Abstract
Commentary to:
Chitosan hydrogel for localized gene silencing
Hee Dong Han, Edna M. Mora, Ju Won Roh, Masato Nishimura, Sun Joo Lee, Rebecca L. Stone, Menashe Bar-Eli, Gabriel Lopez-Berestein and Anil K. Sood
Introduction
An ideal cancer therapy should selectively kill the tumor cells without any collateral damage to normal cells. A number of strategies are being developed to achieve this goal. RNA interference (RNAi), a targeted therapeutic method, is used to knock down or silence key regulatory molecules that are essential for the survival, migration and invasion of cancer cells. Gene silencing can be accomplished by several ways. One approach is to use anti-sense oligos to block the translation of a target mRNA. The second and most widely used strategy is to deliver short interfering RNA (siRNA) into cells. siRNA is usually a 21-nucleotide RNA duplex containing a 2-nucleotide 3′ overhang. Once delivered into the cell, siRNA is assembled into RNA-induced silencing complex (RISC), which is composed of many proteins, including p54 and Argonaute. Activation of the RISC complex results in selection of one of the strands of the RNA duplex (guide RNA) on the basis of thermodynamic instability of the 5′ end. The antiguide strand is destroyed in the RISC. The RISC complex, with the guide strand, then binds to a complementary sequence in mRNA and cleaves the target mRNA. An alternate method to siRNA is to use short hairpin RNA (shRNA). shRNA is delivered into cells as DNA templates using either a plasmid construct or a viral vector. Vector designs incorporate the U6 promoter to drive efficient transcription by RNA polymerase III. The resultant transcript, ranging from fifty to one hundred nucleotides in length, forms a short hairpin loop. Unlike siRNA, shRNA needs to be processed within the target cells before being loaded onto the RISC. Processing is mediated by Drosha in the nucleus and then transported to the cytoplasm by Exportin 5. In the cytoplasm, the DICER complex trims the shRNA further to generate a double stranded RNA, with a 2-nucleotide overhang that resembles siRNA.
Recent studies have shown that the endogenously generated, non-coding RNA (microRNA) can also be used to silence target genes. MicroRNAs (miRNA) are about 22-nucleotides long. They are transcribed as 60–70 nucleotide-long primary miRNAs, which are then processed into pre-miRNA and miRNA by Drosha and Dicer, respectively. miRNAs bind to the 3′UTR of target messenger RNAs and silence these targets by multiple mechanisms, which include degradation, deadenylation and inhibition of translation initiation. A single miRNA can target several gene transcripts depending on sequence complementarity. Conversely, any given mRNA can have multiple miRNA target sites. Thus, miRNA-mediated effects are pleotropic in nature.
siRNA and Cancer Therapy
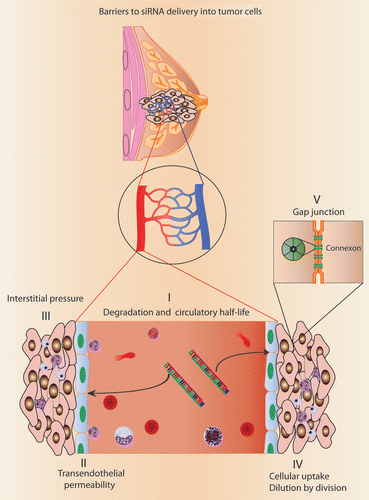
The siRNA-based gene silencing method is highly selective and efficient. However, clinical translation of this approach faces formidable challenges to overcome.Citation1–Citation3 A summary of some of the barriers limiting the efficacy of a systemically administered siRNA is shown in . Unlike systemic delivery, bioavailability is not a problem for siRNA-mediated therapy of localized diseases (e.g., intravitreal and intrathecal sites). However, sustained delivery of siRNA is necessary for efficient gene silencing. Continuous release of siRNA into the local milieu can be accomplished by formulating siRNA into biocompatible and biodegradable matrices. Examples of such components include Alginate (polysaccharide isolated from the cell wall of brown algae), which can polymerize in the presence of calcium or a photoactivable derivative of Alginate.Citation4 Additionally, collagen has been used to prepare siRNA-containing hydrogels. One of the major disadvantages of using collagen is its potential immunogenicity and proinflammatory properties. In the current issue of Cancer Biology & Therapy, Han et al. describe an alternative to collagen-based hydrogels by exploiting the temperature dependent gelling properties of a Chitosan Hydrogel (CH) for siRNA delivery.Citation5 Chitosan (a linear polysaccharide derivative prepared from the exoskeleton [chitin] of crustaceans) hydrogel is biodegradable and biocompatible with no immunoinflammatory response in vivo. These properties are ideal for the local delivery of siRNA. In addition to siRNA containing CH, they have also prepared CH that contained docetaxel as a payload. These preparations were used to determine the efficacy of local, sustained delivery of siRNA to inhibit the growth of tumors in mice. As a proof-of-principle study, Han et al. targeted transglutaminase 2 (TG2) with siRNA. TG2 is overexpressed in metastatic cancer cells and is associated with chemoresistance. In contrast to naked siRNA directed to TG2, CH preparations effectively inhibited tumor growth. When the TG2-siRNA treatment was combined with CH-docetaxel, there was a significant improvement in antitumor activity. These studies validate the versatility of a chitosan hydrogel matrix for the delivery of siRNA and other chemotherapeutic drugs. Samples mixed with the CH remained in solution phase at room temperature and then transitioned into a semi-solid gel when injected into the tumor tissue. A thermogelling property of CH simplifies the practicality of this approach for in situ delivery of siRNA. Furthermore, CH-siRNA showed significant tissue penetration (about 25 cells or 270 nm) making it an ideal vehicle for sustained local release thereby, negating the need for repeated injections.
