Abstract
Objective: To achieve effective delivery of siRNA into target cells in vivo, we have developed a novel approach of siRNA delivery by using local drug delivery systems.
Results: The chitosan hydrogel (CH-HG) displayed a liquid-solid phase transition in a temperature-dependent manner and formed an endothermic hydrogel in tumor tissue after intra-tumoral injection. Additionally, we tested the extent of in vivo delivery following a single intra-tumoral injection of Alexa555 siRNA/CH-HG into A375SM-bearing mice. The Alexa555 siRNA demonstrated higher localization into tumor cells compared to control. The Alexa555 siRNA delivery extends to tumor cells outside of CH-HG and some tumor cells also infiltrated into CH-HG. For therapeutic proof-of-concept studies, CH-HG including TG2-targeted siRNA significantly inhibited tumor growth in melanoma (A375SM) and breast (MDA-MB231) tumor models compared to control (A375SM: 72% reduction and MDA-MB231: 92% reduction, p<0.001).
Experimental Design: we prepared a CH-HG system loaded with siRNA to enhance localized therapeutic efficacy without risk for systemic side effects. Delivery of siRNA into CH-HG was confirmed by fluorescence microscopy. Antitumor efficacy was examined in mouse models of melanoma (A375SM) and breast (MDA-MD231) cancer.
Conclusions: This study developed a novel local delivery method for siRNA therapy using the CH-HG system. This approach could have broad applications for multiple localized diseases.
See commentary:
Hydrogel-siRNA for cancer therapy
Introduction
RNA interference (RNAi)-based approaches hold great potential for cancer therapy for targeted inhibition of gene expression at the post-transcriptional level of specific mRNA molecules.Citation1–Citation3 Therefore, siRNA-based therapy may allow development of a broad array of targeted drugs against pathogenic genes.Citation4 However, effective delivery of siRNA to target cells in vivo remains a significant challenge for realizing its full therapeutic potential because of degradation by RNases. Additionally, targeting and retention of siRNA in normal cells is problematic due to risk of off-target or side effects. Therefore, to overcome these limitations, novel delivery systems are needed.
For localized conditions, drug delivery systems formulated to deliver high drug concentrations for an extended time period could be an ideal strategy to maximize therapeutic benefit while avoiding side effects.Citation5 However, as drugs of low molecular weight pass rapidly into the bloodstream after intra-tumoral injection, and because the time period of retention in the tumor is quite short, new strategies to enhance drug delivery and therapeutic effects in tumor tissues are needed.
Here, we present a new method of siRNA delivery using chitosan hydrogel (CH-HG), which can be injected directly into the tumor site. CH-HG displays liquid-solid phase transition depending on temperature, thus CH-HG can be simply injected at the disease site without requirement for a surgical procedure. Chitosan is particularly attractive for clinical and biological applications due to its low toxicity, biocompatibility and biodegradability.Citation6,Citation7 Thus, CH-HG system should lead to enhanced concentrations of therapeutic payloads at tumor sites, minimize concerns about side effects and ultimately raise the therapeutic index. In addition, CH-HG can be gradually degraded by enzymes at tumor site after the drug is completely released.Citation7 While many cancers result in metastatic spread, there are several malignant (some skin, breast and brain cancers) and non-malignant (psoriasis and cervical dysplasia) diseases that could be amenable to treatment using CH-HG. Moreover, CH-HG could be useful in adjuvant therapy,Citation8 local treatment for chronic periodontitisCitation9 and pain control.
For proof-of-concept studies, we focused on targeting tissue transglutaminase (TG2), which is a critical gene in cancer growth and chemo-resistance.Citation10 TG2 has been shown to activate the RhoA and mitogen-activated protein kinase pathways, which control key downstream signaling steps that affect invasion and growth of malignant tumor cells. TG2 overexpression has been noted in several cancers including malignant melanoma,Citation11 breast,Citation12 ovarianCitation10 and pancreatic cancers.Citation13 Here, we applied siRNA and/or drug-loaded delivery using CH-HG as a novel local delivery system for therapeutic agents.
Results
Characteristics of CH-HG.
