Animal Care. Mice were maintained at 22°C in a 12 hrs light and dark cycle with ad libitum access to water and food. The studies used female NCr nude mice weighing between 18 and 25 g which were obtained from Taconic (Oxnard, CA) and maintained in an SPF-facility. Animals were housed in groups of four or five. Long-term survival was determined based on the time in days when mice were terminated due to tumor ulceration, the presence of tumors exhibiting volumes in excess of 500 mg, and/or signs of deteriorating animal health requiring euthanasia as defined by a health monitoring standard operating procedure. Animals that did not develop tumors as assessed by BLI prior to initiation of treatments were excluded from the study. The studies exhibited in this paper are representative studies where control arms have been repeated at minimum of three times and have been shown to be highly reproducible.
Experimental design, tumor xenografts and treatments.
For the first study (), mice were randomized into three groups of six according to the site of tumor inoculation (i.p., o.t. or i.c.). To initiate the i.p. model, the abdomen of each animal was disinfected using 70% isopropanyl alcohol to clean the injection site and 5 × 106 LCC6WT-luc cells in 500 µl HBSS were injected i.p. using a 25-gauge needle. The o.t. model was initiated via injection of 2 × 106 LCC6WT-luc cells into the mammary fat pad in a volume of 50 µL media using a 28-gauge needle.Citation26 For the i.c. tumor model, disease was initiated by anesthetizing animals using isoflurane and positioning animals so that a 26-gauge needle attached to a 1 mL tuberculin syringe could be inserted at a 30 degree angle immediately caudal to the xyphiod process. The needle was aimed towards the left shoulder. Slight negative pressure was placed on the syringe upon entry and the needle was slowly moved forward until blood appeared in the hub of the needle. Once the needle was in the correct position, 2 × 106 LCC6WT-luc cells in a volume of 100 µl media were slowly (over 30–60 seconds) injected. Animals were monitored for tumor growth, body weight and health status. Tumor growth for all three models, was monitored using an IVIS 200 imaging system (Xenogen, Caliper Life Sciences, MA) as described below. Animal body weights were measured every Monday, Wednesday and Friday.
For the second study (), mice were randomized into three groups (20 mice per group) according to the site of tumor inoculation (i.p., o.t. or i.c.). Tumors were established as described above and on day 7, animals were treated with 1 dose of 10 mg/kg Dt spiked with tritium labeled Dt. Four animals were removed per group at 0.5, 1, 2, 4 and 24 hrs post treatment, at which time blood was collected via cardiac puncture and organs were harvested as outlined in the methods below. For the third study (), mice were randomized into three groups according to the site of tumor inoculation (i.p., o.t. or i.c.) and further randomized into two sub-groups (six mice per group) (vehicle and 5 mg/kg). Tumors were established as described above and readily detectable by luminescence in all animals 24 hrs and 7 days post-inoculation. On day 7, animals were treated with the vehicle or Dt (5 mg/kg) (i.v. Q7D × 4), a well tolerated Dt dose selected to better differentiate between the drug's activity in the models. Animals were monitored for growth, body weight and survival. Tumor growth was monitored using the IVIS 200. For the fourth study (), mice were randomized into two groups according to the site of tumor inoculation (i.p. or i.c.) and further randomized into four sub-groups (six mice per group) according to the dose of docetaxel (5, 10, 15 mg/kg) used. Tumors were established as described above and on day 7, animals were treated with Dt or the vehicle control (i.v. Q7D × 4). Animals were monitored for growth, body weight and survival. Tumor growth was monitored using the IVIS 200. For the fifth study () mice were randomized into two groups to be inoculated with LCC6WT-luc cells and further randomized into four sub-groups according to treatment (vehicle, 10 mg/kg Dt, 200 mg/kg QLT0267 or a combination of 10 mg/kg Dt and 200 mg/kg QLT0267) (five mice per group). The QLT0267 dose (200 mg/kg) and schedule (QD × 28) was selected based on previous studies that showed effective therapy in different human xenograft models.Citation10,Citation11,Citation27 i.c. tumors were established as described above and on day 7, animals were treated with (vehicle; Dt-10 mg/kg [i.v.] [Q7D × 4]; QLT0267-200 mg/kg [p.o.] [QD × 28]; or a combination of Dt-10 mg/kg [i.v.] [Q7D × 4] and QLT0267-200 mg/kg [p.o.] [QD × 28]). Animals were monitored for tumor growth (IVIS 200), body weight and overall health status.
