Abstract
Uveal melanoma is the most common primary intraocular malignancy in adults; however, current therapeutic modalities, including chemotherapy, have not been successful. Oncolytic viruses serve as an emerging gene therapy tool for cancer treatment because they specifically kill tumor cells while sparing normal cells. The oncolytic virus H101 has been approved by the Chinese State Food and Drug Administration for the treatment of certain malignancies. Unfortunately, the monotherapy of adenovirus has demonstrated limited efficacy in a clinical setting. Thus, novel treatment strategies in which an oncolytic virus is combined with existing chemicals are advancing toward potential clinical use. In this study, we chose the combination of oncolytic virus H101 and the alkylating agent dacarbazine (DTIC) to treat uveal melanoma cells in vitro. Our results demonstrated that the combination exerted a synergistic antitumor effect without enhanced toxicity to normal cells via a type of cell cycle block other than the induction of apoptosis. Further investigation is warranted to elucidate the specific underlying mechanisms of this co-treatment therapy. Our study suggests the viro-chemo combination therapy is feasible and is a potentially promising approach for the treatment of uveal melanoma.
Introduction
Uveal melanoma (UM) is the most common primary intraocular malignancy in adults, with an incidence of 0.7 per 100,000 people.Citation1 Survival rates for UM remain poor due to the high frequency of metastases exhibiting significant resistance to systemic therapy, with median survival less than 6 months.Citation2
It is well-established that the primary goal of treating UM is to prevent metastases and save lives, followed secondarily by reserving the eyeball and vision. Currently, enucleation and plaque radiotherapy are the two most frequently used treatments for UM. Other recently developed therapies that have been developed in the past decade include photocoagulation, transpupillary thermotherapy, charged particle irradiation, and immunotherapy.Citation3 However, due to metastatic disease, the advances achieved in the treatment of the primary tumor have not resulted in an improvement in overall survival. Thus, newer options, including systemic chemotherapy, intra-arterial chemotherapy, chemoembolization, and chemoimmunotherapy are presently being investigated.Citation4
Dacarbazine or dimethyl-triazeno-imidazol carboxamide (DTIC), an alkylating agent, is the only U.S. Food and Drug Administration (FDA) approved chemotherapeutic agent for treatment of melanoma. To date, it is the most effective single agent for treatment of advanced melanoma and causes DNA methylation through the active metabolite MTIC [5-(3-Methyltriazen-1-yl)-imidazole-4-carboximide].Citation5,Citation6 Of note, treatment of melanoma with DTIC alone exhibits a response rate of only 10–25% with a complete response rate of less than 5%, whereas studies of polychemotherapy demonstrate a limited improvement in the response rate along with rising toxicity.Citation6,Citation7 Moreover, there is a general consensus that chemotherapy has potentially reached a plateau of efficacy as a primary treatment modality.Citation8 Therefore, novel approaches are needed in order to overcome chemoresistance as well as to reduce toxicity to normal cells.
Oncolytic viral gene therapy offers the strategic advantage of killing cancer cells while sparing normal cells, and it is a promising modality for treatment of cancers resistant to conventional therapy. H101, a recombinant human type-5 adenovirus with E1B and portions of the E3 region deleted, has been approved for the clinical treatment of head and neck malignancies by the Chinese State Food and Drug Administration.Citation9 The lack of E1B allows H101 to selectively replicate in cancer cells rather than normal cells, resulting in tumor specific cell lysis. The deletion of the 78.3–85.8 μm gene segment in the E3 region, which includes the adenovirus death protein, enhances the safety of the product.Citation10 It has been previously demonstrated that H101 exhibits potential antitumor activity with low toxicity and good tolerance in vitro and in vivo.Citation10,Citation11 However, the therapeutic effect of H101 on UM has not been previously investigated.
The combination treatment of oncolytic virus and a chemical is a new method currently under investigation that has demonstrated encouraging results in refractory malignancies. A synergistic tumor cell killing ability has been previously observed in the combination therapy of various adenoviruses with chemicals due to the augmentation of apoptosis.Citation12-Citation15 Chemotherapy, which is mediated by a different mechanism, often induces distinct toxicity and causes multiple side effects, whereas oncolytic adenovirus exhibits greater tumor selectivity. Thus, in combination they potentially act synergistically without overlapping toxicity. In the present study, we investigated the killing ability of H101 on UM cells and examined the combination effect of H101 and DTIC on UM. As we had hypothesized, the combination therapy did exhibit additive activity, and functioned as a cell cycle block rather than by induction of apoptosis. To date, this is the first attempt to evaluate the effect of the combination of H101 and DTIC on UM cells. Our data demonstrated the potential for effective H101 and DTIC combination treatment and indicated that further investigation is warranted.
