Abstract
bcr/abl fusion gene is thought to be a promising target for chronic myelogenous leukemia (CML) patients to enhance immune response after attaining complete remission. In this study, we sought to enhance cellular immunity by co-expression of BCR/ABL and murine IL-12 gene on the tumor cell surface as a glycosyl-phosphatidylinositol (GPI)-form. The successfully constructed plasmid pBudCE4.1-BCR/ABL-GPI-mIL12 resulted in high levels of splenocyte proliferative responses, significant levels of IL-2 and IFNγ, and strong cytotoxic T-lymphocyte (CTL) responses in vitro. In a murine transplant model, the vaccinated mice showed decreased infiltration of leukemia cells and reduced expression of BCR/ABL transcripts and protein in bone marrow cells. Results of the present study indicated that this novel immunization strategy is useful in enhancing immune protection in mice, which would provide new insights into the development of effective vaccines for treating CML.
Introduction
The molecular mechanism responsible for chronic myelogenous leukemia (CML) is the bcr/abl oncogene, which arises from a t (9; 22) (q34; q11) reciprocal translocation commonly referred to as the Philadelphia chromosome (Ph).Citation1 The encoded BCR/ABL fusion protein and its constitutively activated tyrosine kinase activity are essential for malignant progression in CML.Citation2 Hence, the tumor-specific BCR/ABL protein is a favored target for the development of new therapeutics for the treatment of CML. Furthermore, previous studies demonstrate that the BCR/ABL protein may function as a tumor-specific antigen and T cells, which generated specific for this neoantigen, or peptides deduced from its sequence can recognize CML cells expressing BCR/ABL protein.Citation3-Citation6 Based on these results, how to elicit CML-specific T cells capable of eliminating the malignant cells is one of the greatest challenges in treating CML. Thus, peptides plus adjuvants or dendritic cells (DC) generated from Ph positive cells expressing the BCR/ABL products were used as vaccine.Citation4
As is well known, the induction of a specific antitumor immune response is critical for the activation and proliferation of tumor-specific T cells.Citation7 Engagement of the T-cell receptor with antigen-MHC complexes provides the initial signal. Although the BCR/ABL protein has an intracellular location, enzymatic degradation products of the protein could be presented on the cell surface as short peptides and thus recognized by T cells.Citation8 However, only a minority of BCR/ABL fusion peptides contain suitable amino acid sequence for binding to class I molecules and bind with either intermediate or high affinity to purified HLA A3.2, A11 and B8 molecules,Citation8 which can’t be presented continually to produce long-lasting antitumor immune responses. In addition, more and more evidence shows that CML patients in chronic phases had reduced numbers of circulating DCs,Citation9 which always had a lower expression of the co-stimulatory molecules CD80/CD83/CD40 compared with control DCs.Citation10 For T cells to proliferate and respond to an antigen, the absence of co-stimulation during T-cell recognition of a specific antigen results in T-cell unresponsiveness.Citation11 By lacking these co-stimulatory molecules, CML cells may thus escape host immunity. Studies have shown that co-delivery of genes of immunologic molecules such as cytokines and co-stimulatory molecules can drive immune responses in a particular direction. IL-12, which is a 70 kDa heterodimer protein, stimulates T cells and NK cells to increase the secretion of IFNγ and promotes cytotoxic activity by NK cells. As a stimulator of the cellular responses, IL-12 has potential efficacy in malignant diseases. It has been reported that IL-12 might be an effective immune adjuvant for vaccination therapy.Citation12-Citation14
Based on this previous evidence, in this study, we developed a membrane anchored BCR/ABL and murine IL-12 co-expression DNA vaccine by the glycosyl-phosphatidylinositol (GPI). GPI anchoring has been established as a unique mode of protein binding to the plasma membrane via a common lipid structure.Citation15 This GPI-mediated approach by protein transfer has been found to be effective in stimulating immune responses for the co-stimulatory molecules and cytokines.Citation16-Citation19 By applying this approach, we found that when the GPI-anchored BCR/ABL and mIL-12 were injecting into mice, the constructed plasmid expressed BCR/ABL on the cell surface in the GPI-anchored form and was functional and induced a powerful antitumor immunity in bone marrow transplant model with CML-like syndrome.