In vivo imaging system (IVIS).
Imaging was performed once per week to monitor tumor growth. LCC6WT-luc tumor bearing mice were injected i.p. with 500 µl D-luciferin (15 mg/ml) (Xenogen Corp., Alameda, CA). Mice were anaesthetized using isoflurane and at precisely twenty minutes (±2 min.) post luciferin injection mice were imaged. Photographic and luminescence images were taken at exposure times of 1, 2 and 5 seconds and Xenogen IVIS® software was used to quantify nonsaturated bioluminescence in regions of interest (ROI). Light emission between 5.5 × 106–7.0 × 1010 was assumed to be indicative of viable luciferase-labeled tumor cells while emissions below this range were considered as background. Bioluminescence was quantified as photons/second for each ROI.
Dt pharmacokinetics and tumor tissue drug uptake.
Tumor bearing animals were given an i.v. dose of 10 mg/kg Dt, labeled with 3 mCi of [3H]-Dt. Four animals were sacrificed by CO2 asphyxiation at 0.5, 1, 2, 4 and 24 hrs and blood was collected immediately via a cardiac puncture. Blood was rapidly transferred to EDTA containing Microtainers™ and placed on ice. Plasma was separated by centrifugation (15 min at 1,500x g at 4°C) and stored at −80°C. One hundred µL of plasma was added to 5 ml scintillation fluid and the amount of [3H]-Dt present was measured by liquid scintillation counting (Packard 1900TR Liquid Scintillation Analyzer). DPMs were assumed to be due to intact Dt and the results were converted to concentration of Dt ([Dt]) per 1 mL of plasma using the specific activity of the injected Dt solution prior to injection of animals. To determine the amount of drug in the peritoneal cavity, the cavity was lavaged with 5 mL of ice cold saline. 100 µL of collected fluid was assessed for radioactive Dt. The resulting DPMs were used to estimate the amount of drug in the peritoneal cavity per mL, which included cell and non-cell associated [3H]-Dt.
Tumors or tissues containing tumors were harvested and the presence of luciferase positive cells was confirmed by BLI. Tumors or tissues with confirmed evidence of tumor growth (e.g., mammary fat pad for mice given o.t. cell inoculations, pancreas for mice given i.p. cell inoculations and adrenal glands and ovaries for mice given i.c. cell inoculations) were harvested. Harvested tissue was weighed in mg and then solubilized using 500 µL of Solvable (Perkin Elmer, Boston, MA) and incubated overnight at 50°C. Organ homogenates were decolorized using 200 µL H2O2 and 50 µL EDTA and subsequently incubated overnight at room temperature. The samples were assessed for [3H]-Dt using scintillation counting and these data were then used to estimate the amount of Dt per mg of tissue.
Statistical analysis.
All statistical data was collected using GraphPad Prism (San Diego, CA). Parametric analysis was done using standard deviation or standard error of the mean and n, in an unpaired Student's t-test. DT area under the curve analysis was done using Phoenix™ WinNonlin® software (Pharsight, St. Louis, MO). Survival curves were generated using SigmaPlot 10.0 (Dundas Software, Germany) from which median survival times were extrapolated. The survival curves were compared using log-rank statistics using SPSS software (IBM corporation, Somers, NY).
Conclusion
We used three different preclinical breast cancer models derived from a single cell line to help delineate whether the location of disease would influence drug distribution and therapeutic efficacy. The broader implication of the multiple model and multiendpoint approach described here is a more robust predictive power for clinical efficacy of experimental therapeutics.