Results
Several human UM cell lines exhibited a variable response to H101
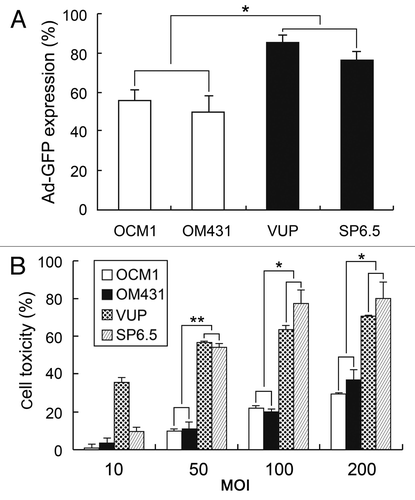
To investigate the effect of oncolytic adenovirus H101 on the UM cell line, we first examined the general response of the UM cell line to adenovirus. We assessed adenovirus infectivity using non-replicating Ad-CMV-GFP using flow cytometry analysis (). According to the efficiency of infectivity, VUP and SP6.5 cells were defined as comprising the comparatively sensitive group whereas OCM1 and OM431 were considered as comprising the insensitive group. Next we evaluated the killing ability of oncolytic adenovirus H101 regarding human UM cell lines. Cells were treated with H101 at various multiplicities of infection (MOI) levels, ranging from 10–200, and cytotoxicity was determined after 72 h using the MTT assay. As expected, H101 exhibited greater toxicity on VUP and SP6.5 cells compared with OCM1 and OM431 cells (), a finding consistent with the infectivity results. To obtain a potentially synergistic effect, we chose the relatively sensitive cell lines VUP and SP6.5 at the MOI of 50 to test the combination treatment.
Figure 1. Sensitivity of human UM cell lines to adenovirus. (A) Infectivity of adenovirus Ad-GFP for human UM cell lines. Cells were infected with Ad-CMV-GFP adenovirus at an MOI of 20 pfu/cell, and GFP values were analyzed by flow cytometry. (B) Cytotoxicity of oncolytic adenovirus H101. Different cells were treated with H101 at an MOI of 50 pfu/cell and cell toxicity was determined by MTT assay 72 h post-infection. The data represent three independent experiments and the p values were determined by comparing VUP and SP6.5 vs. OM431 and OCM1. *p < 0.05, **p < 0.01
Combination of H101 and DTIC exhibited additive cytotoxicity in human UM cell lines
To examine whether the combination of H101 and DTIC enhances the antitumor effect in UM cell lines, SP6.5 and VUP cells were exposed to H101alone, DTIC alone, and H101 plus DTIC, respectively. As observed using light microscopy, co-treatment led to a stronger antitumor effect characterized by an increase in cell death as well as morphological changes (). Cell toxicity, measured by the MTT assay, was determined at 24, 48, 72 and 96 h post-treatment (). When compared with single treatment, the combination of H101 and DTIC demonstrated a moderate increase of cell toxicity in both cell lines, starting at and maintained 48 h post-treatment. For SP6.5 cells, the cell toxicity after co-treatment increased 13–30%, whereas VUP cell toxicity increased 8–27%, demonstrating the cytopathic augmentation exhibited by the combined therapy. At the time of 96 h, however, due to insufficient number of living cells, the overlapping effect of the combination may not be obvious.
Figure 2. Cytotoxicity of combination treatment of H101 and DTIC on UM cells and normal cells. (A) morphological changes under light microscopy. SP6.5 cells were treated with DTIC, H101, and DTIC + H101, respectively. Original magnification × 200. (B) Cytotoxicity of DTIC, H101, and DTIC + H101 on SP6.5 (50 MOI + 5 µg/ml) and VUP cells (50 MOI + 2 µg/ml). Cells were subjected to the MTT assay at 24, 48, 72 and 96 h after treatment. (C) Human normal pigment epithelial cells ARPE-19 were treated exactly as above to evaluate the safety of the co-treatment. The results are representative of three independent experiments. *p < 0.05 compared with the DTIC or H101 treatment group.