Results
PBBGI transfected cells express GPI-anchored BCR/ABL protein on the cell surface
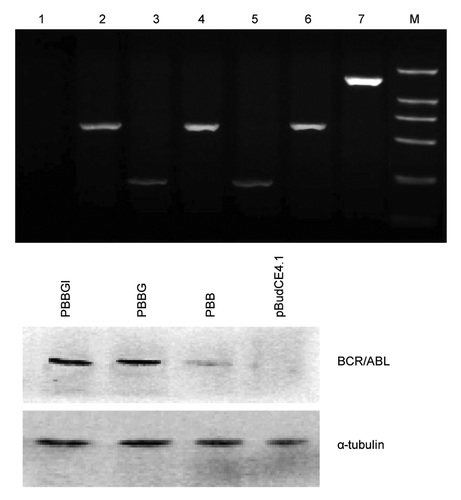
We constructed the plasmids PBB, PBBG and PBBGI, and successfully characterized them by endonuclease digestion analyses and DNA sequencing (data not shown). Subsequently, the plasmids were transiently transfected into the COS-7 cells and the inserted fragments BCR/ABL, GPI and mIL-12 were transcribed normally in COS-7 cells, as detected by RT-PCR shown in . Moreover, protein gel blot was performed to detect GPI-anchored BCR/ABL expressing on COS-7 cell membrane (). To examine mIL-12 expression in transfected COS-7 cells, the supernatants were collected 72 h after transfection. ELISA assay results showed that the mIL-12-expressing level in COS-7 cells transfected with PBBGI was significantly higher than that transfected with PBBG and PBB (p < 0.05, data not shown). All these results demonstrate that the constructed plasmids were stable enough to maintain its genetic stability.
Figure 1. Identification of BCR/ABL and mIL-12 expression in COS-7 cells. (A) Results of RT-PCR in the transfected COS-7 cells. Cells either transfected with pBudCE4.1 (lane 1), PBB (lane 2), PBBG (lane 3 and lane 4), PBBGI (lane 5, lane 6 and lane 7). M: DL2000 (B) Results of protein gel blot in the transfected COS-7 cells. Cells were treated as indicated. The amount of α-tubulin was used as an internal control. PBB, pBudCE4.1-BCR/ABL; PBBG, pBudCE4.1-BCR/ABL-GPI; PBBGI, pBudCE4.1-BCR/ABL-GPI-mIL12.
PBBGI induced cell-mediated immunity in BALB/c mice
In order to test whether PBBGI maintain its biological function, splenocyte proliferation was studied 30 d after the last vaccination. As shown in , the four groups all produced vigorous lymphocyte responses compared with negative control (p < 0.01). In addition, in mice immunized with PBBGI and PBBG, the levels of splenocyte proliferation were significantly higher than those immunized with PBB (p < 0.05).
Table 1. Sequences of primers used in PCR and the anticipated base pair sizes of the PCR products
The cell-mediated immunity produced in the immunized mice was indirectly evaluated by measuring the amounts of cytokines released in the supernatants from the cultures of ConA-stimulated splenocytes. As shown in , PBBGI significantly increased the production of IL-2 and IFNγ as compared with PBBG and PBB (p < 0.05). This profile of cytokine secretion suggests that the co-expression of BCR/ABL and IL-2 on the cell surface can enhance Th1-type immunity.
Figure 2. Cytokine production from splenocytes and CTL activity after ConA stimulation. Mice were administered plasmid pBudCE4.1, PBB, PBBG, PBBGI or PBS. Splenocytes were harvested for cytokine production (A) and CTL activity (B) 30 d post-vaccination. Statistically significant differences are indicated by * compared with PBS control group (p < 0.01), or # compared with PBBG (p < 0.05). (C) Results of protein gel blot in the stably transfected SP2/0 cells. pBudCE4.1, pBudCE4.1-treated group; PBB, pBudCE4.1-BCR/ABL-treated group; PBBGI, pBudCE4.1-BCR/ABL-GPI-mIL12-treated group; PBS, PBS-treated group.