We first examined formation of CH-HG in tumor tissues after intra-tumoral injection in A375SM-bearing mice (). The CH solution () displayed a liquid-solid phase transition in a temperature-dependent manner and formed an endothermic hydrogel in tumor tissue after intra-tumoral injection ( and C). CH-HG including therapeutic payloads shows liquid phase at room temperature; however, it changes to solid phase following injection into the tumor. H&E staining confirmed CH-HG localization in tumor tissues ().
Release of siRNA from CH-HG.
We next examined in vitro release of Alexa555 siRNA from CH-HG. Substantial release of Alexa555 siRNA occurred within 12 h, followed by slower release over time (Sup. Fig. S1A). Prior to performing proof-of-concept in vivo efficacy studies, we tested the extent of in vivo delivery following a single intra-tumoral injection of Alexa555 siRNA/CH-HG into A375SM-bearing mice (). We first confirmed CH-HG localization into tumor tissues by H&E staining (). As shown in , the Alexa555 siRNA (red) demonstrated higher localization into tumor cells compared to control (hydrogel alone) or Alexa555 siRNA alone. The Alexa555 siRNA delivery extends to tumor cells outside of CH-HG and some tumor cells also infiltrated into CH-HG ( and C). In addition, we measured the distance of Alexa555 siRNA extension from CH-HG into the tumor (). Alexa555 siRNA extended >270 µm from CH-HG at 24 h and penetrated into the tumor cells outside of CH-HG (Sup. Fig. S1B).
Antitumor efficacy of CH-HG loaded with TG2 siRNA.
To determine the effectiveness of gene silencing and potential therapeutic efficacy, we focused on TG2 due to its significant role in tumor growth and progression.Citation10 The A375SM and MDA-MB231 cell lines were selected for these experiments as they show high TG2 expression levels (Sup. Fig. S1C). Prior to in vivo experiments, we performed in vitro experiments using TG2 siRNA in the A375SM cells. TG2 expression was decreased by >60% at 48 h after TG2 siRNA transfection (Sup. Fig. S1D). For assessing in vivo gene silencing, a single intra-tumoral injection of TG2 siRNA/CH-HG (150 µg siRNA/kg body weight) was given into A375SM-bearing mice and tumors were harvested for TG2 expression by western blot. TG2 expression was significantly reduced (>80% at 2 days) in tumor tissues following treatment with TG2 siRNA/CH-HG compared to control siRNA/CH-HG (). Notably, TG2 expression level was suppressed by up to 50% until 10 days after a single injection of TG2 siRNA/CH-HG compared to control siRNA/CH-HG ().
We next examined the therapeutic efficacy of TG2 silencing with either TG2 siRNA/CH-HG or docetaxel plus TG2 siRNA/CH-HG in mice bearing A375SM and MDA-MB231 tumors. Two weeks following injection of tumor cells subcutaneously (A375SM) or into the mammary fat pad (MDA-MB231), mice were randomly allocated to the following groups (n = ten mice/group): (1) control siRNA/CH-HG, (2) TG2 siRNA/CH-HG, (3) docetaxel/CH-HG and (4) TG2 siRNA plus docetaxel with CH-HG. All mice were sacrificed when animals in any group became moribund (4–5 weeks after cell injection depending on the cell line). In the A375SM model, TG2 siRNA/CH-HG resulted in significant inhibition of tumor growth compared to control siRNA/CH-HG (48% reduction, p < 0.01). Notably, combination of TG2 siRNA and docetaxel with CH-HG showed the greatest inhibition of tumor growth compared to control siRNA/CH-HG (72% reduction, p < 0.001), TG2 siRNA/CH-HG (24% reduction, p < 0.03) and docetaxel/CH-HG (22% reduction, p < 0.01; ). In the MDA-MB231 model, TG2 siRNA/CH-HG showed significant inhibition of tumor growth compared to control siRNA/CH-HG (66% reduction, p < 0.001). Combination of TG2 siRNA and docetaxel with CH-HG showed the greatest inhibition of tumor growth compared to control siRNA/CH-HG (92% reduction, p < 0.001), TG2 siRNA/CH-HG (32% reduction, p < 0.004) and docetaxel/CH-HG (55% reduction, p < 0.001; ). There were no differences in total body weight, feeding habits or behavior between the groups, suggesting that there were no overt toxicities related to therapy. In addition, the body weight of mice in individual experimental groups was not significantly different, suggesting that the mice demonstrated no obvious side effects (Sup. Fig. S2). There was no inflammation at the injection site, and the animals maintained a healthy appearance after intra-tumoral injection of the CH-HG.