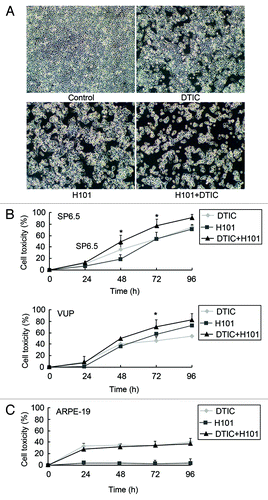
In addition to effectiveness, we investigated the safety of the combination therapy; thus, ARPE-19, a normal human retinal pigment cell line, was treated with H101 and DTIC separately or in combination. Unable to replicate in normal cells, H101 exhibited almost no cell lysis in ARPE-19 cells, hence, the toxic effect of the combination treatment was similar to DTIC alone, exhibiting no overlapping impact (). These findings taken together indicate that combination treatment could achieve an identical antitumor effect at a lower DTIC concentration, decreasing the toxicity to normal cells.
Co-treatment did not affect the apoptotic pathway
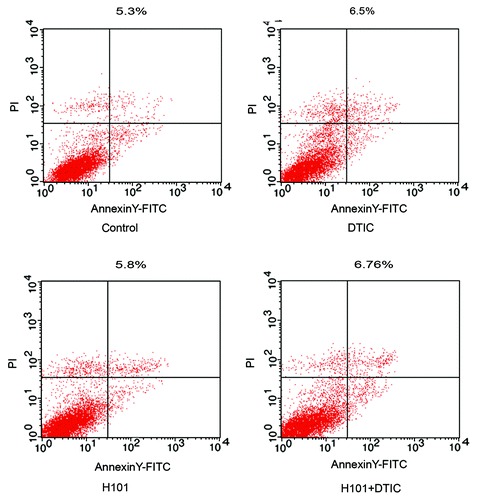
To investigate the underlying mechanism of combination treatment, we analyzed the apoptosis of SP6.5 cells when exposed to the various therapies. Twenty-four hours after treatment, apoptosis was assessed using flow cytometry analysis and an Annexin V-FITC apoptosis kit. As seen in , monotherapy with DTIC (6.5%) or H101 (5.8%) as well as combination treatment (6.76%) did not induce a high level of apoptosis compared with the PBS control (5.3%).
Cell cycle distribution was disturbed by combination treatment
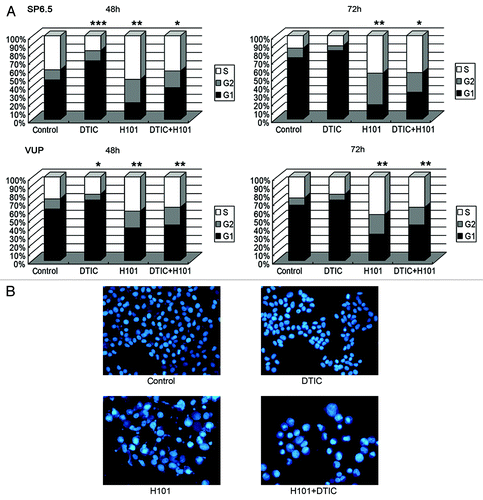
We then investigated whether our treatment scheme would exhibit an impact on the cell cycle. Using PI staining buffer, cell cycle distribution analysis of SP6.5 and VUP cells were conducted 48–72 h post-treatment with flow cytometry. As shown in , in SP6.5 cells, DTIC led to increased cell arrest in the G1 phase beginning 48 h post-treatment through 72 h post-treatment, whereas H101 induced notable cell arrest in the G2 and S phases beginning 48 h post-treatment through 72 h post-treatment. In the co-treatment group, cell cycle distribution exhibited a combination of the findings seen in the two monotherapy groups, with a moderate increase in the G2 and S phases induced by H101; this was most likely due to the neutralization of G1 decrease by DTIC. Similarly, the combination treatment induced the same cell cycle distribution changes in VUP cells. In concordance with the flow cytometry analysis, morphological changes of cells after treatment using DAPI staining are displayed in . Typical nuclear apoptotic changes, including chromatin condensation and nuclear fragmentation, were limited whereas distinct nuclear enlargement appeared in the H101 and co-treatment groups, potentially indicating G2 or S phase arrest.
