Abstract
Rho, a Ras-related small GTPase, and Rho-associated coiled coil-containing protein kinase (Rho kinase, ROCK1 and ROCK2) are key regulators of focal adhesion, actomyosin contraction, and thus cell motility. Rho/ROCK kinases also play roles in proliferation, differentiation, apoptosis and oncogenic transformation. In the present study, we have shown that Rho/ROCK pathway inhibition by fasudil, an orally administered inhibitor of Rho kinases, enhanced cisplatin-induced growth inhibition and apoptosis in human ovarian cancer cell lines. Fasudil inhibited hypoxia inducible factor (HIF)-1α protein expression. Knockdown of RhoA, ROCK1 or ROCK2 also attenuated the expression of HIF-1α. Furthermore, knockdown of HIF-1α using small interfering RNA enhanced cisplatin-induced growth inhibition and apoptosis as did inhibition of the Rho/ROCK pathway by fasudil, the Rho/ROCK inhibitor Y27632, or by Rho/ROCK knockdown. Therefore, the Rho/ROCK pathway may modulate HIF-1α signal transduction and blockade of Rho/ROCK enhances the efficacy of cisplatin by inhibiting HIF-1α in ovarian cancer cells. Our findings suggested that the Rho/ROCK pathway may be a new target for molecular targeting therapies against ovarian cancer.
Introduction
Ovarian cancer is a major cause of death from gynecological malignancy. A platinum-based combination with paclitaxel is now the most effective first-line chemotherapy.Citation1,Citation2 There has been some improvement in survival with the introduction of platinum and paclitaxel therapy; nevertheless, the likelihood of success in the treatment of patients with advanced, recurrent, or persistent ovarian cancer has remained largely unchanged for decades.Citation3 A strong predictive factor for ovarian cancer patients is the sensitivity of their tumor to platinum.Citation4 Therefore, it is very important to understand how cancer becomes platinum-resistant and to develop molecular targeting therapies for platinum-resistant ovarian cancer. Several studies have shown the effect of molecular targeting therapies on ovarian cancers.Citation5 The most promising molecular targeting therapy is the blockade of angiogenesis using antiangiogenic drugs, such as bevacizumab, which is an antibody to vascular endothelial growth factor (VEGF), and a phase III study combining bevacizumab with first-line chemotherapy is currently ongoing to assess its benefits in ovarian cancer patients.
VEGF is an important regulator of angiogenesis in many types of cancer, the expression of which is mediated by the transcription factor hypoxia-inducible factor (HIF)-1α, which plays a key role in regulating the adaptation of tumors to hypoxia.Citation6 Hypoxia contributes to selecting cancer cells that are resistant to apoptosis and mediates resistance to chemotherapy and radiation therapy.Citation7 Clinical studies support the notion that HIF-1α is also an important regulator of angiogenesis and tumor growth, and HIF-1α overexpression is associated with patient mortality and a poor response to treatment in ovarian cancers.Citation8,Citation9
We previously reported that Rho A is activated and affects HIF-1α expression under hypoxia, and fasudil, a specific inhibitor of Rho kinase,Citation10 prevented HIF-1α expression in endothelial cells.Citation11 Rho kinases, also termed Rho-associated coiled coil-containing protein kinases (ROCK1 and ROCK2), were originally isolated as RhoA-GTP interacting proteins.Citation12 Rho kinase promotes actin-myosin-mediated contractile force generation through the phosphorylation of numerous downstream target proteins, including the myosin light chain (MLC)Citation12 and LIM kinase,Citation13 suggesting the involvement of the Rho/Rho kinase pathway in cell migration, invasion, cell-cell adhesion, smooth muscle contraction, cytokinesis, and mitosis.Citation14-Citation17 Rho/ROCK kinases also play roles in proliferation, differentiation, apoptosis, and oncogenic transformation, although these responses can be cell-type-dependent.Citation10 The specific Rho kinase inhibitor Y-27632 inhibits the invasiveness and tumor growth of several human cancer cellsCitation18 including ovarian cancer.Citation19 Therefore, the Rho/ROCK pathway seems to be an attractive molecular target for the development of cancer therapy.
Recently, Ying et al. showed that fasudil inhibits tumor progression in human and rat tumor models.Citation20 In this study, we used fasudil as a Rho kinase inhibitor to investigate the involvement of the Rho/ROCK pathway in ovarian cancer. Fasudil, a well-tested orally available drug, has been approved in Japan for the treatment of cerebral vasospasmCitation21 and is well-tolerated without any severe adverse reactions.Citation22 Therefore, fasudil can be used easily in the clinical setting. Also, blockade of the Rho/ROCK pathway enhanced cisplatin-induced cytotoxicity through suppression of focal adhesion kinase-independent mechanisms in lung carcinoma cells.Citation23 There have been no reports about the effect of Rho/ROCK pathway inhibition on the sensitivity of cisplatin in ovarian cancers. These considerations led us to examine whether fasudil enhances the sensitivity of ovarian cancer to cisplatin in vitro. We also investigated whether the inhibition of HIF-1α, a downstream protein of the Rho/ROCK pathway, modulates cisplatin-induced cytotoxicity.