To determine biological effects of TG2 siRNA/CH-HG therapy in tumor tissues, we examined tumors for TG2 expression and markers of cell proliferation (Ki67), microvessel density (MVD, CD31) and apoptosis (TUNEL). In the A375SM model, TG2 siRNA/CH-HG treatment resulted in significant inhibition of cell proliferation (p < 0.001 vs. control siRNA/CH-HG), MVD (p < 0.001 vs. control siRNA/CH-HG) and increased apoptosis (p < 0.001 vs. control siRNA/CH-HG). The combination group of TG2 siRNA and docetaxel with CH-HG significantly reduced cell proliferation (p < 0.05) and MVD (p < 0.001) and increased apoptosis (p < 0.001) as compared to the control siRNA groups (). In the MDA-MB231 model, TG2 siRNA/CH-HG showed significant inhibition of cell proliferation (p < 0.003), MVD (p < 0.001) and increased cell apoptosis compared to control siRNA/CH-HG (p < 0.004). The combination group of TG2 siRNA and docetaxel with CH-HG showed further decreases in cell proliferation (p < 0.001) and MVD (p < 0.03) and increased apoptosis compared to the control siRNA/CH-HG (p < 0.04, Sup. Fig. S3A). To determine possible direct effects of CH-HG on the tumor stroma, the tumors were stained for α-SMA/Tunel. There was no significant increase in apoptosis of stromal cells (Sup. Fig. S3B). We also examined for macrophage infiltration into the tumors using a F4/80 immunostaining. There were no changes in macrophage infiltration following treatment with TG2 siRNA/CH-HG (Sup. Fig. S3C).
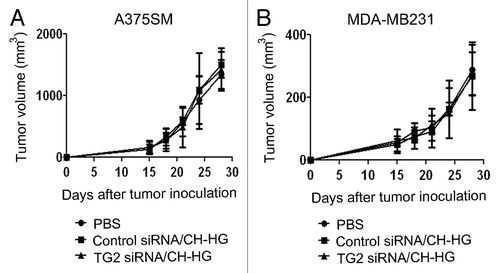
We also examined the therapeutic efficacy of TG2 silencing by naked TG2 siRNA to determine the importance of CH-HG as a delivery method. Two weeks following injection of tumor cells subcutaneously (A375SM) or into the mammary fat pad (MDA-MB231), mice were randomly allocated to the following groups (n = five mice/group): (1) PBS, (2) control siRNA and (3) TG2 siRNA. All mice were sacrificed when animals in any group became moribund (4–5 weeks after cell injection depending on the cell line). As shown in , treatment with naked TG2 siRNA alone through intra-tumoral injection had no significant antitumor effect compared to PBS or control siRNA treatment in both A375SM and MDA-MB231 models ( and B). These data suggest that incorporation of siRNA into CH-HG was indeed required for effective delivery and target modulation.