Figure 4. Cell cycle distribution measurements and morphological changes of UM cells. (A) Cell cycle distribution of SP6.5 and VUP cells. Cell cycle analysis was performed at 48 and 72 h post-treatment with DTIC, H101, and DTIC + H101 by flow cytometry. ***p < 0.001 compared with G1 of control group, **p < 0.01 compared with G2 + S of control group, *p < 0.05 compared with G2 + S of control group in SP6.5 cells, *p < 0.05 compared with G1 of control group in VUP cells. The results are representative of three independent experiments. (B) Morphological changes of the nuclei SP6.5 cells were stained by DAPI 48 h after treatment with DTIC, H101 and DTIC+H101.
DTIC did not interfere with adenovirus replication
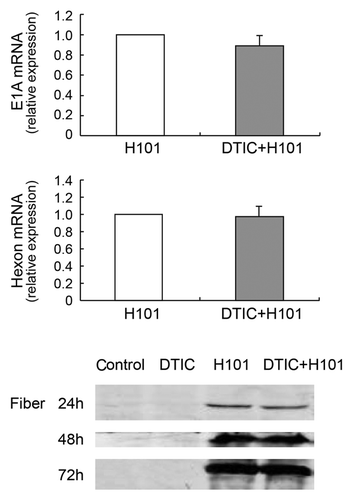
To investigate whether DTIC exhibited an effect on adenovirus replication, we detected early viral gene E1A expression using q-PCR analysis 24 h post-infection (). We found no evident change in E1A gene expression following DTIC treatment. Additionally, we evaluated the expression of late adenoviral gene Hexon and Fiber using q-PCR or western blot, respectively (). Both Hexon and Fiber expression levels were not affected by DTIC, a finding indicating that the mechanism underlying the co-treatment did not involve attenuation or enhancement of adenovirus replication.
Figure 5. Effect of co-treatment on adenoviral DNA replication in SP6.5 cells. (A) Viral early gene E1A expression was detected by q-PCR 24 h post-treatment with H101 or DTIC + H101. (B) Viral late gene Hexon expression was detected by q-PCR 72 h post-treatment with H101 or DTIC + H101. For comparison, the E1A and Hexon expression after H101 treatment was defined as 1. The results are representative of three independent experiments. (C) Western blot analysis of viral late protein Fiber expression 24, 48 and 72 h after DTIC, H101, and DTIC + H101 treatment.
Discussion
Combination of oncolytic adenovirus and chemotherapy present a promising novel treatment strategy in cancer therapy and is rapidly advancing toward clinical use in many malignancies.Citation16 In the present study we investigated the therapeutic effect of the oncolytic adenovirus H101 in combination with an alkylating agent DTIC in the treatment of UM cell lines. Synergistic antitumor activity was observed following combination treatment, without any overlapping cytotoxicity to normal cells ().
Currently, DTIC is the most active single agent for advanced melanoma, however, it rarely produces a durable response or improves UM patient prognosis.Citation17 Gene therapy based on oncolytic adenovirus is a feasible way to reduce chemoresistance. Oncolytic viruses kill cells via virus-mediated cell lysis, which is distinct from chemicals. A synergistic combination is thus possible. H101, a recombinant human type-5 adenovirus with an E1B-55KD deletion, has been used in combination with chemotherapy in patients with advanced cancer and demonstrates potential antitumor activity.Citation18
The infectivity of adenovirus varies in different cells due to CAR or other tumor-associated genes such as CEACAM6.Citation19,Citation20 In the current study, adenovirus also exhibits differential infectivity on UM cell lines in the form of Ad-GFP expression, which is in concordance with the killing ability of oncolytic adenovirus H101 (). We selected relatively H101 sensitive cell lines VUP and SP6.5 for the combination treatment in this study, in which both cell lines received the additive killing effect of DTIC and H101 (). Oncolytic adenovirus H101 selectively replicates in p53-deficient cells rather than normal cells. Therefore, the cytotoxicity of the combination therapy on normal cells should reduce the H101 cytotoxicity ().
The precise underlying mechanism of the combination treatment of chemicals such as DITC and oncolytic adenovirus, however, is not well understood. Some studies have observed a synergistic effect through enhanced apoptosis and autophagy.Citation12,Citation13,Citation21 However; our investigation demonstrated that the tumor cell death had little to do with apoptosis, whether through monotherapy of DTIC or H101, or through co-treatment, which appears to be related to cell cycle blocking (and). It has been previously reported that DTIC (2 mM) kills cells in a manner related to apoptosis,Citation22 and our results exhibited that DTIC (5 ug/ml) primarily led to cell arrest in the G1 phase in UM cell lines. Nevertheless, the differences in action of DTIC may be due to different concentrations used (2 mM vs. 5 μg/ml). In addition, tumor cell lysis caused by oncolytic adenovirus has been previously shown to block the cell cycle at the G2/M phase,Citation10,Citation23 which is consistent with our data and caused a distinct accumulation of cells in S and G2 phases. The combination, however, did not lead to an obvious cell arrest in a certain phase. Instead, we observed a neutralized effect that eventually manifested as a moderate increased cell number in S and G2 phases.
