Abstract
The heat shock proteins (HSPs) represent a class of proteins which are induced under physiologic stress to promote cell survival in the face of endogenous or exogenous injury. HSPs function predominantly as molecular chaperones, maintaining their “client” proteins in the correct conformational state in order to withstand a biologic stressor. Elevated HSP expression is also found in a range of pathologic conditions, notably malignancy. Cancer cells exploit the pro-survival phenotype endowed by HSPs to bolster their proliferative potential. Consequently, developing means of abrogating HSP expression may provide a way to render cancer cells more susceptible to radiation or chemotherapy. Here, we review the members of the HSP class and their roles in malignancy. We focus on attempts to target these proteins, particularly the small HSPs, in developing potent radiation and chemotherapy sensitizers, as well as proposed mechanisms for this sensitization effect.
The Heat Shock Proteins—Overview
In the face of physiologic stress, cells are equipped with a range of mechanisms to successfully withstand such insults. A classic example of one such mechanism is the heat shock response, first described in Drosophila in 1962.Citation1 The heat shock response was found to be orchestrated by a protein class later termed the Heat Shock Proteins (HSPs), whose synthesis, unlike the majority of cellular proteins, increased under conditions of heat shock.Citation2 It was later shown that the HSPs allow cells to survive a wide range of both endogenous and exogenous insults including cytotoxic agents, oxidants, heavy metals and infection.Citation3,Citation4 In response to these stressors, the transcriptional regulator HSF1, in concert with family member HSF2, mediates heat shock gene transcription to enact the stress response and increase cellular HSP levels.Citation5
The HSPs are categorized by molecular weight and include members Hsp100 (this HSP has no mammalian homolog, though is characterized in bacteria and yeast), Hsp90, Hsp70, Hsp60, HSP40 and the small HSPs, which range between 13–42 kDaCitation3,Citation6–Citation8 (). The HSPs serve predominantly as molecular chaperones for other cellular proteins; high molecular weight HSPs require ATP as well, whereas low molecular weight HSPs are ATP-independent.Citation4 Because they interact with a wide range of proteins in their role as molecular chaperones, HSPs have not only been implicated in a variety of cellular functions, but are also regarded as important actors in a range of pathological conditions.
Molecular chaperones function by providing a sequestered folding chamber in which a target or “client” protein can assume its native conformation. Client proteins therefore appropriately mature without risk of forming aggregates or non-specifically associating with unwanted cellular proteins.Citation3 While chaperones are important for cellular physiology even under basal conditions, their role obviously assumes increased importance under stress,Citation4 particularly because such adverse conditions can precipitate protein misfolding or aggregation. Such aberrant proteins can, in turn, disrupt important regulatory complexes. Therefore, HSPs function to restore cellular homeostasis by ensuring proper formation of new proteins, preserving existing complexes, restoring function of denatured proteins, and solubilizing protein aggregates.Citation3,Citation9,Citation10 Their chaperone activity also allows HSPs to prevent inappropriate activation of a client protein's downstream targets, a function referred to as protein “holding.”Citation3,Citation11 This process occurs predominantly in the cytoplasm and ought to be distinguished from that of the glucose-regulated proteins (GRPs). GRPs are a related class of proteins also induced by cellular stress and associated protein damage in the endoplasmic reticulum. They are induced by similar stressors, but act principally on secretory polypeptides such as immunoglobulins and various glycoproteins.Citation12,Citation13 While GRPs have also been studied in relation to tumorigenesis, this review will focus only on the cytoplasmic chaperones.
Although the HSPs have been characterized predominantly as chaperones, they have also been invoked in other cellular processes including apoptosis and the immune response. Like the Bcl-2 protein family, the HSPs include both pro-apoptotic and anti-apoptotic family members and function at a variety of steps in the apoptotic signaling cascade. For example, Hsp27 and Hsp70 have been implicated in anti-apoptotic roles, whereas Hsp60 can have pro-apoptotic function (see detailed discussion to follow). While HSPs' chaperone activity may play some role in their ability to modulate the apoptotic response, studies have also demonstrated effects on apoptosis independent of chaperoning activity. This holds true both for pro-apoptotic and antiapoptotic effects.Citation14 With regard to their immune-modulatory role, HSP members such as Hsp70 and Hsp90 have been found extracellularly to elicit an immune response under conditions of cell necrosis.Citation4,Citation15
The Large HSPs
Hsp90.
Hsp90 is the most abundantly expressed HSP. Even under basal conditions, Hsp90 can represent as much as 2% of the total cellular protein content.Citation10,Citation16 While its high basal expression suggests its importance in cellular homeostasis, Hsp90 has also been heavily studied with regard to its anti-apoptotic function and association with oncogenesis.Citation2 Hsp90's principle function is as molecular chaperone, and it acts in concert with several co-chaperone proteins, namely Hsp70, Hsp40, Hip, Hop, p23, and Cdc37 in an ATP-dependent manner. The Hsp90 complex binds immature client proteins to help them assume their native conformation. Many of Hsp90's client proteins are conformationally-unstable proteins involved in signal transduction pathways important in cell development, growth, and survival. They include transmembrane tyrosine kinases (such as HER-2/neu, EGFR, IGF-1R), signaling proteins (Akt, Raf-1 and IKK), tumor suppressors, (p53, Kit), chimeric signaling proteins (Bcr-Abl), steroid hormone receptors, and cell-cycle regulators (see review by Kamal et al.).Citation17 Therefore, Hsp90 can alter protein activity, participate in cell cycle regulation, influence cell growth, and in so doing, alter cellular behavior to favor proliferation.Citation4
Hsp90 can also promote cell survival through its anti-apoptotic activity, the majority of which relates to its influence on the NF-κB pathway.Citation7 Hsp90 stabilizes RIP, which associates with the TNF-α receptor when it binds its ligand, thereby promoting NF-κB activity.Citation18,Citation19 Downstream in the NF-κB pathway, Hsp90 and its co-chaperone Cdc37 promote proper folding of the IKK and Akt protein complexes, which each enhance I-κB dissociation from NF-κB and subsequently enhance its activity.Citation19 Hsp90 also inhibits the dephosphorylation of Akt to promote cell growth.Citation20,Citation21 Finally, Hsp90 can influence the intrinsic apoptotic pathway as well by inhibiting oligomerization of Apaf-1, thereby preventing the apoptosome complex from forming and consequently, prevent downstream caspase activation.Citation22
Hsp70.