Discussion
RNAi-based cancer therapy is a highly specific method, but hurdles related to systemic in vivo delivery of siRNA need to be overcome to utilize siRNA in clinical settings. Local delivery systems have been considered to increase drug concentration or facilitate uptake into target tissue and to protect therapeutic payloads and inhibit nonspecific delivery.Citation14 Therapeutic payloads packaged into HG may be a clinically viable approach for the development of a local drug delivery system.Citation15,Citation16 The biocompatibility and biodegradability of these systems are key parameters for their eventual medical and pharmaceutical applications. Here, we demonstrate that a novel hydrogel (HG)-based local delivery system loaded with docetaxel and siRNA targeted to a key cancer associated gene leads to potent antitumor efficacy in pre-clinical models of melanoma and breast carcinoma. This study provides a rational approach to improving antitumor efficacy through combination therapy for localized diseases. In addition, the CH-HG system allows for co-delivery of therapeutic payloads, such as chemotherapy, that could further enhance therapeutic efficacy without increasing systemic exposure to the drug. Injection of CH-HG is quite simple because CH-HG is liquid phase at room temperature without need for a surgical procedure to insert hydrogel at the site of disease. Therefore, it does not disrupt the tumor environment. In addition, biodegradation rate of injected hydrogel into tumor tissue can be controlled by injection volume.Citation7,Citation17 In this study, we injected 30 µl of chitosan solution, which is likely to degrade within 3–4 days. Thus, this amount can be used for repeated injections of siRNA. This local delivery system may be attractive for many biomedical applications including infiltration of anesthetic agents after surgery and treatment of certain skin, breast and brain cancers. Such an approach may also be useful for adjuvant therapy,Citation8 or local treatment for chronic periodontitis.Citation9 For systemic treatment, we have already established other delivery methods such as DOPC nanoliposomes or chitosan nanoparticles.Citation4,Citation10,Citation18
One of the major impediments for successful cancer therapy is metastasis and emergence of drug resistance. TG2 is known to be overexpressed in most cancer cells, and is known to play a significant role in drug resistance and survival of cancer cells.Citation10 TG2 plays an important role in multiple steps in cancer growth and is an attractive therapeutic target. In the setting of localized diseases, delivery of a drug to a limited area may be preferable. Such delivery was highly effective in inhibiting tumor growth as demonstrated in the current study.
In summary, we have developed novel method for local delivery that offers the ability to achieve high concentrations of a drug at the local site of interest. Our data further demonstrate that CH-HG-based local siRNA delivery can achieve therapeutic efficacy without systemic side effects related to the biomaterials or drug. This CH-HG based local delivery strategy has broad potential as a delivery platform in human disease and represents an opportunity for further development of RNAi therapeutics.
Materials and Methods
Preparation of chitosan hydrogel (CH-HG) loaded with siRNA.
We have previously demonstrated the physical characteristics of CH-HG as an in vitro or in vivo depot system after intratumoral injection.Citation6,Citation7,Citation17 Briefly, CH solution (medium molecular weight of 161 kDa, viscosity of 200,000 cps and a degree of deacetylation of 80%) was obtained by dissolving 40 mg of CH in 1.8 ml of 0.1 M HCl solution. Glycerol 2-phosphate disodium salt hydrate (β-GP) solution containing siRNA and/or docetaxel was prepared by dissolving 0.2 g of β-GP and predetermined amount of drug in 0.2 ml of distilled water. The CH solution was cooled to 4°C and continuously stirred while adding 0.2 ml of β-GP and drug solutions were added. CH-HG was successfully formed at body temperature and physiological pH in vivo following intra-tumoral injection into tumor-bearing mice ().
Release of siRNA from CH-HG.
The release of Alexa555-labeled siRNA (Alexa555 siRNA) from CH-HG was examined by measuring the fluorescence intensity of Alexa555. One hundred micrograms of Alexa555 siRNA (1 µg/µl) was added in 2 ml of CH-HG solution at room temperature and the mixture was incubated at 37°C to prepare CH-HG. The release of Alexa555 siRNA from CH-HG was measured as a function of time using a fluorescence spectrophotometer. The emission and excitation wavelengths were 565 and 555 nm, respectively.
In vivo delivery of Alexa555 siRNA from CH-HG.
Detection of Alexa555 siRNA uptake from CH-HG was performed, as described previously in reference Citation18. Relevant tissues were harvested after single injection of either control siRNA/CH-HG or Alexa555 siRNA/CH-HG into A375SM-bearing mice. Alexa555 uptake was determined by assessing uptake by fluorescence microscopy of tumor tissues in five random fields at original magnification x200.
Cell lines and siRNA.
The derivation and source of the human melanoma (A375SM) and breast cancer (MDA-MB231) cell lines have been previously described in reference Citation12 and Citation19. The TG2 siRNA (target sequence: 5′-GGG CGA ACC ACC UGA ACA A-3′) and control siRNA (target sequence: 5′-UUC UCC GAA CGU GUC ACG U-3′) were purchased from Sigma.