Previous studies have demonstrated that drugs and cell cycle status have an impact on cell entry of adenovirus.Citation24,Citation25 Lieber et al. reported that drugs may enhance adenovirus replication when added after virus infection.Citation26 Thus; we tested whether DTIC had interfered with the entry or replication of adenovirus H101, which may play a potentially positive role in the combination treatment. DTIC did not affect the early virus gene E1A expression level detected by q-PCR, and it also did not affect the late gene Hexon and Fiber expression detected by q-PCR and western blot, respectively (), which indicated that DTIC may not enhance the killing ability of H101 through upregulation of cell entry or virus replication. It is reported that some viruses overcome chemoresistance by downregulating the DNA repair protein MGMT in human glioma cells.Citation27 Moreover, H101 may enhance the antitumor effect of DTIC through a specific signal pathway. Further studies are needed to verify the hypothesis.
Several studies in animal models have demonstrated that the combination of adenovirus-mediated gene therapy and chemotherapy has synergistic therapeutic benefit.Citation28 Compared with the recent studies applying the combination treatment of oncolytic adenovirus and chemotherapy, our research has the following characteristics: above all, it is the first attempt of combining oncolytic adenovirus H101 and DTIC to treat UM cell lines. Nowadays the clinical treatment of uveal melanoma is mainly surgery and radiotherapy, systemic chemotherapy is only used in some advanced cases with metastasis.Citation1 Here we choose the combination of chemotherapy rather than radiotherapy and adenovirus is intended to explore a new way of topical chemotherapy plus topical injection of oncolytic virus to treat uveal melanoma. Next, some studies of the described combination treatment for other tumor type observed an augmentation of apoptosisCitation12-Citation15 while our investigation demonstrated that whether monotherapy of DTIC or H101, or co-treatment appears to be related to cell cycle blocking instead of apoptosis. Meanwhile we observed DTIC did not enhance the killing ability of H101 through upregulation of cell entry or virus replication. Both of them may indicate a new mechanism under the combination therapy worth further investigations. Besides, the adenovirus we used is a mature clinical product different from that of some other studies. The safety of H101 has been observed in tumor patients,Citation18 and the local injection may avoid the systemic toxicity.
There is always a challenge in the use of adenovirus given the pre-existing host immunity. The oncolytic adenovirus H101 we used in the study, however, has been approved for the clinical treatment of head and neck malignancies by the Chinese official agent and clinical data show that it is well tolerable and has good efficacy in several malignancies.Citation9 On the other hand, improved virotherapy can be achieved by sustaining adenoviral replication using immunosuppression.Citation29 Besides, the ocular immune privilege may actually be attributed to uveal melanoma virotherapy.Citation30,Citation31 Ocular tissues and fluids express a wide variety of anti-inflammatory and immunosuppressive molecules which would help H101 to escape from immune surveillance, resulting in enhanced spread of viral infection and tumor lysis.
Taken together, the combination of DTIC and H101 exerts an efficient UM cell killing effect in vitro via cell cycle blocking. This study demonstrates a potentially promising potential for the effective treatment of UM. The underlying mechanisms need to be elucidated in order to enhance the efficacy of adenovirus-based gene therapy.
Materials and Methods
Cell lines and cell culture
Human uveal melanoma cell lines SP6.5, VUP, OCM1 and OM431 were kindly provided by Professor John F. Marshall (Tumor Biology Laboratory, Cancer Research UK Clinical Center, John Vane Science Center).Citation32 Human retinal pigment epithelium cell line ARPE-19 was generously provided by the Department of Ophthalmology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine. All cell lines were maintained at 37°C with 5% CO2. Human uveal melanoma cell lines were cultured in DMEM supplemented with 10% fetal bovine serum (FBS). ARPE-19 cells were cultured in DMEM/F-12 (1:1) supplemented with 10% fetal bovine serum (FBS).
Chemicals and virus
Dacarbazine (Nanjing Pharmaceutical Factory) was dissolved in 0.9% saline to produce a 1,000 µg/ml stock solution.