Hsp70 is actually a class of several proteins unto itself. It is the most highly conserved and most strongly induced HSP in all organisms from E. coli to man.Citation3,Citation11,Citation23 Hsp70 helps preserve a number of cellular activities in stress conditions including mitosis, meiosis, and cellular differentiation. Similar to Hsp90, Hsp70 acts as a chaperone to maintain unfolded proteins in an intermediate state to prevent inappropriate aggregation, and then promotes refolding to their native conformation.Citation24 This process also depends on ATP as well as other co-chaperones.Citation23 Unlike Hsp90, however, Hsp70 family members are generally expressed at low levels under basal conditions, though are highly inducible. Several members of the Hsp70 sub-family, however, are constitutively expressed.Citation4,Citation7
The Hsp70s promote cell survival by interfering with apoptosisCitation25 and inhibiting permeabilization of the lysosomal membrane.Citation26 This function is in contrast to that of Hsp90, whose predominant role is as a molecular chaperone. As anti-apoptotic molecules, Hsp70s are considered the prototypical inhibitors of apoptosis, blocking both intrinsic and extrinsic pathways.Citation4,Citation9,Citation27,Citation28 They can safeguard cells from death induced by TNFα, monocyte signaling, oxidative damage, chemotherapeutics, radiation, NO, and heat stress (reviewed by Arya et al.).Citation7 Hsp70 also inhibits apoptosome formation through interactions with Apaf-1Citation29 and can impede events downstream of caspase activation such as changes in nuclear morphology and phospholipase A2 activation.Citation30 Moreover, Hsp70s prevent nuclear translocation of apoptosis inducing factor (AIF) by binding the protein upon its release from mitochondria.Citation28 Lastly, Hsp70 prevents cell death through a caspase-independant cell death pathway, preventing lysosomal permeabilization and subsequent release of cathepsin into the cytosol.Citation26
Hsp60.
Hsp60 is less well-characterized than other heat shock proteins. Interestingly, it harbors both pro-apoptotic and antiapoptotic functions.Citation7 For instance, Hsp60 has been investigated in cardiac myocytes as an inhibitor of apoptosis. It works alongside Hsp10 to maintain mitochondrial integrity and binds Bak to prevent downstream activation of apoptotic pathways.Citation31,Citation32 As an apoptosis activator, Hsp60 is found in esophageal carcinoma cells to be highly expressed in correlation with high apoptotic index.Citation33 Hsp60 was also found to be necessary for caspase-mediated apoptosis in Drosophila melanogaster.Citation34
The Small HSPs
The small HSPs are comprised of 10 members including, most notably, Hsp27 (also known as Hsp25 and HspB1), but also MKBP, HspB3, αA-crystallin, αB-crystallin, Hsp20, cvHsp, Hsp22, HspB9, and HspB10. They have been studied in connection to smooth muscle function, platelet regulation, cardiovascular disease, mycobacterial disease, neurological disease, and cancer.Citation35–Citation37 Hsp27 in particular has received attention due to its association with a wide range of malignancies. It consists of a C-terminal domain structurally similar to the α-crystallin proteins found in the lens of the eye. It also harbors an N-terminal hydrophobic WDPF motif required for oligomerization.Citation38 In vivo, Hsp27 is found in 100–800 kDa oligomeric complexesCitation39 which dissociate upon phosphorylation at important regulatory sites S15, S78, and S82.Citation38,Citation40 Hsp27 oligomerization is regulated by MAPKAP kinases 2 and 3, which are themselves induced by various stressors including mitogens, inflammatory cytokines, and a variety of oxidants.Citation38 Hsp27's quaternary structure helps determine its function with various oligomeric forms each performing a different specific role in the cell.Citation41 For example, the protein was found to bind cytochrome c or DAXX only in its hypophosphorylated, oligomeric form.Citation42,Citation43 In fact, some attempts to modulate the activity of Hsp27 as a therapeutic modality in cancer have focused on interfering with its oligomerization,Citation44 the details of which will be discussed in subsequent sections.
The functions of Hsp27 are wide and varied, though the protein is recognized predominantly for its role as a molecular chaperone.Citation45 Of the HSPs, Hsp27 is the most strongly induced chaperone besides Hsp70. Heat, oxidative stress, irradiation, and anti-cancer drugs all promote increased Hsp27 expression.Citation9 Unlike its larger family members, Hsp27 is ATP-independent and its chaperone activity is regulated by its oligomerization state. The large multimer has the highest affinity for client proteins and its level of chaperone activity can thus be tailored by modifying the extent of oligomerization.Citation4,Citation46
Hsp27 also displays strong anti-apoptotic behavior. It has been found to antagonize a range of anti-apoptotic pathways including that induced by staurosporine, the Fas death receptor pathway, deprivation of growth factors, oxidative damage, hyperthermia, UV radiation, and chemotherapeutics.Citation14 Hsp27 interacts with procaspase-9 and procaspase-3, inhibiting upstream cleavage events in the apoptotic cascade.Citation22,Citation43,Citation45 It is also thought to bind and sequester cytochrome c released into the cytoplasm in response to death signals. Consequently, apoptosis is inhibited as the apoptosome cannot associate with Apaf-1.Citation7,Citation37,Citation38,Citation43 Hsp27 also interacts with Daxx, preventing its translocation to the plasma membrane and subsequent Fas-mediated apoptosis.Citation42 Lastly, Hsp27 has been tightly linked to activation of Akt, which further promotes cell survival. An early study of Hsp27 demonstrated a direct interaction of Hsp27 and Akt in neutrophils, the dissociation of which resulted in enhanced neutrophil apoptosis.Citation47 Later, Hsp27 was found to upregulate Akt indirectly through a PI3K-dependent mechanism. This resulted in prevention of Bax-mediated mitochondrial permeabilization and apoptosis.Citation48 Interestingly, in addition to protecting against apoptosis, Hsp27 was shown to prevent cell necrosis, demonstrated in an early study in a murine fibrosarcoma model in which necrosis was induced by TNFα.Citation49
Hsp27 has been investigated as an anti-oxidant, endowed with two mechanisms of preventing oxidative stress. Besides its ability to repair oxidized protein damage through its chaperone activity, it also appears to enhance a cell's ability to withstand oxidative damage by increasing cellular glutathione.Citation45,Citation50 Although the precise mechanism for this cytoprotective role of Hsp27 is currently unclear, it has been postulated that Hsp27 increases glucose-6-phosphate dehydrogenase, glutathione reductase, and glutathione transferase in L929 cells, allowing for a greater store of reduced glutathione with which the cell can ward off oxidative damage.Citation51 Additionally, small oligomers of Hsp27 stabilize polymerized, or F-actin, exerting a protective effect through the cytoskeleton.Citation52–Citation54 The small oligomers have also been found to play a role in protein degradation through the ubiquitinproteasome pathway under cellular stress.Citation4,Citation55 Lastly, small Hsp27 oligomers, which favor the degradation of I-κB and consequently enhance NF-κB activity, contribute to its anti-apoptotic qualities as well.Citation37
Heat Shock Proteins and Cancer
The Role of the Large HSPs.