Western blot analysis.
Preparation of cell and tumor tissue lysates has been previously described in reference 20. Protein concentrations were determined using a BCA Protein Assay Reagent Kit (Pierce Biotech, Rockford, IL) and aliquots of 20 µg protein were subjected to gel electrophoresis on 7.5 or 10% SDS-PAGE gels. Transfer to membranes and immunoblotting was performed as described previously in reference Citation20.
Antitumor efficacy.
Female athymic nude mice (NCr-nu) were purchased from the National Cancer Institute-Frederick Cancer Research and Development Center (Frederick, MD) and maintained as previously described in reference Citation21. All mouse studies were approved by the MD Anderson Cancer Center Institutional Animal Care and Use Committee. The mice used for in vivo experiments were 8–10 weeks old. To produce tumors, A375SM (2 × 106 cells per 50 µl HBSS) was injected subcutaneously (s.c.) and MDA-MB231 cells (2 × 106 cells per 20 µl HBSS) were inoculated into the mammary fat pad by a surgical incision. Mice (n = 10 per group) were monitored daily for adverse effects of therapy and were sacrificed when mice in any group appeared moribund.
To assess tumor growth, treatment began 2 weeks after cell injection into mice (around 5 mm tumor size). TG2 siRNA/CH-HG was given twice weekly at a dose of 150 µg/kg body weight through intra-tumoral injection.Citation10 Additionally, TG2 siRNA/CH-HG loaded with docetaxel was prepared for combination therapy. Docetaxel was given once a week at a dose of 100 µg. Treatment continued until mice became moribund (depending on tumor-cell). Mouse weight and tumor weight were recorded when the mice were sacrificed. The individuals who performed the necropsies, tumor collections and tissue processing were blinded to the treatment group assignments. Tissue specimens were fixed either with formalin or optimum cutting temperature solution (OCT, Miles, Inc., Elkhart, IN) or were snap frozen.
For determination of tumor growth, individual tumor size was measured with calipers and the tumor volume was calculated using the formula: Tumor Volume (mm3) = width × length2/2, as described previously in reference Citation6. In addition, the toxicity of CH-based formulations was also evaluated by determining the body weight of treated mice.
Immunohistochemical staining.
Immunohistochemical (IHC) analysis was performed on tumor tissues from mice that were treated by intra-tumoral injection of either siRNA/CH-HG or docetaxel + siRNA/CH-HG. Procedures for IHC analysis of TG2 expression (TG2 antibody), cell proliferation (Ki67) and microvessel density (CD31) were performed as described previously in reference Citation21. All of these analyses were recorded in five random fields for each slide at x200 magnification. In addition, terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) was performed as described previously to determine cell apoptosis.Citation21 The quantification of apoptotic cells was calculated by the number of apoptotic cells in five random fields at x200 magnification. All staining was quantified by two investigators in a blinded fashion.
Statistical analysis.
Differences in continuous variables were analyzed using the Student's t-test for comparing two groups and ANOVA was performed to compare differences for multiple group comparisons. For values that were not normally distributed, the Mann-Whitney rank sum test was used. The statistical package for the Social Sciences (SPSS, Inc.) was used for all statistical analyses. A p value of <0.05 was considered statistically significant.
Figures and Tables
Figure 1 Localization of CH-HG after intra-tumoral injection. 2 × 106 cells of A375SM in 50 µl were carefully inoculated into mice subcutaneously. After inoculation of tumor cells, 50 µl of CH-HG was injected by intra-tumoral injection. (A) CH-HG solution at room temperature, (B) A375SM tumor-bearing mouse, (C) hydrogel localization into tumor tissue, (D) H&E staining in tumor tissue (x4 magnification).