Oncolytic adenovirus H101, which carries an E1B-55k deletion and a partial deletion of the 78.3–85.8 μm gene segment in the E3 region, has been described previously (provided by Shanghai Sunway Biotech).Citation10
Cell viability
SP6.5, VUP, and ARPE19 cells were seeded in 96-well plates overnight and treated with Dacarbazine, H101, or both agents in combination. Cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma) according to the manufacturer’s instructions at 1–4 d post-treatment, as previously described.Citation11 Each experiment was performed three times.
Cell cycle
SP6.5 and VUP cells were treated with Dacarbazine, H101, or a combination of both agents, and PBS served as the control. Cells were harvested at 48 and 72 h following treatment, washed with cold PBS twice, fixed in 70% ethanol, and stored at 4°C overnight. Next, cells were washed twice with cold PBS and incubated with PI/RNase staining buffer (BD Biosciences) for 15 min at room temperature. Cell cycle distribution was detected and analyzed using the FACScan and CellQuest programs (Becton Dickinson).
Apoptotic analysis
Apoptosis was determined by flow cytometry using the FITC Annexin V Apoptosis Detection Kit (BD Bioscience) and cells were collected in accorded with the manufacturer’s instructions. Samples were examined by FACScan (Becton Dickinson) and data were analyzed using the Cell Quest program (Becton Dickinson).
Western blot
SP6.5 cells treated with Dacarbazine, H101, or a combination of both agents were harvested and separated on 10–12% SDS-PAGE. Next, the proteins were transferred onto a PVDF membrane for 1 h at 13 V. Membranes were blocked using PBS containing 5% non-fat milk and 0.1% Tween 20 for 2 h followed by incubation with the primary antibody overnight at 4°C. Primary antibodies used were: anti-Fiber (Abcam) and anti-β-actin (Santa Cruz Biotechnology). The bands were detected using the Odyssey Infrared imaging system (Odyssey; LI-COR).
Real-Time PCR
Real-Time PCR was conducted using the Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems) and the SYBR Premix Ex Taq (TaKaRa). Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. The cDNA was synthesized from 1 µg of total RNA using PrimeScript RT reagent Kit (TaKaRa). Specific PCR primers were as follows: E1A: 5′-TGCCACGGAGGTGTTATTACCGAA-3′ (forward) and 5′-ACAGTTCGTGAAGGGTAGGTGGTT-3′ (reverse), Hexon: 5′-TAACCAGTCACAGTCGCAAG-3′ (forward) and 5′-GTCAAAGTACGTGGAAGCCAT-3′ (reverse). The denaturation step was conducted at 95°C for 30 sec, and the amplification program was set to 40 cycles consisting of 95°C for 5 sec and 60°C for 34 sec per cycle. Data were analyzed by comparing the 2-△△Ct values of E1A or Hexon mRNA in DTIC+H101 treated cells and H101 treated cells.
Statistical analysis
All experiments were performed in triplicate, and the data were expressed as mean ± SD. The data were analyzed with unpaired 2-tailed t-test and results were considered statistically significant at p < 0.05.
