Abstract
Inducing apoptosis is an attractive antitumor strategy. PERP is an apoptosis-associated target of p53, and its activation alone is sufficient to induce apoptotic pathway leading to cell death. We have previously demonstrated that overexpression of PERP in tumor cell lines with low intrinsic PERP activity suppressed cancer cell growth and enhanced sensitivity to chemotherapeutical agents. We further identified that PERP was present in surgical normal lung tissue, but absent in cancerous tissue of the same patient. Here, we sought to investigate the anti-tumor effects of PERP gene therapy in vivo. Then nude mice were transplanted with p53-mutanted Anip973 human lung cancer xenografts and treated with normal saline, pcDNA3.1 (vector) and pcDNA3.1-PERP, respectively. Successful transfection and robust expression of PERP was detected. Treatment with pcDNA3.1-PERP increased apoptosis and retarded growth in the xenografts, which contributed to a 55% decrease in tumor volume compared with controls. Furthermore, PERP gene therapy activated pro-apoptotic Caspase-3 cascade and upregulated the expression of the second mitochondria-derived activator of caspase (Smac) and human TNF-related apoptosis-inducing ligand (TRAIL), while suppressed vascular endothelial growth factor (VEGF) expression, indicating apoptosis and anti-angiogenesis are involved in the inhibitory effect of the PERP gene therapy. Taken together, our results suggest PERP gene therapy may supply an alternative strategy for lung adenocarcinoma management. Furthermore, Anip973 is a p53-mutanted cell line and the findings of this study provide reference value for other p53-mutanted cancers which is common among malignant tumors.
Introduction
Lung cancer is one of the most common malignancies and leading causes of death in the world. Conventional treatment strategies include surgery, chemotherapy, irradiation and a combination of these. Despite improvements in diagnostic tools and considerable progress in treatment protocols, the low survival rate of patients with lung cancer has hardly changed.Citation1 Thus, alternative therapeutic agents and treatment strategies are necessitated for better management of lung cancer. Many tumor suppress genes are frequently altered in lung cancer and restoration of these gene functions may prevent tumor progression. Therefore, exhaustive searches for the identification of genes altered in carcinogenesis are being conducted. It was initially reported that a major lung cancer susceptibility locus existed within chromosome 6q23–25.Citation2 Interestingly, PERP (TP53 apoptosis effector) gene has been assigned to chromosome 6q24, a region implicated in the development of a large variety of cancers.Citation3 Lower expression of PERP or frequent loss of heterozygosity (LOH) for this region was observed in human malignancies, such as cervical cancer, breast cancer, prostate cancer, pancreatic cancer, malignant melanoma and their metastases.Citation4-Citation8 It also has been demonstrated that PERP loss promotes tumorigenesis in a context specific manner, such as UV-induced skin cancer.Citation9 However, the anti-tumor activities and mechanisms of this gene in vivo have not been studied.
Based on these facts, we hypothesized that overexpression of PERP gene in vivo would suppress the growth of lung cancers with low intrinsic PERP level. Using a non-viral gene therapeutic approach, we analyzed the inhibitory effects of PERP in human lung adenocarcinoma xenografts in vivo. Herein, we demonstrate intra-tumoral delivery of plasmid-PERP construct attenuated tumor growth through induction of apoptosis by activating Caspase-3 activity and enhancing the expression of Smac and TRAIL; in addition, exogenous PERP also suppressed VEGF expression. Hence, these findings exhibited tumor-suppressive activities of PERP gene therapy and held potential for alternative treatment of pulmonary adenocarcinoma.