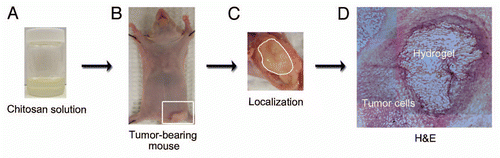
Figure 2 In vivo delivery of siRNA from CH-HG. Tumor tissues were harvested after single injection of either Alexa555 siRNA alone or Alexa555 siRNA/CH-HG into A375SM-bearing mice. Uptake was observed by fluorescence microscopy of Alexa555 siRNA in three random fields (red, magnification x200): (A) H&E staining and (B and C) Alexa555 siRNA delivery from CH-HG (x200 magnification). (D) siRNA delivery from CH-HG into tumor tissue. We measured the distance of Alexa555 siRNA extension from CH-HG to tumor cells. Quantitative difference was determined by measuring distance of Alexa555 siRNA from CH-HG. Error bars represent SEM. *p < 0.05.
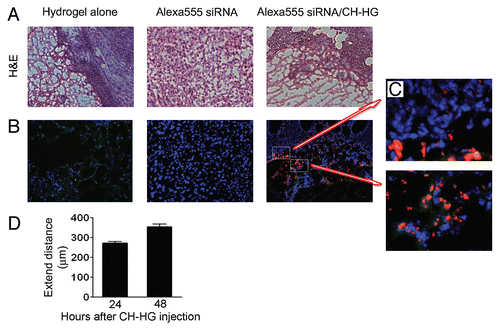
Figure 3 Antitumor efficacy of CH-HG. Effect of TG2 downregulation following intra-tumoral injection of TG2 siRNA and/or docetaxel with CH-HG into A375SM and MDA-MB231-bearing mice. (A) TG2 expression in A375SM tumor tissues was assessed after TG2 siRNA/CH-HG injection (20 µg of protein used). Quantitative differences were determined by densitometry analysis. (B and C) Treatment was started 2 weeks after inoculation of tumor cells into mice: (B) A375SM and (C) MDA-MB231. TG2 siRNA and/or docetaxel with CH-HG was given twice weekly at a dose of 150 µg/kg body weight (TG2 siRNA) through intra-tumoral injection. CH-HG loaded with docetaxel was injected once per week, at a dose of 100 µg. Treatment was continued until mice in any group became moribund (typically 4–5 weeks depending on tumor cell). Error bars represent SEM. *p < 0.05. (D) Immunohistochemical peroxidase analysis for TG2 expression (magnification x200), cell proliferation (Ki67, x200 magnification), microvessel density (CD31, x200 magnification) and TUNEL (x200 magnification) was performed on A375SM-tumor tissues following treatment with TG2 siRNA and/or docetaxel with CH-HG. All of these analyses were recorded in five random fields for each slide. Error bars represent SEM. *p < 0.05.
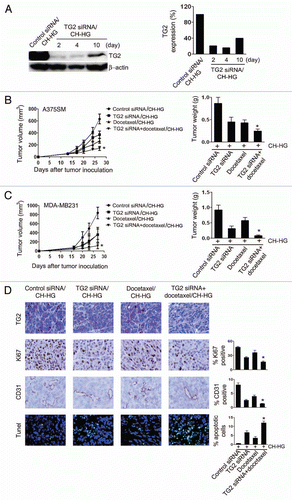
Figure 4 Antitumor efficacy of naked siRNA in A375SM and MDA-MB231-bearing mice by intra-tumoral injection. Treatment was started 2 weeks after tumor cells inoculation into mice. (A) A375SM and (B) MDA-MB231. TG2 siRNA was given twice weekly at a dose of 150 µg/kg body weight through intra-tumoral injection. Treatment was continued until mice in any group became moribund. Error bars represent SEM.
Additional material
Download Zip (1.2 MB)Acknowledgements
Portions of this work were supported by NIH grants (CA110793, P50 CA083639, CA109298, P50 CA098258, CA128797, RC2 GM092599 and U54 CA151668), DOD (OC-073399, W81XWH-10-1-0158 and BC085265), the Ovarian Cancer Research Fund, Inc. (Program Project Development Grant), the Zarrow Foundation and the Betty Anne Asche Murray Distinguished Professorship and the Laura and John Arnold Foundation. MMKS was supported by the Baylor WRHR grant (HD050128) and the GCF Molly-Cade ovarian cancer research grant.