Acknowledgments
This work was supported by the National Key Program for Basic Research of China grant (2010CB529902), The National Natural Science Foundation of China grant (10979034, 81001008), The Science and Technology Commission of Shanghai (10JC1409100), the Shanghai Rising-Star Program(11QA1404000), and the Shanghai Leading Academic Discipline Project grant (S30205).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- van den Bosch T, Kilic E, Paridaens D, de Klein A. Genetics of uveal melanoma and cutaneous melanoma: two of a kind?. Dermatol Res Pract 2010; In press http://dx.doi.org/10.1155/2010/360136; PMID: 20631901
- Singh AD, Borden EC. Metastatic uveal melanoma. Ophthalmol Clin North Am 2005; 18:143 - 50; http://dx.doi.org/10.1016/j.ohc.2004.07.003; PMID: 15763199
- Shields CL, Shields JA. Ocular melanoma: relatively rare but requiring respect. Clin Dermatol 2009; 27:122 - 33; http://dx.doi.org/10.1016/j.clindermatol.2008.09.010; PMID: 19095158
- Coupland SE, Anastassiou G, Stang A, Schilling H, Anagnostopoulos I, Bornfeld N, et al. The prognostic value of cyclin D1, p53, and MDM2 protein expression in uveal melanoma. J Pathol 2000; 191:120 - 6; http://dx.doi.org/10.1002/(SICI)1096-9896(200006)191:2<120::AID-PATH591>3.0.CO;2-P; PMID: 10861569
- Quirin C, Mainka A, Hesse A, Nettelbeck DM. Combining adenoviral oncolysis with temozolomide improves cell killing of melanoma cells. Int J Cancer 2007; 121:2801 - 7; http://dx.doi.org/10.1002/ijc.23052; PMID: 17724714
- Lillehammer T, Engesaeter BO, Prasmickaite L, Maelandsmo GM, Fodstad O, Engebraaten O. Combined treatment with Ad-hTRAIL and DTIC or SAHA is associated with increased mitochondrial-mediated apoptosis in human melanoma cell lines. J Gene Med 2007; 9:440 - 51; http://dx.doi.org/10.1002/jgm.1036; PMID: 17410615
- Bajetta E, Del Vecchio M, Bernard-Marty C, Vitali M, Buzzoni R, Rixe O, et al. Metastatic melanoma: chemotherapy. Semin Oncol 2002; 29:427 - 45; http://dx.doi.org/10.1053/sonc.2002.35238; PMID: 12407508
- Bagnyukova TV, Serebriiskii IG, Zhou Y, Hopper-Borge EA, Golemis EA, Astsaturov I. Chemotherapy and signaling: How can targeted therapies supercharge cytotoxic agents?. Cancer Biol Ther 2010; 10:839 - 53; http://dx.doi.org/10.4161/cbt.10.9.13738; PMID: 20935499
- Yu W, Fang H. Clinical trials with oncolytic adenovirus in China. Curr Cancer Drug Targets 2007; 7:141 - 8; http://dx.doi.org/10.2174/156800907780058817; PMID: 17346105
- Song X, Zhou Y, Jia R, Xu X, Wang H, Hu J, et al. Inhibition of retinoblastoma in vitro and in vivo with conditionally replicating oncolytic adenovirus H101. Invest Ophthalmol Vis Sci 2010; 51:2626 - 35; http://dx.doi.org/10.1167/iovs.09-3516; PMID: 20007825
- Zhang H, Wang H, Zhang J, Qian G, Niu B, Fan X, et al. Enhanced therapeutic efficacy by simultaneously targeting two genetic defects in tumors. Mol Ther 2009; 17:57 - 64; http://dx.doi.org/10.1038/mt.2008.236; PMID: 19018252
- Jiang G, Liu YQ, Wei ZP, Pei DS, Mao LJ, Zheng JN. Enhanced antitumor activity by the combination of a conditionally replicating adenovirus mediated interleukin-24 and dacarbazine against melanoma cells via induction of apoptosis. Cancer Lett 2010; 294:220 - 8; http://dx.doi.org/10.1016/j.canlet.2010.02.003; PMID: 20189296
- Pan Q, Liu B, Liu J, Cai R, Wang Y, Qian C. Synergistic induction of tumor cell death by combining cisplatin with an oncolytic adenovirus carrying TRAIL. Mol Cell Biochem 2007; 304:315 - 23; http://dx.doi.org/10.1007/s11010-007-9514-6; PMID: 17577631
- Zhang Y, Qin X, Zhang Y, Zhao L, Wang Y, Liu X, et al. Combination of ZD55-MnSOD therapy with 5-FU enhances antitumor efficacy in colorectal cancer. J Cancer Res Clin Oncol 2008; 134:219 - 26; http://dx.doi.org/10.1007/s00432-007-0273-2; PMID: 17632733
- Ingemarsdotter CK, Baird SK, Connell CM, Oberg D, Hallden G, McNeish IA. Low-dose paclitaxel synergizes with oncolytic adenoviruses via mitotic slippage and apoptosis in ovarian cancer. Oncogene 2010; 29:6051 - 63; http://dx.doi.org/10.1038/onc.2010.335; PMID: 20729921