Results
Screening of targeted human cancer cell line in vitro and test of clinical samples
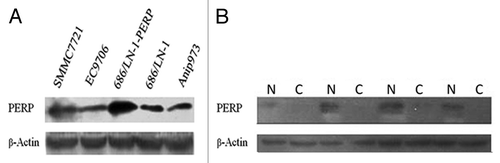
Downregulation of PERP gene expression was observed in human melanoma, mammary carcinoma, ovarian cancer and cervical cancer.Citation7,Citation8 Low expression level of PERP in multiple cancers and its location on chromosome 6q24 strongly suggested that PERP might be a tumor suppressor gene. This notion was further strengthened by the fact that lack of PERP promoted tumorigenesis.Citation9 In order to study the potential anti-tumor effects of PERP, we screened its expression in a series of malignancies other than the ones above-mentioned and found PERP expression in human lung adenocarcinoma cell line Anip973 was significantly lower than others (). More importantly, detection of four-paired surgical lung adenocarcinoma samples showed expression of PERP in normal lung epithelial tissue, but null in cancerous tissue of the same patient (). In addition, Anip973 was a p53-mutated human lung adenocarcinoma cell line with high expression of HER2/neuCitation10 and activation of PERP is sufficient to induce cell death solely.Citation3 Therefore, Anip973 was chosen as targeted cancer cell line for PERP gene therapy.
Figure 1. Screening of targeted human cancer cell line and tests of clinical samples. (A) PERP expression was assayed by immunobloting in some human cancer cell lines. SMMC7721, hepatocellular carcinoma; EC9706, esophageal carcinoma; Anip973, lung adenocarcinoma; 686/LN-1, oral squamous cell carcinoma lymph node metastasis; 686/LN-1-PERP, 686/LN-1 transfected with pcDNA3.1-PERP plasmid. (B) Four-paired samples of human lung adenocarcinoma were detected by PERP immunobloting. C, cancerous tissue of lung adenocarcinoma; N, normal lung tissue of the same patient.
Exogenous PERP expression in vivo
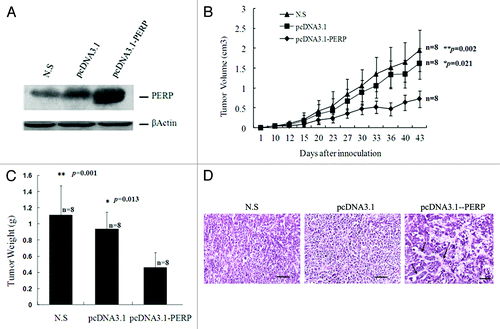
To examine the expression of exogenous PERP in target tumor in vivo, protein gel blot analysis was performed with proteins extracted from xenografts treated with PERP, Vector or N.S. As shown in , the PERP protein level in the PERP-treated xenografts was markedly elevated compared with the other two groups, indicating efficient transfection and robust expression of this gene in the therapeutic group.
Figure 2. PERP overexpression inhibit lung cancer development in BALB/c nude mice. (A) PERP expression was detected in xenografts from N.S, Vector and PERP group, respectively. (B and C) Tumor growth curve (B) and weight (C) for Anip973 xenografts in mice undergoing PERP intervention. *p < 0.05, **p < 0.01. (D) H&E stainning of xenograft sections from N.S, Vector and PERP group, respectively. Arrows denote adenoid structure. Scale bar represents 100 μm.
PERP attenuates lung cancer xenograft in vivo
The mice were regularly monitored and the tumors were measured with caliper prior to sacrifice. Tumor volume (cm3) of N.S group, Vector group, PERP group was 1.9423 ± 1.0828, 1.6141 ± 0.9191, 0.7255 ± 0.5063 (means ± SD), respectively. The inhibition rate of PERP group to Vector group was 55% (inhibition rate = 1-PERP/Vector Volume × 100%). And a significant attenuation of tumor volume was observed in PERP group compared with N.S or Vector group (p = 0.002, 0.021) respectively () with no significant difference between the latter two (p = 0.23). The mice were euthanized by CO2 inhalation, and tumors were excised and weighed on the 43rd day post-transplantation. With PERP treatment, there was a significantly regressive tumor weight compared with control groups (p = 0.001, 0.013) (). Of note, histo-pathological examination of the xenografts section revealed a tendency to forming adenoid structure (grade 2, see arrow) in PERP group, which was not observed in controls where cancer cell arranged in solid structure (grade 3, ). Furthermore, intratumoral injection of the DNA/liposome complex did not induce any systemic toxicity or weight loss (data not shown). To sum up, overexpression of PERP effectively inhibited the progression of the highly tumorigenic cell line Anip973 in vivo.