Authors' Contributions
J.K. designed and executed all experiments and data analysis and wrote the manuscript. M.A. contributed expertise on PK and PD studies. C.W. contributed the LCC6Her2 cell line, executed replicates of in vitro studies, and helped revise the manuscript. H.Y. provided cell culture support. D.S., Y.J.Y., M.O. and D.M. provided animal study support. D.W. helped revise the manuscript. M.B. is Chief Investigator, and assisted in the study design and helped write the manuscript.
Abbreviations
| ILK | = | integrin linked kinase |
| Dt | = | docetaxel |
| o.t. | = | orthotopic |
| i.p. | = | intraperitoneal |
| i.c. | = | intracardiac |
| i.v. | = | intravenous |
| IVIS | = | in vitro imaging system |
| BLI | = | bioluminescent imaging |
| Her2/neu, c-erb-B2 | = | human epidermal grown factor |
| VEGF | = | vasculuar endothelial growth factor |
| NFκB | = | nuclear factor kappa-light-chain-enhancer of activated B cells |
| ROI | = | region of interest |
| LV | = | lentivirus |
| [3H]Dt | = | tritium labled doctetaxel |
| pk | = | pharmacokinetics |
| pd | = | pharmacodynamics |
| DPM | = | disintegrations per minute |
| AUC | = | area under the curve |
| MST | = | median survival time |
| T | = | treated |
| C | = | control |
| MTD | = | maximum tolerated dose |
| EDTA | = | ethylenediaminetetraacetic acid |
| F-actin | = | filamentous actin |
Figures and Tables
Figure 1 Cell growth and sensitivity to Dt in vitro. LCC6WT cells were stably co-transfected using a lenti-virus vector containing the luciferase gene and green fluorescent protein (GFP) (see Materials and Methods). After transfection and selection, selected cells were collected, determined to be mycoplasma free and subsequently grown in vitro. Tumor cell growth rates were determined following plating of 1,000 cells and as described in the methods, the number of viable cells was determined for 7 days using the Alamar blue assay (A). Sensitivity of the transfected cells to increasing concentrations of Dt was determined using the Alamar blue assay on cells that had been exposed to Dt for 72 hrs. The results presented were determined three times in triplicate and the data points represent the mean (±SEM) of the three independent studies.
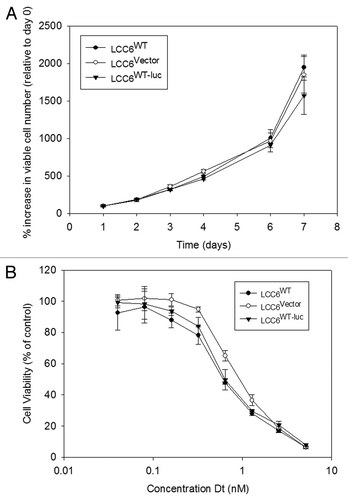
Figure 2 Growth of LCC6WT-Luc cells in female NCr nude mice following inoculation orthotopically (o.t.) (mammary fat pad), intracardiacally (i.c.) (left ventricle) or intraperitoneally (i.p.). LCC6WT-luc cells were serially diluted and placed into wells of a 24 well plate and immediately imaged using the IVIS 200 system to obtain FLUX measurements (A, images). These data were averaged (n = 4) and used to generate a plot of comparing total flux to cell number (A, graph). LCC6WT-Luc cells were inoculated orthotopically (B and C), via intracardiac injection (D and E) or intraperitoneally (F and G) (see Materials and Methods). Bioluminescence imaging was used to monitor tumor growth. Images shown were acquired on days 0, 1, 7, 14, 21 and 28. FLUX values were measured using whole body scans for each animal (representative images shown in B, D and F). FLUX data (shown in C, E and G) was then related to cell number (insets to C, E and G) using the calibration curve (A).
