Abstract
Salivary adenoid cystic carcinoma (SACC) is a frequent subtype of salivary gland malignancy, and it has an important biological behavior for perineural invasion (PNI). Extracellular matrix metalloproteinase inducer (EMMPRIN) is a transmembrane glycoprotein that is involved in the invasive property of many malignancies by stimulating matrix metalloproteinase (MMP) expression. The present study was designed to investigate the role and possible mechanism of EMMPRIN in the PNI of SACC using RNA interference (RNAi) technology. We found that silencing of EMMPRIN expression in the human SACC cell line (SACC-83) suppressed the cell proliferation, adhesion, MMP-2 and MMP-9 secretion, and PNI activity in vitro. EMMPRIN silencing also inhibited the tumor growth and Ki-67 labeled proliferation index in vivo. Using tumor PNI models in nude mice, EMMPRIN silencing inhibited the infiltration, swelling and functional loss of the affected sciatic nerves, as well as the expression of MMP-2 and MMP-9. These results demonstrate that EMMPRIN participates in the PNI of SACC cells by mediating the expression of MMP-2 and MMP-9. Our findings suggest that EMMPRIN is a potential target for anti-PNI treatment in SACC.
Introduction
Salivary adenoid cystic carcinoma (SACC) is a frequently occurring malignant salivary gland neoplasm, constituting approximately 18% of all salivary gland malignancies.Citation1 It has a prolonged clinical course with perineural invasion (PNI), hematogenous metastases, and poor response to classical chemotherapeutic approaches.Citation2 A prominent feature of SACC is the PNI, which is likely caused by the affinity of the neoplasm for the basement membrane rich tissues, such as nerves and blood vessels.Citation3 More than 60% of patients with SACC present with PNI at the time of diagnosis.Citation2,Citation4 PNI is the process by which cancer cells wrap around nerves. PNI of SACC leads to the progressive dysfunction of the infiltrated nerve and may manifest as pain and numbness in the sensory nerves or paralysis of the motor nerves. Moreover, PNI of SACC could prevent complete surgical resection and lead to a poor prognosis.Citation4-Citation6
Matrix metalloproteinases (MMP), a group of zinc-dependent endopeptidases capable of degrading various components of the extracellular matrix (ECM), is believed to play a vital role in tumor invasion.Citation7 In many tumors, MMP expression is mainly regulated by extracellular MMP inducer (EMMPRIN, CD147), which is a transmembrane glycoprotein that belongs to the immunoglobulin superfamily.Citation8 EMMPRIN has been found to facilitate tumor invasion and metastasis by regulating the expression of MMPs within the tumor microenvironment.Citation9,Citation10 Our recent immunohistochemical study showed for the first time that EMMPRIN expression in SACC was positively associated with both the PNI of SACC and the expression of MMP-2 and MMP-9 within the tumor and surrounding stromal compartments.Citation11 Thus, we hypothesized that EMMPRIN may be a key mediator in the PNI of SACC by functionally regulating the expression of MMP-2 and MMP-9.
In the present study, we employed a lentiviral vector system to induce short hairpin RNA (shRNA)-triggered RNA interference (RNAi) to knockdown EMMPRIN expression in the human SACC cell line, SACC-83.Citation12 We evaluated the effects of EMMPRIN silencing on the proliferation and PNI activity of SACC-83 cells in vitro and in vivo. We also studied the effects of EMMPRIN silencing on the adhesion activity of SACC-83 cells and the gelatinolytic activity of MMPs to further explore the function of EMMPRIN in the PNI of SACC.
Results
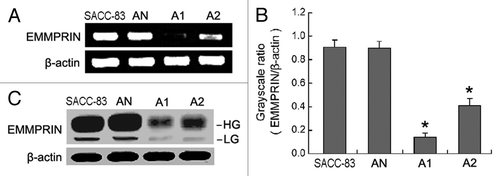
Silencing of EMMPRIN expression by RNAi in SACC-83 cells
To examine the specific effect of EMMPRIN shRNA on EMMPRIN expression in the SACC-83 cell line, three stable cell clones transduced with lentivirus containing negative shRNA, shRNA1, and shRNA2 were obtained and designated AN, A1 and A2, respectively. The RT-PCR results () showed that transduction with EMMPRIN shRNA1 and shRNA2 resulted in the significant inhibition of EMMPRIN mRNA in SACC-83 cells, by 84.53 ± 6.74% in the A1 cells (p < 0.01) and by 54.78 ± 5.16% in the A2 cells (p < 0.01), respectively. While the negative shRNA exhibited no interference effect in the AN cells (p > 0.05). The western blot results () showed that EMMPRIN protein levels were also significantly downregulated in the A1 and A2 cells compared with the SACC-83 or AN cells (p < 0.01). However, EMMPRIN protein levels in A1 cells were reduced more strikingly than in A2 cells.