PERP’s antitumor activities are associated with inducing apoptosis and suppressing VEGF in vivo
Previously our data showed PERP gene augments the apoptotic rate of cancer cell lines in vitro (Fig. S1). Thus, cancer cells transfected with pcDNA3.1-PERP were expected to recapitulate this potency in vivo. Therefore, a TUNEL assay was employed to compare apoptotic index in three groups. As shown in , a few TUNEL-positive cells were detected in tumor tissues obtained from N.S group and Vector group; however, treatment with PERP gene therapy remarkably increased TUNEL-positive cells. The apoptotic index of N.S, Vector, and PERP tumors were 5.6, 8.7, and 29.5, respectively. It suggests that smaller tumors formed in PERP group are due to the increased apoptosis in part.
Figure 3. PERP induces apoptosis and downregulate VEGF. (A) TUNEL staining of Anip973 xenografts from N.S, Vector and PERP group, respectively. Arrows denote TUNEL positive tumor nuclei. Scale bar represents 100 μm. (B–F) Expression of Caspase 3, PARP, Smac, TRAIL and VEGF by immunoblotting were statistically significant in Anip973 xenografts from N.S, Vector and PERP group, respectively.
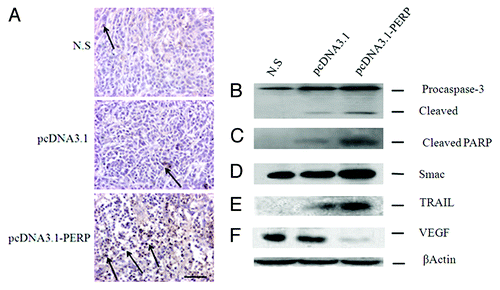
To further evaluate the mechanism of PERP-induced attenuation of tumor growth and induction of apoptosis in lung cancer xenograft models, expression of some proteins associated to apoptosis were analyzed. First, we examined protein level of Caspase-3 in tumors. As shown in , xenografts administrated intratumorally with PERP increased both pro- and cleaved Caspase-3. Second, immunoblotting showed PARP was cleaved in tumors injected with PERP, whereas the controls had no or little such effect (). Third, we determined whether PERP induces the release of proapoptotic proteins from mitochondria. As shown in , Smac in PERP-treated tumor cytosol was more excessive than the controls. Fourthly, to test whether TRAIL might contribute to apoptosis in xenografts, tumor proteins from three groups were also assessed. TRAIL expression in PERP group was found to be upregulated compared with controls () and it suggests that PERP elicit apoptosis in an additional promoting effect. These results demonstrated antitumor activity of PERP is closely associated with apoptosis in human lung cancer xerograft models. In addition, because expression of angiogenic factor, such as VEGF, is critical for the onset of angiogenesis, we examined it and found VEGF was suppressed in PERP group compared with controls ().
Discussion
We demonstrated PERP gene therapy suppress tumor growth in nude mice bearing Anip973 human lung cancer xenograft. PERP is a mediator of p53-dependent apoptosis and a new tumor suppressor gene.Citation7,Citation11 Clinical samples of lung adenocarcinoma demonstrated the PERP expression in normal tissue, but unexpression in cancerous lesion. () Previously our work implicated that PERP inhibits cancer cell growth, sensitizes cancer cells to chemical agents in vitro. (Fig. S1) More recently, it was reported PERP acts as a cell-cell adhesion protein and is essential for epithelial integrity.Citation12,Citation13 Anip973 is a p53-mutated cell line. After PERP gene therapy in the study, xenografts showed higher PERP level than controls (), indicating effectively PERP expression independent of p53 in vivo. The findings of our study offer reference value for other p53-mutanted cancers which is common among malignant tumors.