Figure 3 Docetaxel pharmacokinetics and distribution to sites of disease development following inoculation of LCC6WT-Luc cells via the o.t., i.c. and i.p. injection. 0.5, 1, 2, 4 and 24 hrs after i.v. injection of Dt (10 mg/kg) labeled with [3H]-Dt, animals (n = 4 per time point) were terminated by CO2 asphyxiation and blood was collected by cardiac puncture and placed into an EDTA-containing micro-container placed on ice (see Materials and Methods). Subsequently plasma was separated from blood cells and the amount of [3H]-Dt in 50 mL of plasma was determined by liquid scintillation counting. These data were then used to calculate the plasma concentration of Dt (ng/mL) (A). Dt levels in ascites fluid (cell-associated and non-cell associated) were determined following a 5 mL lavage with serum media (see Materials and Methods). The total amount of fluid recovered from the peritoneal cavity was measured and an aliquot of the ascites was taken for scintillation counting. The amount of Dt per mL of ascites fluid was then determined (B). In animals inoculated with LCC6WT-luc cells, tumors consistently developed in the pancreas. Therefore the pancreas from each mouse was harvested and processed (see Materials and Methods) for assessment of [3H]-Dt. These data were then used to estimate the amount of Dt in pancreatic tissue (ng/mg tissue). For mice bearing systemic disease, BLI data suggested that organs/tissues such as the brain, lung, rib cage and heart (F) exhibited tumor growth, however growth was most consistently observed in the adrenal glands and ovaries thus these organs were chosen as representative organs for metastatic disease. These tissues were collected and analyzed as described above (E). Similarly, tumors harvested from the animals bearing orthotopic tumors were collected and processed (D). All tissue levels were reported as ng Dt per mg wet tissue and the results presented represent the mean (n = 4) ±SD.
![Figure 3 Docetaxel pharmacokinetics and distribution to sites of disease development following inoculation of LCC6WT-Luc cells via the o.t., i.c. and i.p. injection. 0.5, 1, 2, 4 and 24 hrs after i.v. injection of Dt (10 mg/kg) labeled with [3H]-Dt, animals (n = 4 per time point) were terminated by CO2 asphyxiation and blood was collected by cardiac puncture and placed into an EDTA-containing micro-container placed on ice (see Materials and Methods). Subsequently plasma was separated from blood cells and the amount of [3H]-Dt in 50 mL of plasma was determined by liquid scintillation counting. These data were then used to calculate the plasma concentration of Dt (ng/mL) (A). Dt levels in ascites fluid (cell-associated and non-cell associated) were determined following a 5 mL lavage with serum media (see Materials and Methods). The total amount of fluid recovered from the peritoneal cavity was measured and an aliquot of the ascites was taken for scintillation counting. The amount of Dt per mL of ascites fluid was then determined (B). In animals inoculated with LCC6WT-luc cells, tumors consistently developed in the pancreas. Therefore the pancreas from each mouse was harvested and processed (see Materials and Methods) for assessment of [3H]-Dt. These data were then used to estimate the amount of Dt in pancreatic tissue (ng/mg tissue). For mice bearing systemic disease, BLI data suggested that organs/tissues such as the brain, lung, rib cage and heart (F) exhibited tumor growth, however growth was most consistently observed in the adrenal glands and ovaries thus these organs were chosen as representative organs for metastatic disease. These tissues were collected and analyzed as described above (E). Similarly, tumors harvested from the animals bearing orthotopic tumors were collected and processed (D). All tissue levels were reported as ng Dt per mg wet tissue and the results presented represent the mean (n = 4) ±SD.](/cms/asset/8a2592c8-ae95-4e57-b879-f200159b00bb/kcbt_a_10915183_f0004.gif)
Figure 4 Dt treatment of mice (i.v. Q7D × 4 with 5 mg/kg) with established tumors developed following orthotopic (A), intracardiac (B) or intraperitoneal (C) injection of LCC6WT-luc breast cancer cells. The IVIS200 system was used to obtain BLI (representative images provided on top row) and subsequent FLUX analysis was used to assess tumor progression for each model (middle row). Image analysis for total FLUX quantification was determined on 21 days after tumor cell inoculation (following the third treatment with Dt). Results were obtained using at least five mice per treatment group and error bars (on Total Flux data) represent the SEM. An ANOVA analysis of data indicated that the treatment groups were statistically different from controls (*p > 0.05, **p > 0.01 and ***p > 0.005). Survival curves were generated based on the time when animals needed to be terminated due to overall health status or tumor ulceration and tumor size (for the orthotopic tumors only). Log rank statistical analysis of data indicated p = 0.32, p = 0.12 and p = 0.001 for the o.t., i.c. and i.p. models respectively. If animals were terminated due to health status the following day was recorded as the time of death. Median survival time (MST) for each group was estimated and the T-C value was determined by subtracting the MST of control animals, C; from the Dt treated animals, T (D).