Given their pro-proliferative and anti-apoptotic properties, as well as their interaction with a wide variety of cell signaling pathways, HSPs have been heavily studied in the context of cancinogenesis. Several members of the HSP class have shown high correlation to tumor cell expansion, differentiation, and apoptosis.Citation2 Hsp90 in particular has been extensively investigated and studies have identified a variety of its client proteins to be associated with cancer including steroid hormone receptors, tyrosine kinases, SRC family kinases, serine/threonine kinases, cell-cycle regulators, telomerase, transcription factors, and mutant chimeric oncogenes, such as Bcr-Abl (reviewed in Didelot et al.).Citation4 Hsp90 also stabilizes mutant, inactive forms of tumor suppressors and DNA repair proteins such as p53 and MSH2.Citation3 Hsp70's anti-apoptotic effect, impact on lysosomal enzymes, and effect on tumor suppressor proteins such as p53 have implicated a carcinogenic role for this protein as well. Moreover, Hsp70 has been found to inhibit p21- and p53-dependent senescence pathways, thereby further promoting cell proliferation.Citation56
The large HSPs can also contribute to tumorigenesis outside of their cell growth regulatory functions. For instance, Hsp90 and Hsp70 stimulate angiogenesis by promoting endothelial cell mobility and proliferation. This effect is mediated through chaperoning activity with HIF1α and stimulation of nitric oxide synthase and VEGF.Citation3,Citation56,Citation57 Hsp90 can also stimulate tumor metastasis through interaction with MMP-2 to facilitate tumor cell migration. Lastly, extracellular release of Hsp70 stimulates an inflammatory environment in which tumors thrive.Citation3,Citation56
The association between HSPs and cancer is further supported by clinical evidence as well. Hsp90 is overexpressed in human tissue from a range of cancers including breast tumors, lung cancer, leukemia, and Hodgkins and non-Hodgkins B-cell lymphoma.Citation58 Hsp90 expression is also associated with poor prognostic markers in breast cancer such HER-2/neu and estrogen receptor.Citation59 Hsp70 is associated with a poor prognosis in human cancer as well, showing high expression in endometrial cancer, osteosarcoma, renal tumors, breast cancer, gastric cancer, and leukemia.Citation4,Citation9,Citation60,Citation61 One study investigated serum Hsp70 compared with serum PSA in detecting early stage prostate cancer, and demonstrated a significant correlation between serum protein levels and disease.Citation62 Further, patients with CML expressing the chimeric oncogene Bcr-Abl were also found to harbor high levels of Hsp70,Citation63 suggesting a role for the chaperone in the stability of the protein in vivo. Such a finding was particularly striking in patients with imatinib-resistant CML.Citation64
The small HSPs in cancer: Focus on Hsp27.
Hsp27 has been emerging recently as an important player in cancer development. Multiple in vitro experiments have lent support to Hsp27's pro-oncogenic role. Hsp27 expression is particularly high even under basal culture conditions of transformed cells. For example, SQ20B, a radio-resistant head and neck squamous cell carcinoma cell line, exhibits a remarkably elevated cellular concentration of Hsp27.Citation45 Additionally, lung cancer stem cells in culture with elevated Hsp27 demonstrate apoptotic resistance in response to superoxide, cisplatin, gemcitabine, and combination treatments.Citation65 These findings agree with known mechanisms of action of Hsp27 including inactivation of caspase-9 and caspase-3.Citation65 Additionally, confluent cells demonstrate especially high levels of Hsp27 and have proven more resistant than proliferating cells to chemotherapeutic agents, and harbor dramatically lower levels of ROS.Citation66 Lastly, one study comparing primary and metastatic head and neck cancer cell lines showed that the cells proliferate at similar rates, but the latter shows enhanced migration activity. This phenotype correlates with a 22.4-fold higher Hsp27 mRNA level and 25-fold higher protein level.Citation67
The in vitro findings on Hsp27's behavior in transformed cells have paralleled in vivo observations. For instance, the protein is reportedly overexpressed in clinical specimens from oral squamous cell carcinoma, oropharyngial and laryngial cancers.Citation53 Hsp27 has also been shown to provide useful prognostic information for cancer patients.Citation37 For example, high Hsp27 expression was related to poor prognosis following surgery as well as resistance to adjuvant therapy across several cancer types including breast cancer, gastric cancer, osteosarcoma, prostate cancer, head and neck, and colon cancer.Citation45,Citation53 Hsp27 expression can yield prognostic information about chemotherapy response as well. For example, high Hsp27 expression in childhood leukemia can predict a poor response to vincristine.Citation45,Citation68 Hsp27 is also thought to play a role in chemotherapy resistance in breast cancer patients.Citation69
HSPs as Targets for Sensitization to Radiotherapy and Chemotherapy
Radiation and chemotherapy are cornerstones of therapy for many human cancers. As tumors continue to grow in individuals undergoing treatment, genetic and epigenetic alterations within cancer cells promote resistance to these modalities. Moreover, normal tissues can be damaged by these treatments and pose a dose-limiting barrier to complete cure of malignancies. Therefore, significant effort has been invested toward identification of potent means of sensitizing cancer cells to radiation and chemotherapy.Citation70 It stands to reason that HSPs, with their cytoprotective function in the face of stress, may endow tumors with a therapy-resistant phenotype. Thus, these proteins may serve as an “Achilles heel” in cancer cells that can be exploited to sensitize them to radiation or chemotherapy.Citation45 Investigators have probed ways to attack cancer cells by impairing the activity of the HSPs through a variety of means, including small molecule inhibitors, antisense oligonucleotides, and protein aptamers.
HSPs as targets in chemosensitization.
Small molecule inhibitors of HSPs have shown promise in rendering cancer cells more sensitive to chemotherapy. Several Hsp90 inhibitors have been characterized, the most noteworthy being 17-allylaminogeldanamycin (17-AAG), 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG), geldanamycin, and radicicol.Citation71 The latter two have shown potent antitumor activity in preliminary experiments, but expose patients to excessive hepatotoxicity.Citation70 For example, in leukemia, the Hsp90 inhibitor geldanamycin combined with doxorubicin increases apoptosis of cancer cells. The Hsp90 inhibitor 17-AAG has shown tumor growth-inhibitory activity in preclinical studies across a range of cancer types including breast cancer, melanoma, lung cancer, myeloma, and prostate cancer.Citation2 In breast cancer, tumors regress when treated with 17-AAG and angiogenesis inhibitors.Citation3,Citation10 17-AAG was also found to induce Her-2 degradation in breast tumor xenographs with Her-2 overexpression.Citation72 Interestingly, several current chemotherapeutic agents such as taxol, cisplatin, and trichostatin-A possess intrinsic anti-Hsp90 qualities which may contribute to their mechanisms of action (reviewed in Soti et al.).Citation10
The recent development of antisense oligonucleotide and siRNA technology to selectively knock down the expression of target genes has also shown promise in altering HSP expression for cancer cell chemosensitization. In cultured colorectal cancer cells, downregulation of Hsp27 with siRNA enhanced irinotecan sensitivity and high Hsp27 expression correlated with irinotecan resistance. In mouse xenograft prostate cancer studies, systemic administration of Hsp27 siRNA not only decreased tumor progression, but also rendered these tumors more sensitive to paclitaxel,Citation22,Citation73,Citation74 as well as to the Hsp90 inhibitor 17-AAG.Citation45,Citation75 Similarly, the efficacy of antisense oligonucleotides in orthotopic mouse models of bladder cancer, known to harbor elevated Hsp27 expression, have shown promise. Intravesical administration of antisense Hsp27 oligonucleotides to mice with bladder cancer yielded enhanced tumor cell toxicity under concurrent administration of paclitaxel, cisplatin, and gemcitibine.Citation76
Lastly, chemosensitization through HSPs with the peptide aptamer approach has been attempted as well. Aptamers are short peptide sequences mounted on a scaffold protein which force the peptide to maintain a specific conformation. Libraries of aptamers with random peptide sequences can be screened for interaction with a protein of interest. Cancer cell lines treated with protein aptamers that bind Hsp27 showed enhanced cell death in response to chemotherapeutics doxorubicin and cisplatin.Citation41 Protein aptamers represent a novel approach to abrogating Hsp27 activity to radiosensitize tumors. However, only early studies have been reported with this technique.