Apoptosis is a distinct mode of cell death that is responsible for deletion of cells in malignant tumors as well as normal tissues. The rate of apoptosis in malignant tumors is an important factor for tumor growth.Citation14 In the present report, the mass and volume of the xerografts from PERP group were markedly reduced than controls (). The capability of PERP to induce apoptosis and then attenuate tumor growth were demonstrated by TUNEL and protein gel blot. In our mouse models, TUNEL assay showed typical changes characteristic of apoptosis (). And overexpression of PERP elevated the protein level of Smac, TRAIL, executive Caspase-3 and its substrate PARP (). In addition, Smac was shown to act synergistically with TRAIL to induce tumor-selective apoptosis.Citation15 This report is consistent with our result. According to our results, decreased tumor growth was correlated negatively with increased exogenous PERP expression and increased apoptotic activities, suggesting the attenuation of tumor growth by PERP was mediated via an apoptotic pathway partly. Inducing apoptosis of PERP from our study in vivo is in accord with previous work in vitro.Citation3 Besides, it is formally possible that PERP could play roles beyond apoptosis, such as in regulating cell proliferation, which might also hold the possibility to modulate tumor growth.
On the other hand, growth of solid tumor is angiogenesis-dependent. VEGF is a major promoter of tumor angiogenesisCitation16 and increase of tumor-derived VEGF is supposed to promote angiogenesis.Citation17 So we probed it and found a decreased VEGF expression in PERP group (), suggesting PERP prohibit the expression of angiogenic factors which cause suppression of angiogenesis. Meanwhile, the connection between PERP and VEGF remains unclear. And to the best of our knowledge, it is the first report that link PERP gene therapy and VEGF expression. It is necessary to clarify this interaction in future study.
At the same time, the nonspecific tumor inhibitory activity observed in Vector group is not significant and is in agreement with previous reports.Citation18 The antitumor activity was significantly higher in PERP-treated xerografts explained the specificity. In our study, DOTAP transfection reagent is a monolayer liposome suspension and made from DOTAP and neutral auxiliary lipids with specific percentum. And it incorporates DNA to facilitate endocytosis and can be biodegraded. Impressively, all of the treated mice tolerated the intervention very well without any sign of visible toxicity. Moreover, there was a trend of forming adenoid phenotype under light microscopy in PERP group, but not that in controls (), which may suggest a phenotypic reversion after PERP gene therapy. The results implicate that animals receiving in vivo PERP gene transfer are highly susceptible to antitumor effect and no undesirable side effects was observed. Therefore, the reliability of DOTAP transfection reagent was evident thanks to its high transfection rate and low toxicity.
Although we have demonstrated PERP has potent tumor suppressive activities in vivo, the exact function(s) of PERP gene has not been studied in detail. PERP is a bona fide p53 target geneCitation3 and is a member of an expanding family of tetraspan membrane proteins, including the peripheral myelin protein (PMP-22) and epithelial membrane proteins (EMP1–3).Citation19 Both PMP-22 and the EMPs show significant sequence similarity to PERP and have been shown to induce apoptosis when overexpressed.Citation20 However, it is unclear how these tetraspan membrane proteins, including PERP, interface with the canonical apoptotic pathway, and therefore the exact mechanism by which they stimulate apoptosis remains to be elucidated.Citation21 On the other side, the various roles played by other tetraspan proteins may provide clues to the mechanism of action of PERP. Tetraspan membrane proteins have been suggested in a multiplicity of cellular activity, such as trafficking, receptor function, channel activity, and cell adhesion.Citation12,Citation13,Citation22 It is easy to envision that PERP could act in an analogous manner. In addition, TRAIL protein expression in PERP-treated group was undoubtedly higher than controls, implicating PERP at least partially facilitating the shuttling of some critical death receptor proteins. The structure of PERP is different from Bcl-2 family members Bax, Noxa or Puma, but all of them are involving in p53-dependent apoptosis. Now it is not clearly defined whether there is interaction between PERP and Bax, Noxa or Puma. In the coming future, it will be crucial to dissect the mechanisms by which PERP serves or functions in a special way of apoptosis.
Despite significant advances achieved during the past decades for the treatment of lung cancer, the overall 5-y survival rate is poor.Citation1 Hence, there is an urgent need for novel treatment strategies. Our PERP gene therapy in human lung cancer xenograft models will pave the way for future trials to determine the efficacy of combined treatments in preclinical settings. Several studies have reported that ionizing radiationCitation23 and chemotherapyCitation24 improves gene transfer efficacy. By parity of reasoning, combination of PERP-liposome transfer with chemotherapy or radiaotherapy may give rise to more effective tumor regression and be of clinical relevance.