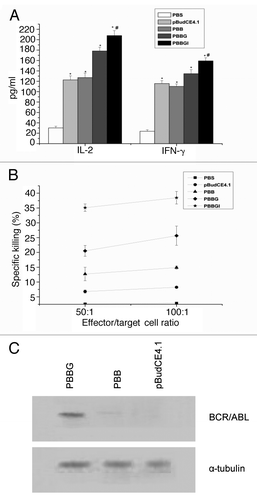
Next, we determined the CTL ability in mice immunized with the mentioned plasmids. Spleen lymphocytes from mice injected with PBBGI, PBBG, PBB or pBudCE4.1 were assayed for their cytolytic activity against syngeneic BALB/c-derived SP2/0 cells transfected with PBBG which stably expressed BCR/ABL protein on the cell membrane (). As shown in , spleen lymphocytes from PBBGI and PBBG group demonstrated a high degree of CTL activity against BCR/ABL-expressing SP2/0 cells, compared with PBB-injected groups (p < 0.05).
Antitumor immune response induced by PBBGI
To investigate the therapeutic value of PBBGI in bone marrow transplant model with CML-like syndrome, the survival condition of each group was monitored after immunization. One mouse died in pBudCE4.1 group and one in PBS control group, 3 d and 12 d after post-vaccination, respectively. No obvious change in body weight was found in each group (data not shown). Blood smears and histological section analyses of immature leukemia cells were performed. The histological section of mice immunized with PBBGI revealed that the level of leukemia cell infiltration was significantly reduced by PBBGI, whereas only moderately reduced by PBBG (). Peripheral WBC counts were also decreased after immunization with PBBGI and PBBG (). In all mice, differential counts showed that the percentage of immature neutrophilic granulocytes in peripheral blood decreased significantly after immunization with PBBGI, PBBG and PBB (and). RT-PCR and protein gel blot analysis were conducted to explore the differential mRNA and protein level of oncogenic BCR/ABL in immunized mice. The results reveal that PBBGI and PBBG can reduce BCR/ABL expression in bone marrow cells to some degree ().
Figure 3. Characteristics of the immunized murine transplant model. (A) Peripheral blood smear before and after immunization with PBBGI (1000 × , Wright staining) (B) HE-stained section of spleen (250 × ). Bone marrow cells were harvested for detecting the change of BCR/ABL transcripts (C) and protein (D). pBudCE4.1, pBudCE4.1-treated group; PBB, pBudCE4.1-BCR/ABL-treated group; PBBGI, pBudCE4.1-BCR/ABL-GPI-mIL12-treated group; PBS, PBS-treated group; Normal, normal BALB/c mice.
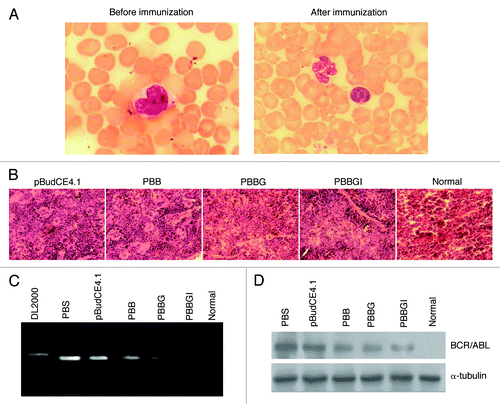
Table 2. Splenocyte proliferation responses in mice immunized with pBudCE4.1, PBB, PBBG, PBBGI or PBS as control
Discussion
In CML, the tyrosine kinase activity of the BCR/ABL fusion protein causes malignant transformation of the CML blasts through deregulation of proliferation, apoptosis and adhesion control pathways. In addition, BCR/ABL represents a unique antigen which offers the opportunity to be specifically targeted by drug-based or immunotherapeutic strategies. Imatinib mesylate (Glivec or Gleevec, Novartis), which was designed as a selective competitive inhibitor of the ABL protein-tyrosine kinase, is a good example of drug-based targeted therapy strategy.Citation20
Another strategy to use of this tumor-specific determinant is to elicit CML-specific T cells capable of eliminating the malignant cells. Accordingly, Osman et al.Citation21 showed that DCs pulsed with BCR/ABL (b3a2) peptide could elicit a potent b3a2-specific CTL response in vitro, which indicated that BCR/ABL can be used as a unique target recognizing by T cells, thus inducing cell-mediated specific immunity. Therefore, BCR/ABL DNA and protein vaccines, synthetic BCR/ABL peptide vaccines or DCs pulsed with these BCR/ABL-derived antigens was used in the treatment of CML.Citation22 However, there still exists many problems in this BCR/ABL-derived antigen induced immune therapy, such as reduced numbers of circulating DCs,Citation9 lower expression of co-stimulatory molecules in DCs,Citation10 and selective affinity with a minority of HLA molecules,Citation8 which were critical factors limiting its clinical application.