HSPs as targets in radiosensitization.
Small molecules to impede HSP function represent one heavily-investigated approach to radiosensitize cancer cells. 17-AAG possesses promising in vitro and in vivo radiosensitization activity and shows clinical promise across several cancer types, including cervical, lung, and colon cancers.Citation2,Citation77 17-AAG has 100-fold higher affinity for Hsp90 in cancer cells compared with normal cells. This phenomenon seems to be related to Hsp90's high chaperoning activity in cancer cells, forcing it to adopt a conformation that favors 17-AAG binding.Citation70,Citation78 Small molecule inhibitors for Hsp70 have also been tested, the most effective of which are quercetin and related chemical derivatives,Citation79 as well as triptolide.Citation80 However, these molecules inhibit the expression of the protein rather than its function, and do not appear to be highly specific for Hsp70.Citation4 Another small molecule approach involves zerumbone, an extract from a subtype of ginger that polymerizes Hsp27 monomers. This compound was shown to sensitize pre-treated cancer cells to radiation in vitro and in a mouse xenograft tumor model by inhibiting Hsp27's anti-apoptotic activity.Citation44
The oligonucletide/RNAi strategy has also been exploited for radiosensitization. For example, RNAi targeting Hsp27 in head and neck cancer cells has made them more radiosensitive in clonogenic survival assays, increases TUNEL positivity and caspase activation after irradiation, increases ROS production, lowers cellular glutathione content, and increases mitochondrial membrane permeability.Citation45 Additionally, downregulating Hsp27 expression using antisense cDNA enhances prostate cancer cells' sensitivity to radiation.Citation81 In vivo, mice treated with Hsp27 antisense oligonucleotides and radiation showed tumor regression and enhanced survival in a xenograft tumor model. Additionally, these investigators noted decreased tumor angiogenesis, a high rate of tumor cell apoptosis, and decreased cellular glutathione in the tumors as well.Citation82
Recently, two protein aptamers that interfere with Hsp27 activity in SQ20B cells also acted as radiosensitizers, increasing clonogenic cell death after irradiation. In the same study, aptamers slowed tumor growth in SQ20B squamous cell carcinoma xenografts in mice. This effect was mediated through cell cycle arrest.Citation41
Mechanisms for radio-sensitization through HSPs.
Clearly, HSPs show potential as targets for radiosensitization and chemosensitization. However, the mechanism by which abrogating expression of HSPs achieves such an effect appears to be complex and multifactorial (). One straightforward hypothesis argues that HSPs simply stabilize signaling molecules that specifically protect cells from radiation- or chemotherapy-induced cell death.Citation71 Studies investigating this mechanism have shown that tumor cell radiosensitization from Hsp90 inhibition was able to cause reduced expression of client proteins Akt, EGFR, Raf-1, ErbB2, IGF-1R and an increase in their ubiquitin-mediated proteasomal degradation.Citation70 Many of these proteins have been specifically linked not only to cell proliferation and survival, but also to protection from cell death induced by radiation.Citation20,Citation83,Citation84 Such a finding fits in nicely with the established role HSPs play in the cell's stress response.
HSP inhibitors may also contribute to cancer therapy sensitization through their antioxidant properties. Ionizing radiation causes DNA damage by generating reactive oxygen species that can cause single or double strand breaks, either by interacting with DNA directly or by exciting other molecules in the vicinity such as H2O.Citation85,Citation86 However, different cell lines may exhibit differential capacity to handling of ROS, leading to a range of levels of ROS in response to radiation. The level of ROS generated in response to radiation in a given cell line, in turn, may dictate the extent to which that cell line is radio-sensitive.Citation87 Hsp27 was first described to play a role in lowering ROS generated in cancer cells in response to TNFα.Citation88 Further work led to the hypothesis that Hsp27 decreases production of ROS in cancer cells by raising intracellular glutathione via glucose-6-phosphate dehydrogenase and glutathione reductase.Citation50 Furthermore, studies in Jurkat cells revealed Hsp27 expression levels to be correlated with a high tolerance for oxidative damage following irradiation and as well as high glutathione content.Citation45 Therefore, HSPs may serve a role in impairing the fundamental mechanisms on of radiation therapy in targeting cancer cells.
Mechanistic studies in HSP-antagonist mediated radiosensitization have raised the possibility that DNA damage response may be a key target of anti-HSP27 modalities. DNA damage induced by ionizing radiation may not kill a target cell if the cell can activate appropriate DNA repair pathways to withstand the damage.Citation87 Hsp90 inhibitors have been studied in particular for their properties inhibiting DNA repair pathways.Citation2 In one study, tumor cells exposed to Hsp90 inhibitor 17-DMAG showed inhibition of DNA double stand break repair and were radiosensitized by this agent. Inhibition of repair was proposed to be caused by DNA-PK phosphorylation as well as suppression of DNA repair protein ATM.Citation71 17-DMAG was shown in another study in non-small cell lung cancer to inhibit base excision repair enzymes apurinic/apyrimidinic endonuclease and DNA polymerase-β, resulting in radiosensitization.Citation89 Lastly, the Hsp90 inhibitor 17-AAG was effective in inhibiting DNA homologous recombination through via Rad51 and BRCA2.Citation86,Citation90 Interestingly, this effect was seen specifically in human prostate and lung cancer cells, but not in normal fibroblasts.Citation90
Still other studies on the radiosensitizing properties of HSP antagonists focus on their effect on tumor angiogenesis. Radiation has been shown to cause elevated expression of HIF-1α in irradiated cellsCitation91 which subsequently upregulates VEGF and promotes angiogenesis and enhanced tumor survival. This effect is partially mediated by Hsp90, which has been shown to stabilize HIF-1α.Citation92 In one study, 17-AAG and 17-DMAG suppressed tumor vascularization by disrupting Hsp90-mediated stabilization of HIF-1α.Citation93,Citation94 A similar effect was shown in irradiated head and neck cancer cells, though the mechanism appeared to proceed through the HSP family member Hsp27. In this study, antisense oligonucleotides to Hsp27 sensitized SQ20B head and neck cancer cells to radiation, an effect attributed to Hsp27's stabilization of Akt, which in turn, stabilizes VEGF as well.Citation82 Lastly, 17-AAG was shown to radiosensitize tumor endothelial cells, rendering the whole tumor less vascularized.Citation70 However, one caveat to these models is that the relationship between angiogenesis, tumor survival, and radiation is a complex one. On one hand, angiogenesis can contribute to tumor survival by shunting a much-needed blood supply to a growing mass, thereby contributing to its growth. Paradoxically, such uncontrolled angiogenesis can create tumor hypoxia as well, as these newly formed vessels lack structural integrity and become leaky, ineffective delivery sources for oxygen. Thus, they may dramatically raise tumor interstitial pressure and consequently decrease perfusion.Citation95,Citation96 Obviously the role of angiogenesis in HSP-mediated radio-resistance requires further elucidation.