- Ottolino-Perry K, Diallo JS, Lichty BD, Bell JC, McCart JA. Intelligent design: combination therapy with oncolytic viruses. Mol Ther 2010; 18:251 - 63; http://dx.doi.org/10.1038/mt.2009.283; PMID: 20029399
- Triozzi PL, Eng C, Singh AD. Targeted therapy for uveal melanoma. Cancer Treat Rev 2008; 34:247 - 58; http://dx.doi.org/10.1016/j.ctrv.2007.12.002; PMID: 18226859
- Lu W, Zheng S, Li XF, Huang JJ, Zheng X, Li Z. Intra-tumor injection of H101, a recombinant adenovirus, in combination with chemotherapy in patients with advanced cancers: a pilot phase II clinical trial. World J Gastroenterol 2004; 10:3634 - 8; PMID: 15534920
- Bergelson JM, Modlin JF, Wieland-Alter W, Cunningham JA, Crowell RL, Finberg RW. Clinical coxsackievirus B isolates differ from laboratory strains in their interaction with two cell surface receptors. J Infect Dis 1997; 175:697 - 700; http://dx.doi.org/10.1093/infdis/175.3.697; PMID: 9041347
- Wang Y, Gangeswaran R, Zhao X, Wang P, Tysome J, Bhakta V, et al. CEACAM6 attenuates adenovirus infection by antagonizing viral trafficking in cancer cells. J Clin Invest 2009; 119:1604 - 15; http://dx.doi.org/10.1172/JCI37905; PMID: 19411761
- Tyler MA, Ulasov IV, Lesniak MS. Cancer cell death by design: apoptosis, autophagy and glioma virotherapy. Autophagy 2009; 5:856 - 7; PMID: 19430207
- Sanada M, Hidaka M, Takagi Y, Takano TY, Nakatsu Y, Tsuzuki T, et al. Modes of actions of two types of anti-neoplastic drugs, dacarbazine and ACNU, to induce apoptosis. Carcinogenesis 2007; 28:2657 - 63; http://dx.doi.org/10.1093/carcin/bgm188; PMID: 17881774
- Cherubini G, Petouchoff T, Grossi M, Piersanti S, Cundari E, Saggio I. E1B55K-deleted adenovirus (ONYX-015) overrides G1/S and G2/M checkpoints and causes mitotic catastrophe and endoreduplication in p53-proficient normal cells. Cell Cycle 2006; 5:2244 - 52; http://dx.doi.org/10.4161/cc.5.19.3263; PMID: 16969092
- Seidman MA, Hogan SM, Wendland RL, Worgall S, Crystal RG, Leopold PL. Variation in adenovirus receptor expression and adenovirus vector-mediated transgene expression at defined stages of the cell cycle. Mol Ther 2001; 4:13 - 21; http://dx.doi.org/10.1006/mthe.2001.0414; PMID: 11472101
- Hemminki A, Kanerva A, Liu B, Wang M, Alvarez RD, Siegal GP, et al. Modulation of coxsackie-adenovirus receptor expression for increased adenoviral transgene expression. Cancer Res 2003; 63:847 - 53; PMID: 12591736
- Bernt KM, Steinwaerder DS, Ni S, Li ZY, Roffler SR, Lieber A. Enzyme-activated prodrug therapy enhances tumor-specific replication of adenovirus vectors. Cancer Res 2002; 62:6089 - 98; PMID: 12414633
- Alonso MM, Gomez-Manzano C, Bekele BN, Yung WK, Fueyo J. Adenovirus-based strategies overcome temozolomide resistance by silencing the O6-methylguanine-DNA methyltransferase promoter. Cancer Res 2007; 67:11499 - 504; http://dx.doi.org/10.1158/0008-5472.CAN-07-5312; PMID: 18089777
- Jiang G, Xin Y, Zheng JN, Liu YQ. Combining conditionally replicating adenovirus-mediated gene therapy with chemotherapy: a novel antitumor approach. Int J Cancer 2011; 129:263 - 74; http://dx.doi.org/10.1002/ijc.25948; PMID: 21509783
- Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips NJ, Wold WS. Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the Syrian hamster model. Mol Ther 2008; 16:1665 - 73; http://dx.doi.org/10.1038/mt.2008.162; PMID: 18665155
- Niederkorn JY. Mechanisms of immune privilege in the eye and hair follicle. J Investig Dermatol Symp Proc 2003; 8:168 - 72; http://dx.doi.org/10.1046/j.1087-0024.2003.00803.x; PMID: 14582667
- Niederkorn JY. Immune escape mechanisms of intraocular tumors. Prog Retin Eye Res 2009; 28:329 - 47; http://dx.doi.org/10.1016/j.preteyeres.2009.06.002; PMID: 19563908
- Jia R, Jiao Z, Xu X, Wang J, Zhou Y, Song X, et al. Functional significance of B7-H1 expressed by human uveal melanoma cells. Mol Med Rep 2011; 4:163 - 5; PMID: 21461580