In an effort to overcome these problems, GPI-anchored protein transfer method was utilized for co-expression of BCR/ABL and mIL-12 on the cell surface. GPI is a post-translationally added lipid anchor and uses a common lipid structure to attach to the cell membranes, irrespective of the proteins links with it.Citation15,Citation23 A number of proteins commonly expressed by cells are attached to the cell membranes via GPI anchors, including enzymes, encoding proteins, surface antigens, cytokines and adhesion molecules.Citation24 GPI anchor signal sequences have been identified for many proteins such as DAF and leukocyte function antigen-3.Citation25 In the present study, we attached a GPI-anchor signal sequence which obtained from CD24 molecule to the intracellular located protein BCR/ABL to convert BCR/ABL to a GPI-anchored form.Citation26 As expected, our RT-PCR and protein gel blot results demonstrated that the fusion protein expressed by COS-7 and SP2/0 cells that were stably transfected with the fusion gene is anchored on the cell surface (and).
It is reported that IL-12 can promote specific antitumor immunity mediated by T cells in several tumors,Citation11,Citation27 through increasing cytokine production, particularly IFNγ, proliferation and cytotoxicity. Nevertheless, systemic administration of IL-12 is associated with toxicity, hampering its further application in clinical. Recently, the application of IL-12 as an immune adjuvant has received attention. Therefore, IL-12 was used as a DNA vaccine adjuvant in our study. The expression of IL-12 was detected in the supernatant of COS-7 cells after transfected with PBBGI, indicating that some of the proteins were released from the cells. To further verified the biological function of PBBGI, splenocyte proliferation ability, cytokine production and CTL activity were studied 30 d after the last vaccination with PBBGI, PBBG, PBB, and the vector counterpart pBudCE4.1 in BALB/c mice. Mice vaccinated with PBBGI and PBBG developed a strong increase of splenocyte proliferative response (p < 0.05), compared with the mice vaccinated with PBB. The production of cytokines in spleen cells were determined by ELISA, suggesting that significant levels of IL-2 and IFNγ were produced in the supernatants of stimulated splenocyte cultures from mice immunized with PBBGI (p < 0.05). Moreover, the immunization of mice with PBBGI and PBBG produced stronger cytotoxic T-lymphocyte (CTL) response than the other groups (, ). These results clearly demonstrated that the GPI-anchored expression of BCR/ABL on the cell surface significantly augmented Th1-type cellular immune responses in BALB/c mice.
In order to investigate whether there is a synergistic antitumor effect when both GPI-anchored BCR/ABL and IL-12 are present simultaneously, we compared the therapeutic value of PBBGI and PBBG in bone marrow transplant model with CML-like syndrome. Consistent with the induced immune response in BALB/c mice, our data illustrate that PBBGI and PBBG could reduce the WBC counts and percentage of immature neutrophilic granulocytes (), downregulate the expression level of BCR/ABL transcripts and protein in bone marrow cells (), and alleviate the infiltration of leukemia cells in peripheral blood and spleen (), while the antitumor effect of PBBGI is more obvious than PBBG. Taken together, it seems that IL-12 gene co-expression with BCR/ABL might participate in inducing a more pronounced antitumor immune response.