In summary, our studies proved the tumor suppressive activity of PERP gene therapy in vivo, which associated with apoptosis and VEGF suppression. And it holds promising for attenuating lung adenocarcinoma growth in clinic.
Materials and Methods
Materials and surgical samples
Rabbit anti-PERP polyclonal antibody was obtained from eBioscience Inc. The antibodies against human Caspase-3, Smac, TRAIL, VEGF, PARP and Actin were purchased from Santa Cruz Biotechnology Inc. Liposome DOTAP and cholesterol with high transfecting rate were purchased from Applygen Technologies Inc. RPMI-1640 medium and bovine serum albumin (BSA) were obtained from GIBCO-BRL-Life Technologies. Four-paired lung adenocarcinoma samples were from Thoracic Surgical Oncology of Cancer Institute and Hospital, Chinese Academy of Medical Sciences. Informed Consent Forms were attained and approval for the study was obtained from the Ethical Committee of our institutes.
Construction of PERP gene into eukaryotic expression vector
pUC57 vector containing PERP cDNA was purchased from Invitrogen Inc. Then PERP cDNA was subcloned into the multiple cloning sites of pcDNA3.1 plasmid (Invitrogen). The presence of the appropriate inserts was confirmed by restriction enzyme mapping and sequencing. The recombinant plasmid was purified using the Maxi prep KIT from Promega according to manufacturer’s protocol (Promega).
Cell lines and culture condition
Anip973 human lung adenocarcinoma cell line and EC9706 human esophageal carcinoma cell line are gifts from Dr. Wang (National Key Laboratory of Molecular Oncology, Chinese Academy of Medical Sciences), SMMC7721 human hepatocarcinoma cell line was provided by the cell bank, Institute of Cell Biology, Chinese Academy of Sciences. 686/LN-1 human oral squamous cell carcinoma lymph node metastasis cell line (a gift from Dr. Hong, MD Anderson Cancer Center) is kept in our laboratory. 686/LN-1-PERP cell line was established by 686/LN-1 transfecting with plasmid pcDNA3.1-PERP. All of these cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum and were grown in 5% CO2 at 37°C in the incubator.
Subcutaneous lung cancer xenograft in nude mice
Twenty-four BALB/C- nude mice (male 12, female 12), 6–8 weeks old, were provided by the Laboratorial Animal Institute of Peking Union Medical College. Nude mice were maintained in a pathogen-free environment and handled according to animal care and use of Ethics Committee of Animal Experimentation in our institutes. Anip973 cells were inoculated (2 x 106cells/0.2ml/mouse) s.c. at the right scapular of the mouse. Tumor sizes were measured with a caliper at each time interval as shown in . The tumor volume was calculated using the formula: Volume = S × S × L/2, where S is the short length of the tumor in cm and L is the long length of the tumor in cm.
Effect of PERP gene on lung cancer xenograft
When the tumor grew up to 0.5 cm in diameter, animals bearing tumors were randomized into three groups (n = 8/group) and treatment was followed. Mice were treated with normal saline (100 µl, N.S group), pcDNA3.1 vector-liposome complex (100 µl = 50 µg DNA + 50 µl liposome, Vector group) and pcDNA3.1-PERP-liposome mixture (100 µl = 50 µg DNA + 50 µl liposome, PERP group), respectively. The DNA-liposome mixture was prepared freshly just before each use. Treatment was processed through intratumoral injection on the 10th, 12th, 15th, 20th, 23rd, 27th day for a total of six doses with 25-gauge needles.
Xenograft histopathologic examination
Xenograft tumor tissues were fixed in 10% neutral buffered formalin. Tissue samples were paraffin embedded, sectioned, and H&E stained for histopathologic evaluation.