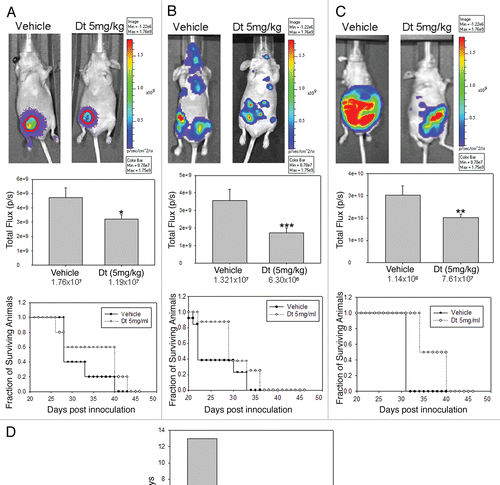
Figure 5 Treatment of mice with Dt at the MTD (i.v. Q7D × 4 with 15 mg/kg). Mice inoculated with LCC6WT-Luc cells i.p. (A) or i.c. (B) (see Materials and Methods) were treated i.v. with 5, 10 or 15 mg/kg of Dt once a week for four weeks. Survival curves were generated based on the time when animals needed to be terminated due to overall health status. If animals were terminated due to health status the following day was recorded as the time of death. Results were obtained using at least five mice per treatment group. Log rank statistical analysis of data indicated p < 0.001, for both the i.c. and i.p. models.
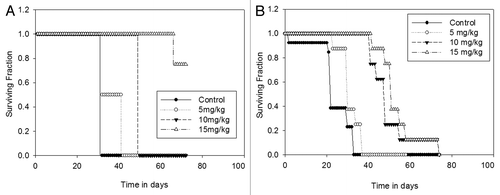
Figure 6 Treatment of mice inoculated i.c. with LCC6WT-luc cells with single agent or a combination of Dt (10 mg/kg Q7D × 4) and the ILK inhibitor QLT0267 (QD × 28). LCC6WT-luc cells were inoculated i.c. (see Materials and Methods) and then treated with vehicle control, Dt (10 mg/kg), QLT0267 (200 mg/kg) or the combination of QLT0267/Dt. BLI was obtained as described in the Materials and Methods 21 days after tumor cell inoculation (representative images have been provided in (A). Total light emission from tumors in animals was quantified at that time (B) and single agent or combination treatment was significantly less when compared to controls (p < 0.0001). Survival curves (C) were generated based on the time when animals needed to be terminated due to overall health status. If animals were terminated due to health status the following day was recorded as the time of death. Results were obtained using at least five mice per treatment group. Log rank statistical analysis of survival data indicated p < 0.003.
Acknowledgements
Funding for this research was from the Canadian Breast Cancer Research Alliance (CBCRA). J.K. is a recipient of the Pacific Century Graduate Scholarship. This work would not have been possible if not for the excellent animal care support that is provided through the BC Cancer Agency's Animal Resource Center. Dr. Robert Clarke (Georgetown University, Washington, DC) generously donated MDA-MB-435 (LCC6) estrogen receptornegative breast cancer cells.