In conclusion, the preliminary results presented here suggest that GPI-anchored BCR/ABL and mIL-12 can enhance distinctly the Th1-type immunity and has evident immunotherapeutic value in the treatment of CML. Nevertheless, the toxicity and dosage of PBBGI, which work as a DNA vaccine, needs further confirmation in animal models before its clinical application. Furthermore, as can be seen from , PBBGI cannot eliminate the expression of BCR/ABL completely which may contribute to the relapse and progression of CML. These questions should be considered in further in vivo studies. Even though the comprehension of the exact mechanism needs more investigations, we believe that the discovery in the present study could be valuable for the improvement of future CML therapy.
Materials and Methods
Animals and cell culture
Female BALB/c mice, 6–8 weeks old, were purchased from the Center of Experimental Animals, Chongqing Medical University, China. All animal experiments were approved by the Center of Laboratory Animals in Chongqing Province. COS-7 cells and mouse myeloma cell line SP2/0 were provided by Molecular Biology Laboratory of Infectious Diseases (Chongqing Medical University, China) and maintained in RPMI 1640 medium, supplemented with 10% fetal calf serum, 2 mM glutamine and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) at 37°C in a humidified atmosphere of 5% CO2.
Construction of GPI-anchored BCR/ABL and IL-12 expression vectors
The mammalian expression vector pBudCE4.1 for simultaneous expression of two genes was purchased from invitrogen life technologies company, USA. BCR/ABL-expressing retroviral vector MIGR1-p210 was a gift from Dr. Warren S. Pear. The plasmid pcIL-12 coding for IL-12 was provided by Dr. Jianming Zeng. cDNA fragment (654 bp) encoding the BCR/ABL was generated by PCR from MIGR1-p210 using primer bcr/abl-forward and bcr/abl-reverse and inserted into the Hind III/SalI sites of pBudCE4.1, resulting in plasmid pBudCE4.1-BCR/ABL (PBB). To insert GPI into PBB, lymphocytes were purified from human peripheral blood mononuclear cells and GPI-anchor signal sequence of CD24 was cloned and inserted into SalI/ BamH I sites of pBudCE4.1 to obtain plasmid pBudCE4.1-BCR/ABL-GPI (PBBG). To construct the GPI-anchored mouse IL-12 expression vector, cDNA encoding the murine IL-12 was amplified from pcIL-12 and inserted into PBBG between the BglII and Not I sites. Therefore, the generating plasmid pBudCE4.1-BCR/ABL-GPI-mIL12 (PBBGI) will express the GPI-anchored BCR/ABL and mIL-12 simultaneously. The sequence for each construct was confirmed by DNA sequencing analysis. All the sequences synthesized to construct the above plasmids were shown in .
Table 3. Peripheral WBC counts and percentage of immature neutrophilic granulocytes before and after immunization
Immunization
Mice were randomly assigned to one of the following administration groups: (1) received pBudCE4.1, (2) received PBB, (3) received PBBG, (4) received PBBGI, (5) received PBS as control. All mice were immunized with the mixture of 100 μg plasmid and 0.75% lidocaine in a ratio of 1:4 into both hind legs three times every 10 d. Animals were killed 30 d after the final immunization.
Lymphocyte proliferation assay
Splenocytes suspensions were prepared from mice at day 30 post-vaccination, and 3 × 105 cells/well were cultured in 96-well plates in triplicate in RPMI-1640. Cultures were stimulated with 5 μg/ml concanavalin A (ConA) for 68 h at 37°C in 5% CO2. Controls were left unstimulated. Ten microliters of 5 mg/ml methyl thiazolyl tetrazolium (MTT; Sigma) was then added to each well and incubated for another 4 h. Supernatants were then removed from each well and discarded. Following addition of 150 μl dimethyl sulfoxide (DMSO) to solubilize the sediment, absorbances were read at 570 nm in a Bio-Rad ELISA reader. Lymphocyte proliferative responses were quantitated by stimulation index (SI), calculated as the ratio of OD570nm of stimulated cells to OD570nm of unstimulated ones.