Results
Effects of the combination of cisplatin with fasudil on cell proliferation in cisplatin-sensitive and -resistant ovarian cancer cells
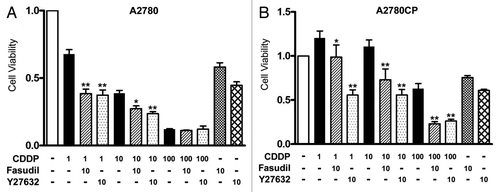
We investigated the effect of fasudil on cell viability in cisplatin-treated cells using the MTS assay (). The cells were treated with various concentrations of cisplatin, alone or in combination with 10 μM fasudil or 10 μM Y-27632, a specific ROCK inhibitor for 72 h. In a preliminary experiment, we performed a concentration- and time-dependent study of the use of fasudil and Y-27632 using an MTS assay. The cells were treated with 10 μM cisplatin in combination with 1, 10 or 100 μM fasudil or Y-27632 for 24, 48, 72 or 96 h. Fasudil and Y-27632 at 10 μM and for 72 h showed the maximal synergistic effect with cisplatin.Citation26 We therefore used 10 μM as the concentration of fasudil and Y-27632 and an incubation time of 72 h in the following experiments. Cisplatin significantly inhibited cell viability in a dose-dependent manner in cisplatin-sensitive A2780 cells, but not in cisplatin-resistant A2780CP cells as reported previously.Citation25 After 72 h treatment, cell survival decreased from 100–68, 45 and 15% when cisplatin was administered at 1, 10 and 100 μM respectively in A2780 cells (, black bars). Cell survival was not decreased by 1 or 10 μM cisplatin, while treatment with 100 μM cisplatin resulted in 62% cell survival in A2780CP cells (, black bars), Cisplatin at 100 μM exhibited a toxic effect that induced marked growth inhibition even in A2780CP cells. There was significant synergistic growth inhibition in both cell lines when they were treated with 1 or 10 μM cisplatin in combination with fasudil or Y27632 (, lateral striped bars and dotted bars). Treatment with cisplatin at 100 μM together with fasudil or Y-27632 showed a synergistic growth inhibitory effect in A2780CP cells, but not in A2780 cells, in which 100 μM cisplatin alone showed maximal growth inhibition. The fact that the synergistic growth inhibitory effect induced by fasudil was found in both cells suggested that fasudil sensitizes human ovarian cancer cells regardless of their sensitivity to cisplatin.
Figure 1. Fasusil enhances cisplatin-induced inhibition of cell proliferation. Cells were seeded in Dulbecco modified Eagle medium, before being treated with the indicated concentrations of cisplatin (CDDP) with or without 10 μM fasusil or Y-27632 for 72 h, and cell viability was assessed by the MTS assay (eight wells in each group) in A2780 (A) and A2780CP (B) cells. Cell viability was expressed as a ratio of the absorbance of cells treated with various concentrations of cisplatin with or without fasusil or fasudil to those cultured without these reagents (control group) (mean ± S.E; n = 8). *p < 0.05 and **p < 0.01 vs cells treated with cisplatin alone. Experiments were repeated at least three times with consistent results, and a representative result is shown.
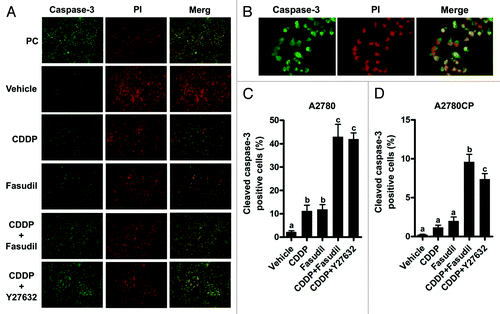
The MTS assay measures the number of viable cells; thus, it is possible that fasudil might affect not only cell proliferation but also apoptosis. Caspase-3 has been implicated as a key mediator of apoptosis in mammalian cells.Citation27 Therefore, we investigated whether fasudil increases the efficacy in cisplatin-induced apoptosis using cleaved caspase-3 immunofluorescent staining in both cells (). Using immunofluorescent staining for cleaved caspase-3, we observed apoptosis in A2780 cells treated with cisplatin alone, fasudil alone, cisplatin + fasudil, or cisplatin + Y-27632 (, immunofluorescent staining data in A2780CP cells not shown). Apoptosis was quantitated as a percentage of the cleaved caspase-3 positive cells compared with the total number of cells for both A2780 and A2780CP cells (). Immunofluorescent reactivity for cleaved caspase-3 in cells treated with cisplatin + fasudil or + Y-27632 was enhanced compared with that in cells treated with cisplatin or fasudil alone. Treatment with cisplatin or fasudil alone resulted in a 15- and 16-fold increase, respectively in the proportion of apoptotic cells compared with the vehicle in A2780 cells (). Treatment with cisplatin in combination with fasudil or Y-27632 significantly increased the percentage of cells positive for cleaved caspase-3 compared with either reagent alone: a 42-fold increase compared with the vehicle group in A2780 cells (). Treatment with cisplatin or fasudil alone resulted in a 5- and 10-fold increase, respectively, in the proportion of apoptotic cells compared with treatment with the vehicle (). A2780CP treated with cisplatin in combination with fasudil or Y-27632 showed 48- or 36-fold increase, respectively, compared with treatment with the vehicle in A2780CP cells (). Although the number of apoptotic A2780CP cells was small in comparison with the number of apototic A2780 cells in all groups, treatment with cisplatin and fasudil or Y-27632 consistently showed a significant increase in apoptosis compared with cisplatin alone for both A2780 and A2780CP cells. These data suggested that fasudil inhibited cell proliferation and enhanced cisplatin-induced apoptosis in both cisplatin-sensitive and -resistant cell lines.