Future Directions and Challenges
Local delivery of siRNA, however, has limited clinical applications. It is important to develop novel vehicles for the systemic delivery of therapeutic payloads. Major advances have been made in the chemical modification and encapsulation strategies to improve bioavailability of systemically delivered siRNA. Tumor selective uptake of siRNA still remains a major challenge. It is exciting to note that some of the recent developments in ‘sonodynamic therapy’ can be used to facilitate local availability of siRNA at the tumor tissue. Ultrasound Contrast agents (microbubbles, 2.5–4.0 um in diameter) contain high molecular weight gases that are least soluble and diffusible in biofluids and are found to be excellent carriers for therapeutic gene delivery. Their smaller size precludes them from getting trapped in highly vascularized tissues such as liver and lungs. Microbubbles can be decorated with targeting moieties for selective homing onto tumor tissues. Targeted microbubbles are successfully used for anticancer gene therapy (reviewed in ref. Citation6). Microbubbles can be burst open to release their cargo at a local site by ultrasound (clinically applicable). Phase III clinical trials have shown that microbubbles are safe for systemic delivery and stay intact for several passages in circulation.Citation7 It remains to be seen whether siRNA can be packaged into microbubbles and released locally by ultrasonic waves at tumor tissues.
Endothelial barriers limit how much of the encapsulated siRNA can reach tumor cells. Transendothelial transport of siRNA via carriers needs further improvement. Tumor endothelium is leaky and fenestrated when compared to the vasculature of the surrounding normal tissue. Tumor vascular endothelium abundantly expresses certain integrins, which can be targeted for improved delivery of encapsulated siRNA. In addition to increasing tissue specific accumulation, it is important to distribute the siRNA into the entire tumor mass for an effective therapy. Dispersion into the tumor is prevented by higher interstitial fluid pressure within the tumors. This problem can be overcome by ‘normalizing’ the tumor vasculature using anti-angiogenic or vascular disrupting agents to improve siRNA delivery into tumors.
The success of siRNA-mediated cancer therapy is highly dependent on delivering the therapeutic siRNA into all of the tumor cells. If only a fraction of the tumor cells were transduced with siRNA, the neighboring cells could escape from selection pressure and continue to grow. This issue can be addressed by modulating intercellular connections and gap junction function. The cytoplasm of a cell is separated from an adjacent cell (4 nm) by specialized intercellular connections at the gap junctions. Each gap junction is made of a connexon, in which, half comes from each of the two adjoining cells. Connexons are made of six homomeric or heteromeric subunits, forming a channel between two cells. Connexons are believed to allow molecules less than 1,000 Daltons to permeate through its hydrophilic channel. Because siRNA are much larger, with an average molecular weight of 13,000 to 14,000 Daltons, they are not likely to be transported across connexons. Two recent publications challenge this notion by elucidating intercellular transfer of microRNAs,Citation8,Citation9 which are similar in size to siRNA. Tumor cells express different connexins (subunits of connexons) and the composition of a connexon may very well decide intercellular transfer of siRNA. Alternatively, cytoplasmic siRNA can be exocytosed and taken up by neighboring cells. Further studies to exploit these cellular phenomena can improve delivery of siRNA into all tumor cells. siRNA-based gene silencing methods hold greater promise for cancer therapy in the near future. Metastatic growth and chemoresistance of cancer cells can be eliminated by improving siRNA delivery methods and by modulating the tumor microenvironment.
Figures and Tables
Figure 1 Schematic representation showing barriers to siRNA-mediated cancer therapy. Stability problems associated with naked siRNA delivery can be prevented by chemical modifications and encapsulation. Improved delivery of siRNA has been achieved by preparing nanocrystals (e.g., Graphene oxide), nanocarriers and microcarriers. Cellular uptake of the encapsulated siRNA is dependent on hydrophobicity and endocytosis. Biodegradable and biocompatible Hydrogel preparations are used for the sustained release of therapeutic siRNAs.
Acknowledgements
This work is supported by a grant from NIH (CA 114340) and the Sparboe Endowment for Women's cancer research. I want to thank Hemant Joshi and Urmila Ramakrishnan for editorial help.
Commentary to:
References
- Chowdhury EH. Strategies for tumor-directed delivery of siRNA. Expert Opin Drug Deliv 2011; 8:389 - 401
- Katakowski JA, Palliser D. Optimizing siRNA delivery to the genital mucosa. Discov Med 2011; 11:124 - 132
- Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. RNA interference in the clinic: challenges and future directions. Nat Rev Cancer 2010; 11:59 - 67
- Krebs MD, Alsberg E. Localized, targeted and sustained siRNA delivery. Chem Eur J 2011; 17:3054 - 3062
- Han HD, Mora EM, Roh JW, Nishimura M, Lee SJ, Stone RL, et al. Chitosan hydrogel for localized gene silencing. Cancer Biol Ther 2011; 11:839 - 845
- Dash R, Azab B, Shen XN, Sokhi UK, Sarkar S, Su ZZ, et al. Developing an effective gene therapy for prostate cancer: New technologies with potential to translate from the laboratory into the clinic. Discov Med 2011; 11:46 - 56
- Bhatia VK, Senior R. Contrast echocardiography: evidence for clinical use. J Am Soc Echocardiogr 2008; 21:409 - 416
- Katakowski M, Buller B, Wang X, Rogers T, Chopp M. Functional microRNA is transferred between glioma cells. Cancer Res 2010; 70:8259 - 8263
- Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA, et al. Gap junction-mediated import of microrna from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res 2011; 71:1550 - 1560