Conclusion
The HSPs represent a promising target for cancer therapy owing to their increased levels and/or enhanced activity in cancer cells, as well as their potent and multi-factorial pro-survival and proliferative properties. Inhibiting their activity, particularly in the context of chemosensitization and radiosensitization, represents an attractive approach to cancer therapy. Such a strategy makes biologic sense given the physiologic stress that such treatment modalities place on cancer cells and the variety of ways that the HSPs enable cells to survive under stress. While Hsp90 inhibitors have already received extensive attention in the clinic as adjuncts to radiation and chemotherapy, recent studies demonstrating radio/chemosensitization of transformed cells through modifying the activity of the small HSPs have opened new avenues for therapeutic intervention. A major challenge for targeting small HSPs (especially those which do not utilize ATP), is the development of competitive small molecule inhibitors. Additional challenges in integrating HSP antagonists into cancer treatment in the clinic include more carefully understanding their mechanisms of action, as well as determining means of selectively inhibiting their function without toxicity to the host or off-target effects.
Abbreviations
| HSP | = | heat shock protein |
| 17-AAG | = | 17-allylamino-geldanamycin |
| 17-DMAG | = | 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin |
Figures and Tables
Figure 1 Proposed mechanisms of action for HSP inhibitors in radiosensitization and chemosensitization.
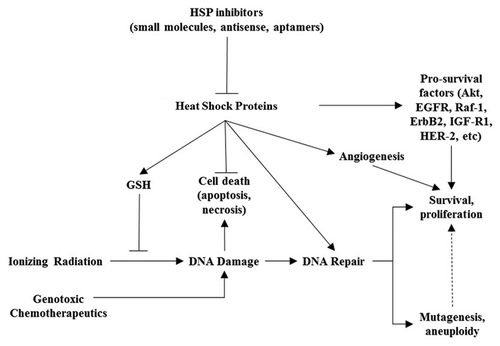
Table 1 Major heat shock proteins involved in radiosensitization and chemosensitization
Acknowledgments
This article was written through support by the NIH Institutional Clinical and Translational Science Award (TL1 RR024133) to David M. Guttmann, as well as a Sponsored Project grant by Enzon Pharmaceuticals.
References
- Ritossa F. A new puffing pattern induced by temperature and DNP in Drosophila. Experimentia 1962; 18:571 - 573; http://dx.doi.org/10.1007/BF02172188
- Giménez Ortiz A, Montalar Salcedo J. Heat shock proteins as targets in oncology. Clin Transl Oncol 2010; 12:166 - 173; PMID: 20231121; http://dx.doi.org/10.1007/s12094-010-0486-8
- Almeida MB, do Nascimento JL, Herculano AM, Crespo-Lopez ME. Molecular chaperones: Toward new therapeutic tools. Biomed Pharmacother 2011;
- Didelot C, Lanneau D, Brunet M, Joly AL, De Thonel A, Chiosis G, et al. Anti-cancer therapeutic approaches based on intracellular and extracellular heat shock proteins. Curr Med Chem 2007; 14:2839 - 2847; PMID: 18045130; http://dx.doi.org/10.2174/092986707782360079
- Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol 2010; 11:545 - 555; PMID: 20628411; http://dx.doi.org/10.1038/nrm2938
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet 1988; 22:631 - 677; PMID: 2853609; http://dx.doi.org/10.1146/annurev.ge.22.120188.003215
- Arya R, Mallik M, Lakhotia SC. Heat shock genes - integrating cell survival and death. J Biosci 2007; 32:595 - 610; PMID: 17536179; http://dx.doi.org/10.1007/s12038-007-0059-3
- Stetler RA, Gan Y, Zhang W, Liou AK, Gao Y, Cao G, et al. Heat shock proteins: cellular and molecular mechanisms in the central nervous system. Prog Neurobiol 2010; 92:184 - 211; PMID: 20685377; http://dx.doi.org/10.1016/j.pneurobio.2010.05.002
- Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: antiapoptotic proteins with tumorigenic properties. Cell Cycle 2006; 5:2592 - 2601; PMID: 17106261; http://dx.doi.org/10.4161/cc.5.22.3448
- Sôti C, Nagy E, Giricz Z, Vigh L, Csermely P, Ferdinandy P. Heat shock proteins as emerging therapeutic targets. Br J Pharmacol 2005; 146:769 - 780; PMID: 16170327; http://dx.doi.org/10.1038/sj.bjp.0706396
- Powers MV, Jones K, Barillari C, Westwood I, van Montfort RL, Workman P. Targeting HSP70: the second potentially druggable heat shock protein and molecular chaperone?. Cell Cycle 2010; 9:1542 - 1550; PMID: 20372081; http://dx.doi.org/10.4161/cc.9.8.11204
- Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell 1991; 66:191 - 197; PMID: 1855252; http://dx.doi.org/10.1016/0092-8674(91)90611-2
- Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett 2007; 581:3641 - 3651; PMID: 17481612; http://dx.doi.org/10.1016/j.febslet.2007.04.045
- Garrido C, Gurbuxani S, Ravagnan L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun 2001; 286:433 - 442; PMID: 11511077; http://dx.doi.org/10.1006/bbrc.2001.5427
- Arispe N, Doh M, Simakova O, Kurganov B, De Maio A. Hsc70 and Hsp70 interact with phosphatidylserine on the surface of PC12 cells resulting in a decrease of viability. FASEB J 2004; 18:1636 - 1645; PMID: 15522909; http://dx.doi.org/10.1096/fj.04-2088com
- Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer 2005; 5:761 - 772; PMID: 16175177; http://dx.doi.org/10.1038/nrc1716
- Kamal A, Boehm MF, Burrows FJ. Therapeutic and diagnostic implications of Hsp90 activation. Trends Mol Med 2004; 10:283 - 290; PMID: 15177193; http://dx.doi.org/10.1016/j.molmed.2004.04.006
- Lewis J, Devin A, Miller A, Lin Y, Rodriguez Y, Neckers L, et al. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-kappaB activation. J Biol Chem 2000; 275:10519 - 10526; PMID: 10744744; http://dx.doi.org/10.1074/jbc.275.14.10519
- Chen G, Cao P, Goeddel DV. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell 2002; 9:401 - 410; PMID: 11864612; http://dx.doi.org/10.1016/S1097-2765(02)00450-1
- Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci USA 2000; 97:10832 - 10837; PMID: 10995457; http://dx.doi.org/10.1073/pnas.170276797
- Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem 2002; 277:39858 - 39866; PMID: 12176997; http://dx.doi.org/10.1074/jbc.M206322200