Cytokine assays
To evaluate cytokine production levels in vivo, spleen lymphocytes were collected and stimulated with 5 μg/ml ConA. Cell-free supernatants were harvested and assayed for IL-2 activity at 24 h, and for IFNγ activity at 72 h. Cytokine quantification was performed with a cytokine ELISA kit (Shenzhen Jingmei Bio-engineering Co., Ltd.). Cytokine concentrations were determined by reference to standard curves constructed with known amounts of mouse recombinant IL-2 or IFNγ.
CTL assay
Four weeks after vaccination, spleens were removed for the preparation of single-cell suspensions. Spleen lymphocytes were co-cultured with 5 μg/ml ConA in each well of a 96-well plate. Three days later, spleen lymphocytes were harvested and used as effector cells. SP2/0 cells transfected with PBBG were used as target cells. The effector cells were incubated with the target cells at two different effector/target (E/T) ratios of 50:1 and 100:1 for 24 h at 37°C in 5% CO2. At every E/T ratio, triplicate wells of effector and target cells alone were established in parallel. CTL cytotoxicity was detected by MTT assay and its killing effect was calculated by the formula: Killing effect (%) = [1-(OD570nm in experimental group- OD570nm of effector cells in control group) / OD570nm of target cells in control group] × 100%.
Protein gel blot analysis
Protein was loaded on 8% SDS-PAGE and separated proteins were transferred to PVDF membranes followed by blocking with 5% non-fat dry milk. The membranes were incubated with primary antibodies against c-ABL, α-tubulin (Cell Signaling), followed by HRP-conjugated secondary antibody (Cell Signaling). The membranes were then washed and detection was performed using the enhanced chemiluminescence protein gel blot detection system (Cell Signaling). Images of each band were analyzed by Bio-Rad Gel Imaging System and normalized to α-tubulin. Representative images of three independent experiments are shown in the results section.
Bone marrow transplantation and in vivo tumor study
The bone marrow transplant model with CML-like syndrome was generated as previously described.Citation28 Briefly, BALB/c donor mice were treated for 4 d with 5-fluorouracil (200 mg/kg; intravenous injection). Bone marrow cells from donor mice were collected by flushing femurs and tibias. Cells were counted and cultured in pre-stimulation medium (RPMI, 15% FBS, 5%WEHI-3B conditioned medium, 6 ng/ml recombinant murine IL-3, 10 ng/ml recombinant murine IL-6, and 50–100 ng/ml recombinant stem cell factor). Cells were transduced with p210BCR/ABL expressing retrovirus in the presence of 4 μg/ml polybrene (Sigma-Aldrich) by two rounds of spinoculation. Cells were collected, resuspended in Hanks balanced salt solution and injected into lateral tail veins of lethally irradiated (450 c-Gy) BALB/c recipient mice. The constructed bone marrow transplant models were randomly divided into five groups as mentioned in section 4.3. All mice were immunized with the mixture of 100 μg plasmid and 0.75% lidocaine in a ratio of 1:4 into both hind legs three times every 10 d. Thirty days post-vaccination, hematopoietic organs (spleen and bone marrow) were harvested and analyzed by histology, reverse-transcription-PCR (RT-PCR) and protein gel blot. The measurement of hematologic variables was done upon sacrifice and blood smears were stained with a Wright stain. Spleen was fixed in 10% buffered formalin for 48 h. Paraffin-embedded sections were then stained with hematoxylin-eosin.
Statistical analysis
The results were expressed as means ± standard deviations of three or more experiments performed in duplicate. Statistical analyses were performed using ANOVA to determine differences between three or more groups and a Student t-test for determining the differences between two groups. A p value of less than 0.05 was considered as statistically significant.