Figure 2. Fasudil enhances cisplatin-induced apoptosis in ovarian cancer cell lines. Cells were plated on chamber slides, allowed to attach, and made quiescent. The cells were treated with various reagents for 48 h. (A) Treated cells were excised and double-stained with anti-active caspase 3 followed by FITC-conjugated goat anti-mouse IgG (green) and PI staining (red). Photos were taken at 100× magnification, and a representative finding is shown in A2780. (B) Photos were taken at x100 magnification of the objective lens. Data concerning immunofluorescent staining in A2780 and A2780CP cells were not shown. The caspase-positive cells were expressed as a percentage of all cells. Three random fields per sample were recorded at x100 magnification, and A2780 (B) and A2780CP cells were counted (C). The values shown represent the mean ± SE (n = 3). Different letters above the bars indicate a significant difference (p < 0.05, n = 3, in each group).
The inhibitory effect on HIF-1α expression induced by cisplatin and fasudil
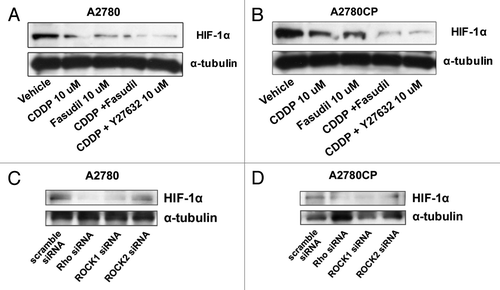
To investigate whether fasudil inhibits HIF-1α expression, we measured the amount of the HIF-1α protein by western blot analysis (). Cisplatin inhibited HIF-1α protein expression compared with the vehicle control in both A2780 and A2780CP cells () as previously reported.Citation28 The inhibitory effect was stronger in cisplatin-sensitive A2780 cells. Cisplatin-induced downregulation of HIF-1α expression was enhanced by combination with fasudil or Y-27632 in both cell lines.
Figure 3. The inhibitory effect on HIF-1α expression of cisplatin and fasudil on ovarian cancer cell lines. Cells were serum-starved overnight and treated with drug-free medium (lane: vehicle), 10 μM cisplatin (lane: CDDP), 10 μM fasudil (lane: Fasudil), 10 μM cisplatin + 10 μM fasudil (CDDP + Fasudil), or 10 μM cisplatin + 10 μM Y27632 (CDDP + Y27632) for 6 h. The lysates were subjected to western blotting using anti-HIF-1α or anti-α-tubulin antibody in A2780 (A) and A2780CP (B) cells as described in Materials and Methods. The efficiency of siRNA on the inhibition of HIF-1α protein expression was examined by western blotting. Cells were transfected with 100 nmol/L scramble, anti-Rho, -ROCK1 or -ROCK2 siRNA. After 24 h incubation, each lysate was subjected to western blotting using anti-HIF-1α or anti-α-tubulin antibody in A2780 (C) and A2780CP (D) cells as described in Materials and Methods.
The Rho/ROCK pathway is involved in HIF-1α expression in ovarian cancer cell lines
To directly assess the role of the Rho/ROCK pathway in HIF-1α expression, we tested whether HIF-1α expression was inhibited by introducing siRNA for RhoA, ROCK1, or ROCK2 in A2780 () and A2780CP () cells. In cells transfected with anti-RhoA and ROCK1 siRNA, the HIF-1α protein expression was strongly inhibited, compared with that in scramble siRNA transfected cells in A2780 (). In the ROCK2 transfected A2780 cells, the HIF-1α protein expression was slightly decreased (, lane ROCK2 siRNA). In cells transfected with anti-RhoA, ROCK1 and ROCK2 siRNA, HIF-1α protein expression was strongly inhibited, compared with that in the scramble siRNA transfected cells (). These results suggested that the Rho/ROCK pathway is essential for HIF-1α expression in ovarian cancer cell lines.
Knockdown of HIF-1α increases the efficacy of cisplatin in ovarian cancer cell lines
Fasudil inhibited the expression of HIF-1 in ovarian cancer cells (), and the inhibition of the Rho/ROCK pathway decreased HIF-1α expression (). These results were consistent with the results in our previous report in human endothelial cellsCitation11 and suggested that HIF-1α is involved in the downstream signaling of the Rho/ROCK pathway in ovarian cancer cells. These considerations led us to examine whether the knockdown of HIF-1α results in the enhancement of cisplatin-induced growth inhibition and apoptosis. In cells transfected with anti-HIF-1α siRNA, the HIF-1α protein expression was strongly inhibited, compared with that in scramble siRNA transfected cells in both cell lines (, right panels). We investigated the effect of anti-HIF-1α siRNA transfection on the viability of cisplatin-treated cells using the MTS assay (). Treatment with cisplatin showed significant growth inhibition in both scramble and anti-HIF-1α siRNA transfected A2780 cells (, left panel). The degree of the decrease in cell viability caused by cisplatin in cells transfected with anti-HIF-1α siRNA was more marked than that in cells transfected with scramble siRNA in A2780 (, left panel).