TUNEL assay
To determine the fate of tumor cell following treatments, cancer cells and subcutaneous tumor tissues harvested from animals were subjected to Terminal dUTP Nick-End Labeling (TUNEL) assay as recommended by the manufacturer (Promega). Briefly, sections were deparaffinized and rehydrated, and the endogenous peroxidase activity was blocked. The sections were incubated in proteinase K and then incubated with TUNEL reaction mixture. Slides were incubated with converter-peroxidase solution for a further incubation. The reaction was visualized with diaminobenzidine/H2O2. Slides were subsequently washed, counterstained with hematoxylin and mounted. Negative control sections included the above process except the enzyme solution and positive control were prepared by treating sections with 1 µg/ml DNase for 10 min before the above protocol. Cells exhibiting nuclear staining dark brown without cytoplasmic background were regarded as undergoing apoptosis. To calculate the percentage of apoptotic cells in 10 high-power fields (1,000 cells), apoptotic index was estimated as follows: apoptotic cells/total number of cells × 100. Slide examination was performed by four independent observers with minimal interobserver variability.
Protein gel blot analysis
Cellular, tissue and xenograft protein were prepared. Equal amounts of protein (50–100 µg) were electrophoresized onto sodium dodecylsulfate (SDS)-polyacrylamide gels and transferred to a PVDF membrane (Amersham). The membrane was first incubated in a dilution of 1:500–2,000 with the following primary antibodies: PERP, Caspase-3, PARP, Smac, TRAIL, VEGF; and then developed with the secondary antibodies. Enhanced chemiluminescence reagents were used to detect the signals according to the manufacturer’s instructions (Pierce).
Statistical analysis
The data from three groups were compared statistically by one-way ANOVA test using SPSS 10.0 statistical software. p < 0.05 was considered statistically significant.
| Abbreviations: | ||
| LOH | = | loss of heterozygosity |
| PARP | = | poly (ADP-ribose) polymerase |
| PERP | = | TP53 apoptosis effector |
| Smac | = | second mitochondria-derived activator of caspase |
| TRAIL | = | TNF-related apoptosis-inducing ligand |
| VEGF | = | vascular endothelial growth factor |
Additional material
Download Zip (274.7 KB)Acknowledgments
This work was supported by a grant from National Natural Science Foundation of China (81101940). We thank Jing Wu for editing the manuscript and Jietao Song for help with animal care.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69 - 90; http://dx.doi.org/10.3322/caac.20107; PMID: 21296855
- Bailey-Wilson JE, Amos CI, Pinney SM, Petersen GM, de Andrade M, Wiest JS, et al. A major lung cancer susceptibility locus maps to chromosome 6q23-25. Am J Hum Genet 2004; 75:460 - 74; http://dx.doi.org/10.1086/423857; PMID: 15272417
- Attardi LD, Reczek EE, Cosmas C, Demicco EG, McCurrach ME, Lowe SW, et al. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev 2000; 14:704 - 18; PMID: 10733530
- Mazurenko N, Attaleb M, Gritsko T, Semjonova L, Pavlova L, Sakharova O, et al. High resolution mapping of chromosome 6 deletions in cervical cancer. Oncol Rep 1999; 6:859 - 63; PMID: 10373671
- Noviello C, Courjal F, Theillet C. Loss of heterozygosity on the long arm of chromosome 6 in breast cancer: possibly four regions of deletion. Clin Cancer Res 1996; 2:1601 - 6; PMID: 9816339
- Davies L, Gray D, Spiller D, White MR, Damato B, Grierson I, et al. P53 apoptosis mediator PERP: localization, function and caspase activation in uveal melanoma. J Cell Mol Med 2009; 13:1995 - 2007; http://dx.doi.org/10.1111/j.1582-4934.2008.00590.x; PMID: 19040420