Acknowledgments
This work was supported by Natural Science Foundation Project of CQ CSTC (2010BB5369). We thank Professor Tongchuan He, the director of the Molecular Oncology Laboratory of the University of Chicago Medical Center in America, for the critical reading of this manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer 2005; 5:172 - 83; http://dx.doi.org/10.1038/nrc1567; PMID: 15719031
- Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science 1990; 247:1079 - 82; http://dx.doi.org/10.1126/science.2408149; PMID: 2408149
- Clark RE, Dodi IA, Hill SC, Lill JR, Aubert G, Macintyre AR, et al. Direct evidence that leukemic cells present HLA-associated immunogenic peptides derived from the BCR-ABL b3a2 fusion protein. Blood 2001; 98:2887 - 93; http://dx.doi.org/10.1182/blood.V98.10.2887; PMID: 11698267
- Grünebach F, Mirakaj V, Muller MR, Brummendorf T, Brossart P. BCR-ABL is not an immunodominant antigen in chronic myelogenous leukemia. Cancer Res 2006; 66:5892 - 900; http://dx.doi.org/10.1158/0008-5472.CAN-05-2868; PMID: 16740729
- Yotnda P, Firat H, Garcia-Pons F, Garcia Z, Gourru G, Vernant JP, et al. Cytotoxic T cell response against the chimeric p210 BCR-ABL protein in patients with chronic myelogenous leukemia. J Clin Invest 1998; 101:2290 - 6; http://dx.doi.org/10.1172/JCI488; PMID: 9593785
- Sun JY, Krouse RS, Forman SJ, Senitzer D, Sniecinski I, Chatterjee S, et al. Immunogenicity of a p210(BCR-ABL) fusion domain candidate DNA vaccine targeted to dendritic cells by a recombinant adeno-associated virus vector in vitro. Cancer Res 2002; 62:3175 - 83; PMID: 12036931
- Shashidharamurthy R, Bozeman EN, Patel J, Kaur R, Meganathan J, Selvaraj P. Immunotherapeutic strategies for cancer treatment: A novel protein transfer approach for cancer vaccine development. Med Res Rev 2011; http://dx.doi.org/10.1002/med.20237; PMID: 21241001
- Bocchia M, Wentworth PA, Southwood S, Sidney J, McGraw K, Scheinberg DA, et al. Specific binding of leukemia oncogene fusion protein peptides to HLA class I molecules. Blood 1995; 85:2680 - 4; PMID: 7742526
- Boissel N, Rousselot P, Raffoux E, Cayuela JM, Maarek O, Charron D, et al. Defective blood dendritic cells in chronic myeloid leukemia correlate with high plasmatic VEGF and are not normalized by imatinib mesylate. Leukemia 2004; 18:1656 - 61; http://dx.doi.org/10.1038/sj.leu.2403474; PMID: 15343347
- Dong R, Cwynarski K, Entwistle A, Marelli-Berg F, Dazzi F, Simpson E, et al. Dendritic cells from CML patients have altered actin organization, reduced antigen processing, and impaired migration. Blood 2003; 101:3560 - 7; http://dx.doi.org/10.1182/blood-2002-06-1841; PMID: 12506035
- Sin JI, Kim JJ, Arnold RL, Shroff KE, McCallus D, Pachuk C, et al. IL-12 gene as a DNA vaccine adjuvant in a herpes mouse model: IL-12 enhances Th1-type CD4+ T cell-mediated protective immunity against herpes simplex virus-2 challenge. J Immunol 1999; 162:2912 - 21; PMID: 10072541
- Portielje JE, Kruit WH, Eerenberg AJ, Schuler M, Sparreboom A, Lamers CH, et al. Subcutaneous injection of interleukin 12 induces systemic inflammatory responses in humans: implications for the use of IL-12 as vaccine adjuvant. Cancer Immunol Immunother 2005; 54:37 - 43; http://dx.doi.org/10.1007/s00262-004-0574-0; PMID: 15693137