Figure 4. The effect of HIF-1α blockade on cell proliferation and apoptosis in ovarian cancer cell lines transfected with anti-HIF-1α siRNA. Western blot analysis for the expression of HIF-1α and an MTS assay in A2780 (A) and A2780CP (B) cells transfected with scramble (lane: scramble siRNA) or anti-HIF-1α (lane: HIF-1α siRNA) siRNA. The cells were harvested 24 h after transfection. Cells transfected with scramble or anti-HIF-1α siRNA were treated with drug-free medium or the indicated concentrations of cisplatin for 24 h, and cell viability was assessed using the MTS assay (eight wells in each group). Cell viability was calculated from the ratio of the absorbance of siRNA transfected cells treated with or without cisplatin to that of scramble siRNA transfected cells cultured with drug-free medium set as 1 (mean ± S.E; n = 8). Experiments were repeated at least three times with consistent results, and a representative result is shown. Different letters above the bars indicate a significant difference at p < 0.05. The effect of HIF-1α blockade on cisplatin-induced cleavage of PARP in A2780 (C) and A2780CP (D) cells transfected with scramble (lane: scramble siRNA) or anti-RhoA (lane: RhoA siRNA) siRNA. The cells were transfected with scramble or anti-HIF-1α siRNA and then treated with or without cisplatin for 24 h. The lysates were subjected to western blotting using anti-PARP or anti-α-tubulin antibody.
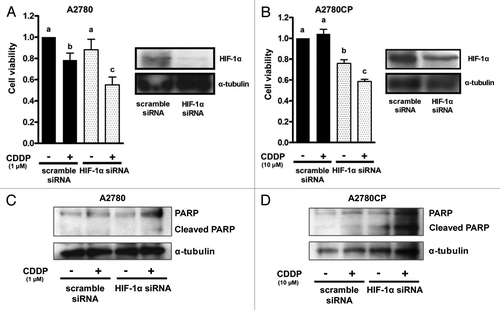
Cell viability was not inhibited by treatment with cisplatin in the scramble siRNA transfected A2780CP cells, while significant growth inhibition was observed in the anti-HIF-1α siRNA transfected A2780CP cells (, left panel).
Furthermore, we examined whether transfection with anti-HIF-1α siRNA increased the expression of cleaved PARP in both cell lines using western blotting (). The expression of cleaved PARP was increased by treatment with cisplatin in both scramble and anti-HIF-1α siRNA transfected A2780 cells (). The degree of increase in cleaved PARP expression caused by cisplatin was stronger in cells transfected with anti-HIF-1α siRNA than that in cells transfected with scramble siRNAwesternin A2780 (). No increase in cleaved PARP expression induced by treatment with cisplatin was observed inwesternthe scramble siRNA transfected A2780CP cells, while an increase in cleaved PARP expression was induced by cisplatin in anti-HIF-1α siRNA transfected A2780CP cells. (). These results suggest that the inhibition of HIF-1α sensitizes ovarian cancer cells to cisplatin.
Confirmation of the involvement of the Rho/ROCK pathway in the enhancement of cisplatin-induced growth inhibition and apoptosis
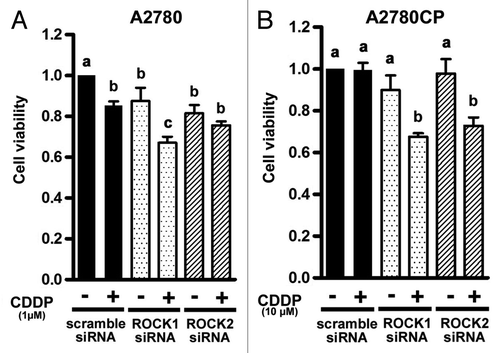
To examine whether the enhancement of anti-tumor effects by fasudil is Rho/ROCK pathway dependent, both cells were transfected with anti-RhoA, ROCK1 or ROCK2 siRNA. We investigated the effect of anti-ROCK1 or ROCK2 siRNA transfection on the viability of cisplatin-treated cells using the MTS assay (). The degree of the decrease in cell viability caused by cisplatin in A2780 cells transfected with anti-ROCK1 siRNA was more marked than that in cells transfected with scramble siRNA, while no enhancement of cisplatin-induced growth inhibition was observed in anti-ROCK2 transfected A2780 cells (). In comparison with A2780CP cells transfected with the scramble siRNA, the degree of the decrease in cell viability caused by cisplatin was significantly higher in both anti-ROCK1 and ROCK2 transfected A2780CP cells ().
Figure 5. The effect of knockdown of ROCK on cell proliferation in ovarian cancer cell lines. The effect of knockdown of ROCK on cell proliferation was assessed by the MTS assay in A2780 (A) and A2780CP (B) cells. The cells were transfected with 100 nmol/L scramble, anti-ROCK1, or -ROCK2 siRNA. After 24 h incubation, the cells transfected with scramble, anti- ROCK1, or -ROCK2 siRNA were treated with drug-free medium or the indicated concentrations of cisplatin for 24 h, and cell viability was assessed using the MTS assay (eight wells in each group). Cell viability was calculated from the ratio of the absorbance of siRNA transfected cells treated with or without cisplatin to that of scramble siRNA transfected cells cultured with drug-free medium set as 1 (mean ± S.E; n = 8). Experiments were repeated at least three times with consistent results, and a representative result is shown. Different letters above the bars indicate a significant difference at p < 0.05.