- Pandey P, Saleh A, Nakazawa A, Kumar S, Srinivasula SM, Kumar V, et al. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J 2000; 19:4310 - 4322; PMID: 10944114; http://dx.doi.org/10.1093/emboj/19.16.4310
- Pandita TK, Pandita S, Bhaumik SR. Molecular parameters of hyperthermia for radiosensitization. Crit Rev Eukaryot Gene Expr 2009; 19:235 - 251; PMID: 19883367
- Zhang Y, Huang L, Zhang J, Moskophidis D, Mivechi NF. Targeted disruption of hsf1 leads to lack of thermotolerance and defines tissue-specific regulation for stress-inducible Hsp molecular chaperones. J Cell Biochem 2002; 86:376 - 393; PMID: 12112007; http://dx.doi.org/10.1002/jcb.10232
- Aghdassi A, Phillips P, Dudeja V, Dhaulakhandi D, Sharif R, Dawra R, et al. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res 2007; 67:616 - 625; PMID: 17234771; http://dx.doi.org/10.1158/0008-5472.CAN-06-1567
- Nylandsted J, Gyrd-Hansen M, Danielewicz A, Fehrenbacher N, Lademann U, Hoyer-Hansen M, et al. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med 2004; 200:425 - 435; PMID: 15314073; http://dx.doi.org/10.1084/jem.20040531
- Gabai VL, Mabuchi K, Mosser DD, Sherman MY. Hsp72 and stress kinase c-jun N-terminal kinase regulate the bid-dependent pathway in tumor necrosis factor-induced apoptosis. Mol Cell Biol 2002; 22:3415 - 3424; PMID: 11971973; http://dx.doi.org/10.1128/MCB.22.10.3415-3424.2002
- Gurbuxani S, Schmitt E, Cande C, Parcellier A, Hammann A, Daugas E, et al. Heat shock protein 70 binding inhibits the nuclear import of apoptosis-inducing factor. Oncogene 2003; 22:6669 - 6678; PMID: 14555980; http://dx.doi.org/10.1038/sj.onc.1206794
- Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol 2000; 2:476 - 483; PMID: 10934467; http://dx.doi.org/10.1038/35019510
- Jäättelä M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J 1998; 17:6124 - 6134; PMID: 9799222; http://dx.doi.org/10.1093/emboj/17.21.6124
- Lin KM, Lin B, Lian IY, Mestril R, Scheffler IE, Dillmann WH. Combined and individual mitochondrial HSP60 and HSP10 expression in cardiac myocytes protects mitochondrial function and prevents apoptotic cell deaths induced by simulated ischemia-reoxygenation. Circulation 2001; 103:1787 - 1792; PMID: 11282911
- Kirchhoff SR, Gupta S, Knowlton AA. Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation 2002; 105:2899 - 2904; PMID: 12070120; http://dx.doi.org/10.1161/01.CIR.0000019403.35847.23
- Faried A, Sohda M, Nakajima M, Miyazaki T, Kato H, Kuwano H. Expression of heat-shock protein Hsp60 correlated with the apoptotic index and patient prognosis in human oesophageal squamous cell carcinoma. Eur J Cancer 2004; 40:2804 - 2811; PMID: 15571964; http://dx.doi.org/10.1016/j.ejca.2004.08.013
- Arya R, Lakhotia SC. Hsp60D is essential for caspase-mediated induced apoptosis in Drosophila melanogaster. Cell Stress Chaperones 2008; 13:509 - 526; PMID: 18506601; http://dx.doi.org/10.1007/s12192-008-0051-3
- Zeng L, Hu Z, Lu W, Tang X, Zhang J, Li T, et al. Small heat shock proteins: recent advances in neuropathy. Curr Neurovasc Res 2010; 7:155 - 166; PMID: 20438447; http://dx.doi.org/10.2174/156720210791184934
- Wang A, Liu X, Sheng S, Ye H, Peng T, Shi F, et al. Dysregulation of heat shock protein 27 expression in oral tongue squamous cell carcinoma. BMC Cancer 2009; 9:167; PMID: 19497117; http://dx.doi.org/10.1186/1471-2407-9-167
- Lo Muzio L, Campisi G, Farina A, Rubini C, Ferrari F, Falaschini S, et al. Prognostic value of HSP27 in head and neck squamous cell carcinoma: a retrospective analysis of 57 tumours. Anticancer Res 2006; 26:1343 - 1349; PMID: 16619543
- Garrido C. Size matters: of the small HSP27 and its large oligomers. Cell Death Differ 2002; 9:483 - 485; PMID: 11973606; http://dx.doi.org/10.1038/sj.cdd.4401005
- Bova MP, McHaourab HS, Han Y, Fung BK. Subunit exchange of small heat shock proteins. Analysis of oligomer formation of alphaA-crystallin and Hsp27 by fluorescence resonance energy transfer and site-directed truncations. J Biol Chem 2000; 275:1035 - 1042; PMID: 10625643; http://dx.doi.org/10.1074/jbc.275.2.1035
- Lelj-Garolla B, Mauk AG. Self-association of a small heat shock protein. J Mol Biol 2005; 345:631 - 642; PMID: 15581903; http://dx.doi.org/10.1016/j.jmb.2004.10.056
- Gibert B, Hadchity E, Czekalla A, Aloy MT, Colas P, Rodriguez-Lafrasse C, et al. Inhibition of heat shock protein 27 (HspB1) tumorigenic functions by peptide aptamers. Oncogene 2011;
- Charette SJ, Lavoie JN, Lambert H, Landry J. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol 2000; 20:7602 - 7612; PMID: 11003656; http://dx.doi.org/10.1128/MCB.20.20.7602-7612.2000
- Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol 2000; 2:645 - 652; PMID: 10980706; http://dx.doi.org/10.1038/35023595
- Choi SH, Lee YJ, Seo WD, Lee HJ, Nam JW, Kim J, et al. Altered cross-linking of HSP27 by zerumbone as a novel strategy for overcoming HSP27-mediated radioresistance. Int J Radiat Oncol Biol Phys 2011; 79:1196 - 1205; PMID: 21353161; http://dx.doi.org/10.1016/j.ijrobp.2010.10.025
- Aloy MT, Hadchity E, Bionda C, Diaz-Latoud C, Claude L, Rousson R, et al. Protective role of Hsp27 protein against gamma radiation-induced apoptosis and radiosensitization effects of Hsp27 gene silencing in different human tumor cells. Int J Radiat Oncol Biol Phys 2008; 70:543 - 553; PMID: 17980509; http://dx.doi.org/10.1016/j.ijrobp.2007.08.061
- Shashidharamurthy R, Koteiche HA, Dong J, McHaourab HS. Mechanism of chaperone function in small heat shock proteins: dissociation of the HSP27 oligomer is required for recognition and binding of destabilized T4 lysozyme. J Biol Chem 2005; 280:5281 - 5289; PMID: 15542604; http://dx.doi.org/10.1074/jbc.M407236200
- Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, et al. Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem 2003; 278:27828 - 27835; PMID: 12740362; http://dx.doi.org/10.1074/jbc.M303417200