- Rodolfo M, Colombo MP. Interleukin-12 as an adjuvant for cancer immunotherapy. Methods 1999; 19:114 - 20; http://dx.doi.org/10.1006/meth.1999.0836; PMID: 10525447
- Lee P, Wang F, Kuniyoshi J, Rubio V, Stuges T, Groshen S, et al. Effects of interleukin-12 on the immune response to a multipeptide vaccine for resected metastatic melanoma. J Clin Oncol 2001; 19:3836 - 47; PMID: 11559721
- Ikezawa H. Glycosylphosphatidylinositol (GPI)-anchored proteins. Biol Pharm Bull 2002; 25:409 - 17; http://dx.doi.org/10.1248/bpb.25.409; PMID: 11995915
- Ji J, Li J, Holmes LM, Burgin KE, Yu X, Wagner TE, et al. Synergistic anti-tumor effect of glycosylphosphatidylinositol-anchored IL-2 and IL-12. J Gene Med 2004; 6:777 - 85; http://dx.doi.org/10.1002/jgm.547; PMID: 15241785
- McHugh RS, Nagarajan S, Wang YC, Sell KW, Selvaraj P. Protein transfer of glycosyl-phosphatidylinositol-B7-1 into tumor cell membranes: a novel approach to tumor immunotherapy. Cancer Res 1999; 59:2433 - 7; PMID: 10344754
- Nagarajan S, Selvaraj P. Glycolipid-anchored IL-12 expressed on tumor cell surface induces antitumor immune response. Cancer Res 2002; 62:2869 - 74; PMID: 12019166
- Zhao F, Dou J, Wang J, Chu L, Tang Q, Wang Y, et al. Investigation on the anti-tumor efficacy by expression of GPI-anchored mIL-21 on the surface of B16F10 cells in C57BL/6 mice. Immunobiology 2010; 215:89 - 100; http://dx.doi.org/10.1016/j.imbio.2009.02.003; PMID: 19457579
- Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science 2000; 289:1938 - 42; http://dx.doi.org/10.1126/science.289.5486.1938; PMID: 10988075
- Osman Y, Takahashi M, Zheng Z, Koike T, Toba K, Liu A, et al. Generation of bcr-abl specific cytotoxic T-lymphocytes by using dendritic cells pulsed with bcr-abl (b3a2) peptide: its applicability for donor leukocyte transfusions in marrow grafted CML patients. Leukemia 1999; 13:166 - 74; http://dx.doi.org/10.1038/sj.leu.2401311; PMID: 10025889
- Schwartz J, Pinilla-Ibarz J, Yuan RR, Scheinberg DA. Novel targeted and immunotherapeutic strategies in chronic myeloid leukemia. Semin Hematol 2003; 40:87 - 96; http://dx.doi.org/10.1016/S0037-1963(03)70046-8; PMID: 12563615
- Englund PT. The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Annu Rev Biochem 1993; 62:121 - 38; http://dx.doi.org/10.1146/annurev.bi.62.070193.001005; PMID: 8352586
- Bütikofer P, Malherbe T, Boschung M, Roditi I. GPI-anchored proteins: now you see 'em, now you don't. FASEB J 2001; 15:545 - 8; http://dx.doi.org/10.1096/fj.00-0415hyp; PMID: 11156970
- Caras IW, Weddell GN. Signal peptide for protein secretion directing glycophospholipid membrane anchor attachment. Science 1989; 243:1196 - 8; http://dx.doi.org/10.1126/science.2466338; PMID: 2466338
- van der Schoot CE, Huizinga TW, van't Veer-Korthof ET, Wijmans R, Pinkster J, von dem Borne AE. Deficiency of glycosyl-phosphatidylinositol-linked membrane glycoproteins of leukocytes in paroxysmal nocturnal hemoglobinuria, description of a new diagnostic cytofluorometric assay. Blood 1990; 76:1853 - 9; PMID: 2145990
- Shi M, Su L, Hao S, Guo X, Xiang J. Fusion hybrid of dendritic cells and engineered tumor cells expressing interleukin-12 induces type 1 immune responses against tumor. Tumori 2005; 91:531 - 8; PMID: 16457153
- Li S, Ilaria RL Jr., Million RP, Daley GQ, Van Etten RA. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J Exp Med 1999; 189:1399 - 412; http://dx.doi.org/10.1084/jem.189.9.1399; PMID: 10224280