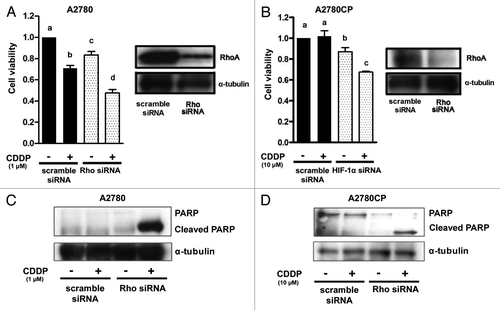
ROCK activation is modulated by RhoA.Citation12,Citation29 Thus, we next investigated the effect of anti-RhoA siRNA transfection on the viability of cisplatin-treated cells using the MTS assay (). RhoA protein expression was strongly inhibited in cells transfected with anti-RhoA siRNA compared with that in scramble siRNA transfected cells in both cell lines (, right panels). Cell viability was not inhibited by treatment with cisplatin in the scramble siRNA transfected A2780CP cells, while significant growth inhibition was observed in the anti-RhoA siRNA transfected A2780CP cells (, left panel). Furthermore, we examined whether transfection with anti-RhoA siRNA increased cisplatin-induced apoptosis in both cell lines using anti-PARP antibody (). The expression of cleaved PARP was increased by the treatment with cisplatin in both scramble and anti-RhoA siRNA transfected A2780 cells (). The degree of the increase in the cleaved PARP expression caused by cisplatin was stronger in cells transfected with anti-RhoA siRNA than in cells transfected with scramble siRNA in A2780 (). No increase in cleaved PARP expression induced by treatment with cisplatin was no observed in the scramble siRNA transfected A2780CP cells, while the anti-RhoA siRNA transfected A2780CP cells did show an increase in cleaved PARP expression after cisplatin treatment. (). These results confirmed the involvement of the Rho/ROCK pathway in the enhancement of the effect of cisplatin in ovarian cancer cell lines.
Figure 6. The effect of RhoA blockade on cell proliferation and apoptosis in ovarian cancer cell lines using transfection with anti-RhoA siRNA. Western blot analysis for the expression of RhoA and an MTS assay in A2780 (A) and A2780CP (B) cells transfected with scramble (lane: scramble siRNA) or anti-RhoA (lane: RhoA siRNA) siRNA. The cells were harvested 24 h after transfection. Cells transfected with scramble or anti-RhoA siRNA were treated with drug-free medium or the indicated concentrations of cisplatin for 24 h, and cell viability was assessed by the MTS assay (eight wells in each group). Cell viability was calculated from the ratio of the absorbance of siRNA transfected cells treated with or without cisplatin to that of scramble siRNA transfected cells cultured with drug-free medium set as 1 (mean ± S.E; n = 8). Experiments were repeated at least three times with consistent results, and a representative result is shown. Different letters above the bars indicate a significant difference at p < 0.05. The effect of RhoA blockade on cisplatin-induced cleavage of PARP in A2780 (C) and A2780CP (D) cells transfected with scramble (lane: scramble siRNA) or anti-RhoA (lane: RhoA siRNA) siRNA. The cells were transfected with scramble or RhoA siRNA and then treated with or without cisplatin for 24 h. The lysates were subjected to western blotting using anti-PARP or anti-α-tubulin antibody.
Discussion
The results of the present study have shown that the Rho kinase inhibitor fasudil enhanced cisplatin-induced growth inhibition and apoptosis in both cisplatin-sensitive and -resistant ovarian cancer cell lines. Inhibition of the Rho/ROCK pathway was involved in the enhancement of the effect of cisplatin by inhibition of HIF-1α signal transduction in ovarian cancer cells. As the prognosis for patients with relapse ovarian cancer depends on the sensitivity of the tumor to platinum, it is very important to understand how tumors become refractory to platinum in order to develop molecular targeting therapies for platinum-refractory ovarian cancer. The Rho/ROCK pathway may be a new target for molecular targeting therapy for ovarian cancer.
The ROCK inhibitor fasudil increased the cisplatin-induced growth inhibition and apoptosis in ovarian cancer cell lines (and). Several studies have reported that ROCK inhibitors increase growth inhibition and apoptosis in hepatocellular carcinomaCitation18 and some specialized cell types.Citation30,Citation31 However, there have been no reports concerning whether Rho/ROCK inactivation enhances the growth inhibition induced by cisplatin in ovarian cancer. In lung carcinoma cells, inhibition of the Rho/ROCK pathway by Y-27632 enhanced cisplatin-induced apoptosis through suppression of a focal adhesion kinase (FAK)-independent mechanism.Citation23
How did fasudil enhance the ability of cisplatin to inhibit cell growth and apoptosis? We have shown that fasudil attenuated HIF-1α protein expression () and that increased sensitivity to cisplatin was observed in cisplatin-sensitive and -resistant ovarian cancer cells when an anti-HIF-1α siRNA was used (). These suggested that fasudil enhanced the efficacy of cisplatin against cell proliferation and apoptosis through the suppression of HIF-1α signaling. HIF-1α regulates gene expression including that of erythropoietin (EPO), insulin-like growth factor (IGF) 2, and transforming growth factor (TGF),Citation32 which are involved in autocrine cell growth. The ATP-binding cassette (ABC) and the breast cancer-resistance protein (BCRP), which promotes cell survival are also regulated by HIF-1α.Citation33
Knockdown of RhoA, ROCK1, and ROCK2 suppressed HIF-1α expression (), suggesting that the Rho/ROCK pathway is an upstream regulator of HIF-1α accumulation in ovarian cancer. Although stimulation of tyrosine kinase activity induces HIF-1α expression in colon cancer cells through activation of PI3K/Akt pathways,Citation34 fasudil did not show any significant effect on the phosphorylation of Akt in the present study (data not shown). The Rho kinase inhibitor fasudil may inhibit HIF-1α signaling independent of PI3K/Akt.