References
- Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev 2007; 59:75 - 86
- Merritt WM, Lin YG, Han LY, Kamat AA, Spannuth WA, Schmandt R, et al. Dicer, Drosha and outcomes in patients with ovarian cancer. N Engl J Med 2008; 359:2641 - 2650
- Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov 2009; 8:129 - 138
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 2006; 12:939 - 944
- Walter KA, Tamargo RJ, Olivi A, Burger PC, Brem H. Intratumoral chemotherapy. Neurosurgery 1995; 37:1128 - 1145
- Han HD, Song CK, Park YS, Noh KH, Kim JH, Hwang T, et al. A chitosan hydrogel-based cancer drug delivery system exhibits synergistic antitumor effects by combining with a vaccinia viral vaccine. Int J Pharm 2008; 350:27 - 34
- Seo SH, Han HD, Noh KH, Kim TW, Son SW. Chitosan hydrogel containing GMCSF and a cancer drug exerts synergistic anti-tumor effects via the induction of CD8+ T cell-mediated anti-tumor immunity. Clin Exp Metastasis 2009; 26:179 - 187
- Willmott LJ, Monk BJ. Cervical cancer therapy: current, future and anti-angiogensis targeted treatment. Expert Rev Anticancer Ther 2009; 9:895 - 903
- Johannsen A, Tellefsen M, Wikesjo U, Johannsen G. Local delivery of hyaluronan as an adjunct to scaling and root planing in the treatment of chronic periodontitis. J Periodontol 2009; 80:1493 - 1497
- Hwang JY, Mangala LS, Fok JY, Lin YG, Merritt WM, Spannuth WA, et al. Clinical and biological significance of tissue transglutaminase in ovarian carcinoma. Cancer Res 2008; 68:5849 - 5858
- Fok JY, Ekmekcioglu S, Mehta K. Implications of tissue transglutaminase expression in malignant melanoma. Mol Cancer Ther 2006; 5:1493 - 1503
- Mangala LS, Fok JY, Zorrilla-Calancha IR, Verma A, Mehta K. Tissue transglutaminase expression promotes cell attachment, invasion and survival in breast cancer cells. Oncogene 2007; 26:2459 - 2470
- Verma A, Wang H, Manavathi B, Fok JY, Mann AP, Kumar R, et al. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res 2006; 66:10525 - 10533
- Varghese OP, Sun W, Hilborn J, Ossipov DA. In situ cross-linkable high molecular weight hyaluronan-bisphosphonate conjugate for localized delivery and cell-specific targeting: a hydrogel linked prodrug approach. J Am Chem Soc 2009; 131:8781 - 8783
- Krebs MD, Jeon O, Alsberg E. Localized and sustained delivery of silencing RNA from macroscopic biopolymer hydrogels. J Am Chem Soc 2009; 131:9204 - 9206
- Singh A, Suri S, Roy K. In-situ crosslinking hydrogels for combinatorial delivery of chemokines and siRNA-DNA carrying microparticles to dendritic cells. Biomaterials 2009; 30:5187 - 5200
- Han HD, Nam DE, Seo DH, Kim TW, Shin BC, Choi HS. Preparation and biodegradation of thermosensitive chitosan hydrogel as a function of pH and temperature. Macromol Res 2004; 12:507 - 511
- Landen CN Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res 2005; 65:6910 - 6918
- Scott J, Dorr RT, Samulitis B, Landowski TH. Imexon-based combination chemotherapy in A375 human melanoma and RPMI 8226 human myeloma cell lines. Cancer Chemother Pharmacol 2007; 59:749 - 757
- Mangala LS, Zuzel V, Schmandt R, Leshane ES, Halder JB, Armaiz-Pena GN. Therapeutic Targeting of ATP7B in Ovarian Carcinoma. Clin Cancer Res 2009; 15:3770 - 3780
- Lee JW, Han HD, Shahzad MM, Kim SW, Mangala LS, Nick AM, et al. EphA2 immunoconjugate as molecularly targeted chemotherapy for ovarian carcinoma. J Natl Cancer Inst 2009; 101:1193 - 1205