- Hildebrandt T, Preiherr J, Tarbe N, Klostermann S, Van Muijen GN, Weidle UH. Identification of THW, a putative new tumor suppressor gene. Anticancer Res 2000; 20:2801 - 9; PMID: 11062687
- Hildebrandt T, van Dijk MC, van Muijen GN, Weidle UH. Loss of heterozygosity of gene THW is frequently found in melanoma metastases. Anticancer Res 2001; 21:1071 - 80; PMID: 11396142
- Beaudry VG, Jiang D, Dusek RL, Park EJ, Knezevich S, Ridd K, et al. Loss of the p53/p63 regulated desmosomal protein Perp promotes tumorigenesis. PLoS Genet 2010; 6:e1001168; http://dx.doi.org/10.1371/journal.pgen.1001168; PMID: 20975948
- Ma LL, Sun WJ, Wang Z, Zh GY, Li P, Fu SB. Effects of silencing of mutant p53 gene in human lung adenocarcinoma cell line Anip973. J Exp Clin Cancer Res 2006; 25:585 - 92; PMID: 17310850
- Ihrie RA, Reczek E, Horner JS, Khachatrian L, Sage J, Jacks T, et al. Perp is a mediator of p53-dependent apoptosis in diverse cell types. Curr Biol 2003; 13:1985 - 90; http://dx.doi.org/10.1016/j.cub.2003.10.055; PMID: 14614825
- Ihrie RA, Marques MR, Nguyen BT, Horner JS, Papazoglu C, Bronson RT, et al. Perp is a p63-regulated gene essential for epithelial integrity. Cell 2005; 120:843 - 56; http://dx.doi.org/10.1016/j.cell.2005.01.008; PMID: 15797384
- Ihrie RA, Attardi LD. A new Perp in the lineup: linking p63 and desmosomal adhesion. Cell Cycle 2005; 4:873 - 6; http://dx.doi.org/10.4161/cc.4.7.1836; PMID: 15970683
- Rossi D, Gaidano G. Messengers of cell death: apoptotic signaling in health and disease. Haematologica 2003; 88:212 - 8; PMID: 12604411
- Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med 2002; 8:808 - 15; PMID: 12118245
- Lassoued W, Murphy D, Tsai J, Oueslati R, Thurston G, Lee WM. Effect of VEGF and VEGF Trap on vascular endothelial cell signaling in tumors. Cancer Biol Ther 2011; 10:1326 - 33; http://dx.doi.org/10.4161/cbt.10.12.14009; PMID: 21079419
- Larsen AK, Ouaret D, El Ouadrani K, Petitprez A. Targeting EGFR and VEGF(R) pathway cross-talk in tumor survival and angiogenesis. Pharmacol Ther 2011; 131:80 - 90; http://dx.doi.org/10.1016/j.pharmthera.2011.03.012; PMID: 21439312
- Ito I, Saeki T, Mohuiddin I, Saito Y, Branch CD, Vaporciyan A, et al. Persistent transgene expression following intravenous administration of a liposomal complex: role of interleukin-10-mediated immune suppression. Mol Ther 2004; 9:318 - 27; http://dx.doi.org/10.1016/j.ymthe.2004.01.007; PMID: 15006598
- Jetten AM, Suter U. The peripheral myelin protein 22 and epithelial membrane protein family. Prog Nucleic Acid Res Mol Biol 2000; 64:97 - 129; http://dx.doi.org/10.1016/S0079-6603(00)64003-5; PMID: 10697408
- Wilson HL, Wilson SA, Surprenant A, North RA. Epithelial membrane proteins induce membrane blebbing and interact with the P2X7 receptor C terminus. J Biol Chem 2002; 277:34017 - 23; http://dx.doi.org/10.1074/jbc.M205120200; PMID: 12107182
- Ihrie RA, Attardi LD. Perp-etrating p53-dependent apoptosis. Cell Cycle 2004; 3:267 - 9; http://dx.doi.org/10.4161/cc.3.3.722; PMID: 14726658
- Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol 2003; 19:397 - 422; http://dx.doi.org/10.1146/annurev.cellbio.19.111301.153609; PMID: 14570575
- Yamini B, Yu X, Pytel P, Galanopoulos N, Rawlani V, Veerapong J, et al. Adenovirally delivered tumor necrosis factor-alpha improves the antiglioma efficacy of concomitant radiation and temozolomide therapy. Clin Cancer Res 2007; 13:6217 - 23; http://dx.doi.org/10.1158/1078-0432.CCR-07-1421; PMID: 17947489
- Yan J, Tie G, Hoffman A, Yang Y, Nowicki PT, Messina LM. Oral tetrahydrobiopterin improves the beneficial effect of adenoviral-mediated eNOS gene transfer after induction of hindlimb ischemia. Mol Ther 2010; 18:1482 - 9; http://dx.doi.org/10.1038/mt.2010.109; PMID: 20551918