We confirmed that inhibition of the Rho/ROCK pathway sensitizes the cell to cisplatin by knockdown of RhoA, ROCK1 and ROCK2 in ovarian cancer cells (and). The enhancement of cisplatin-induced growth inhibition was observed in anti-ROCK1 transfected but not in anti-ROCK2 transfected A2780 cells (). That depends on the degree of HIF-1α inhibition because the HIF-1α protein expression was slightly decreased in anti-ROCK2 transfected cells (). There was no difference between ROCK1 and ROCK2 in their effects on cell growth and HIF-1α protein expression in A2780CP cells. These results suggested that the roles of ROCK1 are different from those of ROCK2 in cell growth and vary with cell type. However, the detailed roles of ROCK1 and ROCK2 in cell growth and apoptosis are still unknown. Further studies should be performed to determine the roles of ROCK1 and ROCK2 in cell growth and apoptosis.
Treatment with Rho/ROCK inhibitors might make it possible to decrease the dose of first-line chemotherapy, resulting in better compliance by patients, in addition to the improved efficacy of second-line therapy for cisplatin-refractory patients. Moreover, inhibition of the Rho/ROCK pathway in combination with cisplatin may be more effective than single agent therapy for improving patient outcomes. Several studies have reported that the Rho/ROCK pathway is involved in cancer invasion and angiogenesis in ovarian cancer.Citation19,Citation35 Rho/ROCK inactivation may suppress angiogenesis and metastasis and be useful for tumor dormancy therapy in ovarian cancer.
We used fasudil as a Rho kinase inhibitor to study the involvement of the Rho/ROCK pathway in ovarian cancer. Fasudil enhanced cisplatin-induced growth inhibition and apoptosis in ovarian cancer cell lines. Fasudil is a well-tested oral treatment that has been approved in Japan for the treatment of cerebral vasospasm,Citation21 and is well tolerated without any severe adverse reactions.Citation22 Therefore, fasudil can be easily used in the clinical setting. In vivo studies are necessary to test the combination of fasudil and cisplatin.
In summary, we have clarified that inhibition of Rho/ROCK enhanced the efficacy of cisplatin by suppressing HIF-1α in ovarian cancer cell lines. Our findings provide valuable information for preclinical development protocols for ovarian cancer that target the Rho/ROCK/HIF-1α pathways.
Materials and Methods
Materials
Clinical-grade gefitinib was kindly provided by AstraZeneca (Macclesfield). Cisplatin, fasudil, and Y-27632 were purchased from Sigma-Aldrich (Dorset). The anti-PARP antibodies were obtained from Cell Signaling. The anti-HIF-1α and RhoA antibodies were obtained from Santa Cruz. The anti-caspase3 antibody was purchased from Promega. FITC-conjugated goat anti-rabbit antibody was purchased from Jackson ImmunoResearch Laboratories, Inc.
Cell cultures
The human ovarian cancer A2780 cell line, which was derived from a patient prior to treatment, was kindly provided by Dr. Tsuruo (Institute of Molecular and Cellular Biosciences) and Drs. R.F. Ozols and T.C. Hamilton (NCI, National Institutes of Health).Citation24 The A2780 cells were maintained in RPMI1640 medium (Nissui) with 10% fetal bovine serum. The A2780CP cells were cultured at 37°C in Dulbecco modified Eagle medium with 10% fetal bovine serum in a water-saturated atmosphere of 95% air and 5% CO2.
Cell viability assessment
Cell proliferation was assessedCitation25 after the addition of cisplatin at the indicated concentrations with or without fasudil for 72 h, after the cells had been plated in 96-well plates, allowed to attach, and made quiescent. The number of viable cells was determined by measuring A490 of a dissolved formazan product after the addition of MTS [3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] for 1 h as described by the manufacturer (Promega). All experiments were performed in quadruplicate, and the viability was expressed as the ratio of the number of viable cells after cisplatin treatment to that without treatment.
Western blotting
The cells were incubated without serum for 16 h and then treated with various agents, before being washed twice in PBS and scraped into lysis buffer [50 mM HEPES (pH 7.5), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EDTA, 10 mM sodium pyrophosphate, 100 µM sodium orthovanadate, 100 mM NaF, 10 µg/ml aprotinin, 10 µg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride]. The protein concentrations of the supernatants were determined using a protein assay reagent (Bio-Rad Laboratories). Equal amounts of proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. Blocking was performed in 5% skimmed milk powder in 1X TBS. Western blot analyses were performed with various specific primary antibodies.
Apoptosis assay
Detection of ovarian cancer cell apoptosis was performed using the caspase-3 immunofluorescence staining method according to the manufacturer's instructions (Promega). The cells were plated on chamber slides, allowed to attach, and made quiescent, before being treated with various reagents for 48 h. The treated cells were then fixed in 4% formaldehyde for 25 min at 4°C, permeabilized with 0.2% Triton X-100, and incubated with anti-active caspase-3 antibody (Promega, 1:250) at 4°C overnight. After being washed, the samples were stained with rhodamine (TRITC)-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch, 1:200). The cells were washed and then incubated in TUNEL reaction mixture for 1 h at 37°C (DNA strand breaks were labeled with fluorescein-12-dUTP). After stopping the reaction and washing the cells, the nuclei were stained with propidium iodide (1 µg/mL) for 15 min at room temperature. The fluorescent staining was visualized using a fluorescence microscope (Olympus). Three random fields per sample were recorded at x100 magnification, and the cells were counted using image J software. The apoptotic cells were expressed as the percentage of the stained cells compared with the total number of cells counted.