- Havasi A, Li Z, Wang Z, Martin JL, Botla V, Ruchalski K, et al. Hsp27 inhibits Bax activation and apoptosis via a phosphatidylinositol 3-kinase-dependent mechanism. J Biol Chem 2008; 283:12305 - 12313; PMID: 18299320; http://dx.doi.org/10.1074/jbc.M801291200
- Mehlen P, Kretz-Remy C, Preville X, Arrigo AP. Human hsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFalpha-induced cell death. EMBO J 1996; 15:2695 - 2706; PMID: 8654367
- Arrigo AP, Virot S, Chaufour S, Firdaus W, Kretz-Remy C, Diaz-Latoud C. Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid Redox Signal 2005; 7:414 - 422; PMID: 15706088; http://dx.doi.org/10.1089/ars.2005.7.414
- Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP. Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol 2002; 22:816 - 834; PMID: 11784858; http://dx.doi.org/10.1128/MCB.22.3.816-834.2002
- Pivovarova AV, Mikhailova VV, Chernik IS, Chebotareva NA, Levitsky DI, Gusev NB. Effects of small heat shock proteins on the thermal denaturation and aggregation of F-actin. Biochem Biophys Res Commun 2005; 331:1548 - 1553; PMID: 15883049; http://dx.doi.org/10.1016/j.bbrc.2005.04.077
- Lee JH, Sun D, Cho KJ, Kim MS, Hong MH, Kim IK, et al. Overexpression of human 27 kDa heat shock protein in laryngeal cancer cells confers chemoresistance associated with cell growth delay. J Cancer Res Clin Oncol 2007; 133:37 - 46; PMID: 16906418; http://dx.doi.org/10.1007/s00432-006-0143-3
- Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol 1995; 15:505 - 516; PMID: 7799959
- Parcellier A, Schmitt E, Gurbuxani S, Seigneurin-Berny D, Pance A, Chantome A, et al. HSP27 is a ubiquitin-binding protein involved in I-kappaBalpha proteasomal degradation. Mol Cell Biol 2003; 23:5790 - 5802; PMID: 12897149; http://dx.doi.org/10.1128/MCB.23.16.5790-5802.2003
- Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci 2006; 31:164 - 172; PMID: 16483782; http://dx.doi.org/10.1016/j.tibs.2006.01.006
- Shiota M, Kusakabe H, Izumi Y, Hikita Y, Nakao T, Funae Y, et al. Heat shock cognate protein 70 is essential for Akt signaling in endothelial function. Arterioscler Thromb Vasc Biol 2010; 30:491 - 497; PMID: 20018937; http://dx.doi.org/10.1161/ATVBAHA.109.193631
- Ghobrial IM, McCormick DJ, Kaufmann SH, Leontovich AA, Loegering DA, Dai NT, et al. Proteomic analysis of mantle-cell lymphoma by protein microarray. Blood 2005; 105:3722 - 3730; PMID: 15650054; http://dx.doi.org/10.1182/blood-2004-10-3999
- Pick E, Kluger Y, Giltnane JM, Moeder C, Camp RL, Rimm DL, et al. High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res 2007; 67:2932 - 2937; PMID: 17409397; http://dx.doi.org/10.1158/0008-5472.CAN-06-4511
- Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR. Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer 1998; 79:468 - 475; PMID: 9761114; http://dx.doi.org/10.1002/(SICI)1097-0215(19981023)79:5,468::AID-IJC4.3.0.CO;2-Z
- Brondani Da Rocha A, Regner A, Grivicich I, Pretto Schunemann D, Diel C, Kovaleski G, et al. Radioresistance is associated to increased Hsp70 content in human glioblastoma cell lines. Int J Oncol 2004; 25:777 - 785; PMID: 15289883
- Abe M, Manola JB, Oh WK, Parslow DL, George DJ, Austin CL, et al. Plasma levels of heat shock protein 70 in patients with prostate cancer: a potential biomarker for prostate cancer. Clin Prostate Cancer 2004; 3:49 - 53; PMID: 15279691
- Ray S, Lu Y, Kaufmann SH, Gustafson WC, Karp JE, Boldogh I, et al. Genomic mechanisms of p210BCR-ABL signaling: induction of heat shock protein 70 through the GATA response element confers resistance to paclitaxel-induced apoptosis. J Biol Chem 2004; 279:35604 - 35615; PMID: 15155749; http://dx.doi.org/10.1074/jbc.M401851200
- Pocaly M, Lagarde V, Etienne G, Ribeil JA, Claverol S, Bonneu M, et al. Overexpression of the heat-shock protein 70 is associated to imatinib resistance in chronic myeloid leukemia. Leukemia 2007; 21:93 - 101; PMID: 17109025; http://dx.doi.org/10.1038/sj.leu.2404463
- Hsu HS, Lin JH, Huang WC, Hsu TW, Su K, Chiou SH, et al. Chemoresistance of lung cancer stemlike cells depends on activation of Hsp27. Cancer 2011; 117:1516 - 1528; PMID: 21425153; http://dx.doi.org/10.1002/cncr.25599
- Garrido C, Ottavi P, Fromentin A, Hammann A, Arrigo AP, Chauffert B, et al. HSP27 as a mediator of confluence-dependent resistance to cell death induced by anticancer drugs. Cancer Res 1997; 57:2661 - 2667; PMID: 9205074
- Zhu Z, Xu X, Yu Y, Graham M, Prince ME, Carey TE, et al. Silencing heat shock protein 27 decreases metastatic behavior of human head and neck squamous cell cancer cells in vitro. Mol Pharm 2010; 7:1283 - 1290; PMID: 20540527; http://dx.doi.org/10.1021/mp100073s
- Verrills NM, Liem NL, Liaw TY, Hood BD, Lock RB, Kavallaris M. Proteomic analysis reveals a novel role for the actin cytoskeleton in vincristine resistant childhood leukemia-an in vivo study. Proteomics 2006; 6:1681 - 1694; PMID: 16456880; http://dx.doi.org/10.1002/pmic.200500417
- Han J, Kioi M, Chu WS, Kasperbauer JL, Strome SE, Puri RK. Identification of potential therapeutic targets in human head & neck squamous cell carcinoma. Head Neck Oncol 2009; 1:27; PMID: 19602232; http://dx.doi.org/10.1186/1758-3284-1-27
- Kabakov AE, Kudryavtsev VA, Gabai VL. Hsp90 inhibitors as promising agents for radiotherapy. J Mol Med (Berl) 2010; 88:241 - 247; PMID: 19946660
- Dote H, Burgan WE, Camphausen K, Tofilon PJ. Inhibition of hsp90 compromises the DNA damage response to radiation. Cancer Res 2006; 66:9211 - 9220; PMID: 16982765; http://dx.doi.org/10.1158/0008-5472.CAN-06-2181
- Basso AD, Solit DB, Munster PN, Rosen N. Ansamycin antibiotics inhibit Akt activation and cyclin D expression in breast cancer cells that overexpress HER2. Oncogene 2002; 21:1159 - 1166; PMID: 11850835; http://dx.doi.org/10.1038/sj.onc.1205184