References
- Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol 2007; 170:793 - 804
- Palmieri D, Lockman PR, Thomas FC, Hua E, Herring J, Hargrave E, et al. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin Cancer Res 2009; 15:6148 - 6157
- Cespedes MV, Casanova I, Parreno M, Mangues R. Mouse models in oncogenesis and cancer therapy. Clin Transl Oncol 2006; 8:318 - 329
- Bibby MC. Orthotopic models of cancer for preclinical drug evaluation: advantages and disadvantages. Eur J Cancer 2004; 40:852 - 857
- Tseng W, Leong X, Engleman E. Orthotopic mouse model of colorectal cancer. J Vis Exp 2007; 10:484
- Hoffman RM. Invest New Drugs 1999; 17:343 - 359
- Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft and mouse allograft preclinical cancer models. Clin Cancer Res 2003; 9:4227 - 4239
- Sathornsumetee S, Rich JN. Vandetanib, a novel multitargeted kinase inhibitor, in cancer therapy. Drugs Today (Barc) 2006; 42:657 - 670
- Bergh J, Jönsson PE, Glimelius B, Nygren P. SBU-group. A systematic overview of chemotherapy effects in breast cancer. Acta Oncol 2001; 40:253 - 281
- Kalra J, Warburton C, Fang K, Edwards L, Daynard T, Waterhouse D, et al. QLT0267, a small molecule inhibitor targeting integrin-linked kinase (ILK) and docetaxel can combine to produce synergistic interactions linked to enhanced cytotoxicity, reductions in P-AKT levels, altered F-actin architecture and improved treatment outcomes in an orthotopic breast cancer model. Breast Cancer Res 2009; 11:R25
- Edwards LA, Woo J, Huxham LA, Verreault M, Dragowska WH, Chiu GL, et al. Suppression of VEGF secretion and changes in glioblastoma multiforme microenvironment by inhibition of integrin-linked kinase (ILK). Mol Cancer Ther 2008; 7:59 - 70
- McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase—essential roles in physiology and cancer biology. J Cell Sci 2008; 121:3121 - 3132
- Saloustros E, Mavroudis D, Georgoulias V. Paclitaxel and docetaxel in the treatment of breast cancer. Expert Opin Pharmacother 2008; 9:2603 - 2616
- Crown J, O'Leary M, Ooi WS. Docetaxel and paclitaxel in the treatment of breast cancer: a review of clinical experience. Oncologist 2004; 9:24 - 32
- Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion and metastasis. J Cell Biol 2000; 148:779 - 790
- Leonessa F, Green D, Licht T, Wright A, Wingate-Legette K, Lippman JF, et al. MDA435/LCC6 and MDA435/LCC6MDR1: ascites models of human breast cancer. Br J Cancer 1996; 73:154 - 161
- Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, et al. Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet 2000; 24:227 - 235
- Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD. MDA-MB-435 cells are derived from M14 melanoma cells—a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res Treat 2007; 104:13 - 19
- Sellappan S, Grijalva R, Zhou X, Yang W, Eli MB, Mills GB, et al. Lineage infidelity of MDA-MB-435 cells: expression of melanocyte proteins in a breast cancer cell line. Cancer Res 2004; 64:3479 - 3485
- Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006; 10:515 - 527
- Dragowska WH, Warburton C, Yapp DT, Minchinton AI, Hu Y, Waterhouse DN, et al. HER-2/neu overexpression increases the viable hypoxic cell population within solid tumors without causing changes in tumor vascularization. Mol Cancer Res 2004; 2:606 - 619
- Montel V, Suzuki M, Galloy C, Mose ES, Tarin D. Expression of melanocyte-related genes in human breast cancer and its implications. Differentiation 2009; 78:283 - 291
- Bachmeier BE, Nerlich AG, Mirisola V, Jochum M, Pfeffer U. Lineage infidelity and expression of melanocytic markers in human breast cancer. Int J Oncol 2008; 33:1011 - 1015
- Zhang Q, Fan H, Shen J, Hoffman RM, Xing HR. Human breast cancer cell lines co-express neuronal, epithelial and melanocytic differentiation markers in vitro and in vivo. PLoS One 2010; 5:9712
- Chambers AF. MDA-MB-435 and M14 cell lines: identical but not M14 melanoma. Cancer Res 2009; 69:5292 - 5293
- Conley FK. Development of a metastatic brain tumor model in mice. Cancer Res 1979; 39:1001 - 1007
- Younes MN, Kim S, Yigitbasi OG, Mandal M, Jasser SA, Dakak Yazici Y. Integrin-linked kinase is a potential therapeutic target for anaplastic thyroid cancer. Mol Cancer Ther 2005; 4:1146 - 1156