Small interfering RNA analysis
Cells seeded at 2 × 105 cells per well in 6-well plates were transfected with either a small interfering RNA (siRNA) specific for RhoA (final concentration of 100 nmol/L; Dharmacon, Inc.), HIF-1α (final concentration of 100 nmol/L; Dharmacon, Inc.) or scramble siRNA (siCONTROL Non-targeting siRNA; Dharmacon, Inc.) using DharmaFECT. After 24 h, the transfection medium was removed and replaced with serum-free medium. Inhibition of RhoA and HIF-1α expression was verified by western blot analysis.
Statistics
Statistical analysis was performed using one-way ANOVA followed by Fisher's least significant difference test, and p < 0.05 was considered significant. Data are expressed as the mean ± SE.
Acknowledgments
This work was supported by a Grant-in-Aid from the Global COE program of the Japan Society for the Promotion of Science, and in part, by Grants-in-Aid Scientific Research No. 17390445 (H.K.) and No.18591822 (K.T.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin versus paclitaxel and cisplatin: a phase III randomized trial in patients with suboptimal stage III/IV ovarian cancer (from the Gynecologic Oncology Group). Semin Oncol 1996; 23:suppl 12 40 - 7; PMID: 8941409
- Vasey PA, Atkinson R, Coleman R, Crawford M, Cruickshank M, Eggleton P, et al. Docetaxel-carboplatin as first line che motherapy for epithelial ovarian cancer. Br J Cancer 2001; 84:170 - 8; http://dx.doi.org/10.1054/bjoc.2000.1572; PMID: 11161372
- Ducton CJ. New options for the treatment of advanced ovarian cancer. Semin Oncol 1997; 24:Suppl 5 2 - 11
- Dizon DS, Hensley ML, Poynor EA, Sabbatini P, Aghajanian C, Hummer A, et al. Retrospective analysis of carboplatin and paclitaxel as initial second-line therapy for recurrent epithelial ovarian carcinoma: application toward a dynamic disease state model of ovarian cancer. J Clin Oncol 2002; 20:1238 - 47; http://dx.doi.org/10.1200/JCO.20.5.1238; PMID: 11870166
- Chon HS, Hu W, Kavanagh JJ. Targeted therapies in gynecologic cancers. Curr Cancer Drug Targets 2006; 6:333 - 63; http://dx.doi.org/10.2174/156800906777441799; PMID: 16848724
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999; 399:271 - 5; http://dx.doi.org/10.1038/20459; PMID: 10353251
- Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res 1998; 58:1408 - 16; PMID: 9537241
- Osada R, Horiuchi A, Kikuchi N, Yoshida J, Hayashi A, Ota M, et al. Expression of hypoxia-inducible factor 1alpha, hypoxia-inducible factor 2alpha, and von Hippel-Lindau protein in epithelial ovarian neoplasms and allelic loss of von Hippel-Lindau gene: nuclear expression of hypoxia-inducible factor 1alpha is an independent prognostic factor in ovarian carcinoma. Hum Pathol 2007; 38:1310 - 20; http://dx.doi.org/10.1016/j.humpath.2007.02.010; PMID: 17555795
- Birner P, Schindl M, Obermair A, Breitenecker G, Oberhuber G. Expression of hypoxia-inducible factor 1alpha in epithelial ovarian tumors: its impact on prognosis and on response to chemotherapy. Clin Cancer Res 2001; 7:1661 - 8; PMID: 11410504
- Olson MF. Application for ROCK kinase inhibition. Curr Opin Cell Biol 2008; 20:242 - 8; http://dx.doi.org/10.1016/j.ceb.2008.01.002; PMID: 18282695
- Takata K, Morishige K, Takahashi T, Hashimoto K, Tsutsumi S, Yin L, et al. Fasudil-induced hypoxia-inducible factor-1alpha degradation disrupts a hypoxia-driven vascular endothelial growth factor autocrine mechanism in endothelial cells. Mol Cancer Ther 2008; 7:1551 - 61; http://dx.doi.org/10.1158/1535-7163.MCT-07-0428; PMID: 18566226
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behavior. Nat Rev Mol Cell Biol 2003; 4:446 - 56; http://dx.doi.org/10.1038/nrm1128; PMID: 12778124
- Scott RW, Olson MF. LIM kinase: function, regulation and association with human disease. J Mol Med 2007; 85:555 - 68; http://dx.doi.org/10.1007/s00109-007-0165-6; PMID: 17294230
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 2003; 83:1325 - 58; PMID: 14506307
- Zicha D, Dobbie IM, Holy MR, Monypenny J, Soong DY, Gray C, et al. Rapid actin transport during cell protrusion. Science 2003; 300:142 - 5; http://dx.doi.org/10.1126/science.1082026; PMID: 12677069
- Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res 2000; 261:44 - 51; http://dx.doi.org/10.1006/excr.2000.5046; PMID: 11082274
- Redowicz MJ. Rho-associated kinase: involvement in the cytoskeleton regulation. Arch Biochem Biophys 1999; 364:122 - 4; http://dx.doi.org/10.1006/abbi.1999.1112; PMID: 10087173