- Rocchi P, So A, Kojima S, Signaevsky M, Beraldi E, Fazli L, et al. Heat shock protein 27 increases after androgen ablation and plays a cytoprotective role in hormone-refractory prostate cancer. Cancer Res 2004; 64:6595 - 6602; PMID: 15374973; http://dx.doi.org/10.1158/0008-5472.CAN-03-3998
- Choi DH, Ha JS, Lee WH, Song JK, Kim GY, Park JH, et al. Heat shock protein 27 is associated with irinotecan resistance in human colorectal cancer cells. FEBS Lett 2007; 581:1649 - 1656; PMID: 17395183; http://dx.doi.org/10.1016/j.febslet.2007.02.075
- McCollum AK, Teneyck CJ, Sauer BM, Toft DO, Erlichman C. Up-regulation of heat shock protein 27 induces resistance to 17-allylamino-demethoxygeldanamycin through a glutathione-mediated mechanism. Cancer Res 2006; 66:10967 - 10975; PMID: 17108135; http://dx.doi.org/10.1158/0008-5472.CAN-06-1629
- Matsui Y, Hadaschik BA, Fazli L, Andersen RJ, Gleave ME, So AI. Intravesical combination treatment with antisense oligonucleotides targeting heat shock protein-27 and HTI-286 as a novel strategy for high-grade bladder cancer. Mol Cancer Ther 2009; 8:2402 - 2411; PMID: 19625496; http://dx.doi.org/10.1158/1535-7163.MCT-09-0148
- Machida H, Matsumoto Y, Shirai M, Kubota N. Geldanamycin, an inhibitor of Hsp90, sensitizes human tumour cells to radiation. Int J Radiat Biol 2003; 79:973 - 980; PMID: 14713575; http://dx.doi.org/10.1080/09553000310001626135
- Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 2003; 425:407 - 410; PMID: 14508491; http://dx.doi.org/10.1038/nature01913
- Wang RE, Kao JL, Hilliard CA, Pandita RK, Roti Roti JL, Hunt CR, et al. Inhibition of heat shock induction of heat shock protein 70 and enhancement of heat shock protein 27 phosphorylation by quercetin derivatives. J Med Chem 2009; 52:1912 - 1921; PMID: 19296652; http://dx.doi.org/10.1021/jm801445c
- Westerheide SD, Kawahara TL, Orton K, Morimoto RI. Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J Biol Chem 2006; 281:9616 - 9622; PMID: 16469748; http://dx.doi.org/10.1074/jbc.M512044200
- Teimourian S, Jalal R, Sohrabpour M, Goliaei B. Down-regulation of Hsp27 radiosensitizes human prostate cancer cells. Int J Urol 2006; 13:1221 - 1225; PMID: 16984557; http://dx.doi.org/10.1111/j.1442-2042.2006.01483.x
- Hadchity E, Aloy MT, Paulin C, Armandy E, Watkin E, Rousson R, et al. Heat shock protein 27 as a new therapeutic target for radiation sensitization of head and neck squamous cell carcinoma. Mol Ther 2009; 17:1387 - 1394; PMID: 19436268; http://dx.doi.org/10.1038/mt.2009.90
- Schulte TW, Blagosklonny MV, Ingui C, Neckers L. Disruption of the Raf-1-Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J Biol Chem 1995; 270:24585 - 24588; PMID: 7592678; http://dx.doi.org/10.1074/jbc.270.41.24585
- Dote H, Cerna D, Burgan WE, Camphausen K, Tofilon PJ. ErbB3 expression predicts tumor cell radiosensitization induced by Hsp90 inhibition. Cancer Res 2005; 65:6967 - 6975; PMID: 16061682; http://dx.doi.org/10.1158/0008-5472.CAN-05-1304
- Hall E, Giaccia A. Radiobiology for the Radiologist 2006; Philadelphia Lippincott Williams and Wilkins
- Dungey FA, Caldecott KW, Chalmers AJ. Enhanced radiosensitization of human glioma cells by combining inhibition of poly(ADP-ribose) polymerase with inhibition of heat shock protein 90. Mol Cancer Ther 2009; 8:2243 - 2254; PMID: 19671736; http://dx.doi.org/10.1158/1535-7163.MCT-09-0201
- Lee YS, Chang HW, Jeong JE, Lee SW, Kim SY. Proteomic analysis of two head and neck cancer cell lines presenting different radiation sensitivity. Acta Otolaryngol 2008; 128:86 - 92; PMID: 17917836; http://dx.doi.org/10.1080/00016480601110196
- Mehlen P, Kretz-Remy C, Briolay J, Fostan P, Mirault ME, Arrigo AP. Intracellular reactive oxygen species as apparent modulators of heat-shock protein 27 (hsp27) structural organization and phosphorylation in basal and tumour necrosis factor alpha-treated T47D human carcinoma cells. Biochem J 1995; 312:367 - 375; PMID: 8526844
- Koll TT, Feis SS, Wright MH, Teniola MM, Richardson MM, Robles AI, et al. HSP90 inhibitor, DMAG, synergizes with radiation of lung cancer cells by interfering with base excision and ATM-mediated DNA repair. Mol Cancer Ther 2008; 7:1985 - 1992; PMID: 18645008; http://dx.doi.org/10.1158/1535-7163.MCT-07-2104
- Noguchi M, Yu D, Hirayama R, Ninomiya Y, Sekine E, Kubota N, et al. Inhibition of homologous recombination repair in irradiated tumor cells pretreated with Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Biochem Biophys Res Commun 2006; 351:658 - 663; PMID: 17083915; http://dx.doi.org/10.1016/j.bbrc.2006.10.094
- Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell 2004; 5:429 - 441; PMID: 15144951; http://dx.doi.org/10.1016/S1535-6108(04)00115-1
- Sharp S, Workman P. Inhibitors of the HSP90 molecular chaperone: current status. Adv Cancer Res 2006; 95:323 - 348; PMID: 16860662; http://dx.doi.org/10.1016/S0065-230X(06)95009-X
- Kim WY, Oh SH, Woo JK, Hong WK, Lee HY. Targeting heat shock protein 90 overrides the resistance of lung cancer cells by blocking radiation-induced stabilization of hypoxia-inducible factor-1alpha. Cancer Res 2009; 69:1624 - 1632; PMID: 19176399; http://dx.doi.org/10.1158/0008-5472.CAN-08-0505
- Bisht KS, Bradbury CM, Mattson D, Kaushal A, Sowers A, Markovina S, et al. Geldanamycin and 17-allylamino-17-demethoxygeldanamycin potentiate the in vitro and in vivo radiation response of cervical tumor cells via the heat shock protein 90-mediated intracellular signaling and cytotoxicity. Cancer Res 2003; 63:8984 - 8995; PMID: 14695217
- Mazeron R, Anderson B, Supiot S, Paris F, Deutsch E. Current state of knowledge regarding the use of antiangiogenic agents with radiation therapy. Cancer Treat Rev 2011; 37:476 - 486; PMID: 21546163
- Lee CG, Heijn M, di Tomaso E, Griffon-Etienne G, Ancukiewicz M, Koike C, et al. Anti-Vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res 2000; 60:5565 - 5570; PMID: 11034104