- Xue F, Takahara T, Yata Y, Xia Q, Nonome K, Shinno E, et al. Blockade of Rho/Rho-associated coiled coil-forming kinase signaling can prevent progression of hepatocellular carcinoma in matrix metalloproteinase-dependent manner. Hepatol Res 2008; 38:810 - 7; http://dx.doi.org/10.1111/j.1872-034X.2008.00333.x; PMID: 18507693
- Sawada K, Morishige K, Tahara M, Ikebuchi Y, Kawagishi R, Tasaka K, et al. Lysophosphatidic acid induces focal adhesion assembly through Rho/Rho-associated kinase pathway in human ovarian cancer cells. Gynecol Oncol 2002; 87:252 - 9; http://dx.doi.org/10.1006/gyno.2002.6831; PMID: 12468322
- Ying H, Sandara LB, Li WW, Alicke B, Xuan JA, Pagila R, et al. The Rho kinase inhibitor fasudil inhibits tumor progression in human and rat tumor models. Mol Cancer Ther 2006; 5:2158 - 64; http://dx.doi.org/10.1158/1535-7163.MCT-05-0440; PMID: 16985048
- Tachibana E, Harada T, Shibuya M, Saito K, Takayasu M, Suzuki Y, et al. Intra-arterial infusion of fasudil hydrochloride for treating vasospasm following subarachnoid haemorrhage. Acta Neurochir (Wien) 1999; 141:13 - 9; http://dx.doi.org/10.1007/s007010050260; PMID: 10071681
- Shibuya M, Hirai S, Seto M, Satoh S, Ohtomo E, Fasudil Ischemic Stroke Study Group. Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. J Neurol Sci 2005; 238:31 - 9; http://dx.doi.org/10.1016/j.jns.2005.06.003; PMID: 16005902
- Igishi T, Mikami M, Murakami K, Matsumoto S, Shigeoka Y, Nakanishi H, et al. Enhancement of cisplatin-induced cytotoxicity by ROCK inhibitor through suppression of focal adhesion kinase-independent mechanism in lung carcinoma cells. Int J Oncol 2003; 23:1079 - 85; PMID: 12963988
- Hamilton TC, Winker MA, Louie KG, Batist G, Behrens BC, Tsuruo T, et al. Augmentation of adriamycin, melphalan, and cisplatin cytotoxicity in drug-resistant and –sensitive human ovarian carcinoma cell lines by buthionine sulfoximine mediated glutathione depletion. Biochem Pharmacol 1985; 34:2583 - 6; http://dx.doi.org/10.1016/0006-2952(85)90551-9; PMID: 4040369
- Mabuchi S, Ohmichi M, Nishio Y, Hayasaka T, Kimura A, Ohta T, et al. Inhibition of NFkappaB increases the efficacy of cisplatin in invitro and in vivo ovarian cancer models. J Biol Chem 2004; 279:23477 - 85; http://dx.doi.org/10.1074/jbc.M313709200; PMID: 15026414
- Yin L, Morishige K, Takahashi T, Hashimoto K, Ogata S, Tsutsumi S, et al. Fasudil inhibits vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Mol Cancer Ther 2007; 6:1517 - 25; http://dx.doi.org/10.1158/1535-7163.MCT-06-0689; PMID: 17513600
- Kothakota S, Azuma T, Reinhard C, Kippel A, Tang J, Chu K, et al. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science 1997; 278:294 - 8; http://dx.doi.org/10.1126/science.278.5336.294; PMID: 9323209
- Duyndam MC, Berkel MPA, Dorsman JC, Rockx DA, Pinedo HM, Boven E. Cisplatin and doxorubicin repress Vascular Endothelial Growth Factor expression and differentially down-regulate Hypoxia-inducible Factor I activity in human ovarian cancer cells. Biochem Pharmacol 2007; 74:191 - 201; http://dx.doi.org/10.1016/j.bcp.2007.04.003; PMID: 17498666
- Lowery DM, Clauser KR, Hjerrild M, Lim D, Alexander J, Kishi K, et al. Proteomic screen defines the Polo-box domain interactome and identifies Rock2 as a Plk1 substrate. EMBO J 2007; 26:2262 - 73; http://dx.doi.org/10.1038/sj.emboj.7601683; PMID: 17446864
- Shibata R, Kai H, Seki Y, Kusaba K, Takemiya K, Koga M, et al. Rho-kinase inhibition reduces neointima formation after vascular injury by enhancing Bax expression and apoptosis. J Cardiovasc Pharmacol 2003; 42:Suppl 1 S43 - 7; http://dx.doi.org/10.1097/00005344-200312001-00011; PMID: 14871028
- Moore M, Marroquin BA, Gugliotta W, Tse R, White SR. Rho kinase inhibition initiates apoptosis in human airway epithelial cells. Am J Respir Cell Mol Biol 2004; 30:379 - 87; http://dx.doi.org/10.1165/rcmb.2003-0019OC; PMID: 12933355
- Minet E, Michel G, Remacle J, Michiels C. Role of HIF-1 as a transcription factor involved in embryonic development, cancer progression and apoptosis. [review] Int J Mol Med 2000; 5:253 - 9; PMID: 10677565
- Comerford KM, Wallance TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible Factor-1-dependent regulation of multidrug resistance (MDR1) gene. Cancer Res 2002; 62:3387 - 94; PMID: 12067980
- Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem 2002; 277:38205 - 11; http://dx.doi.org/10.1074/jbc.M203781200; PMID: 12149254
- Song Y, Wu J, Oyesanya RA, Lee Z, Mukherjee A, Fang X. Sp-1 and c-Myc mediate lysophosphatidic acid-induced expression of vascular endothelial growth factor in ovarian cancer cells via a hypoxia-inducible factor-1-independent mechanism. Clin Cancer Res 2009; 15:492 - 501; http://dx.doi.org/10.1158/1078-0432.CCR-08-1945; PMID: 19147754