Figure 1. EMMPRIN-specific shRNAs result in the silencing of EMMPRIN mRNA and protein in SACC-83 cells. AN, A1 and A2 were stable cell clones of SACC-83 cells transduced with lentivirus containing negative shRNA, EMMPRIN shRNA1, and EMMPRIN shRNA2, respectively. (A) The level of EMMPRIN mRNA was assessed by RT-PCR. (B) Comparison of gray scale ratio of EMMPRIN mRNA/β-actin mRNA. *p < 0.01. (C) The level of EMMPRIN protein was assessed by western blotting. HG, high glycosylated form; LG, low glycosylated form.
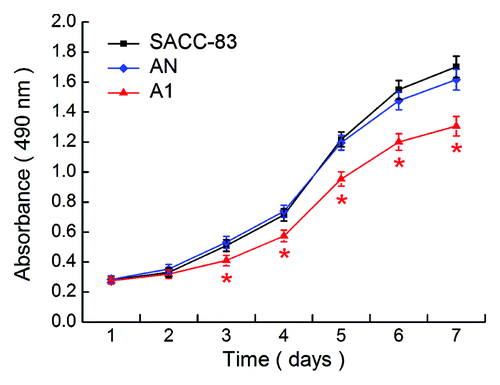
EMMPRIN silencing inhibited tumor cell proliferation
Next, we determined the cell proliferation of SACC-83, AN, and A1, respectively. As shown in , the proliferation of A1 cells was significantly decreased at days 3–7 compared with the SACC-83 or AN cells (p < 0.01). There was no significant difference between the SACC-83 cells and the AN cells (p > 0.05).
EMMPRIN silencing inhibited tumor cell adhesion, MMPs secretion, and perineural invasion activity in vitro
After 45 min of incubation, a significant decrease in the amount of cells attached to the Matrigel-coated plates was observed in the A1 cells compared with the SACC-83 or AN cells (p < 0.01, ). A significant decrease in the amount of cells attached to the neural cells monolayer was also observed in the A1 cells compared with the other two control cells (p < 0.01, ). The results of the gelatin zymography showed that the secretion of MMP-2 and MMP-9 was both reduced markedly in the A1 cells compared with the SACC-83 or AN cells (p < 0.01; ).
Figure 3. EMMPRIN silencing inhibits the adhesion, MMP-2 and MMP-9 secretion, and perineural invasion (PNI) activity of SACC-83 cells in vitro. (A) Adhesion of tumor cells to the Matrigel (magnification × 400). (B) Adhesion of tumor cells to the neural cells (magnification × 400). (C) The secretion of MMP-2 and MMP-9 was measured by gelatin zymography and gray scale analysis. (D) PNI in vitro was evaluated using modified Boyden chambers. Top, H&E images of tumor cells that invaded through the Matrigel coated filte (magnification × 400); bottom, the number of the invaded tumor cells. *p < 0.01.
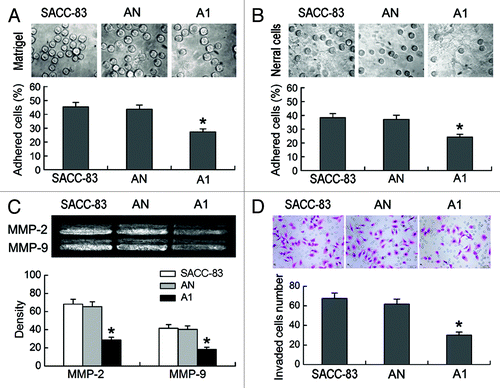
The effects of EMMPRIN silencing on the PNI activity of SACC-83 cells in vitro were evaluated using modified Boyden chambers. The A1 cells exhibited significantly lower PNI activity compared with the SACC-83 or AN cells (p < 0.01; ). There was no significant difference between the SACC-83 cells and the AN cells (p > 0.05).
Effects of EMMPRIN silencing on tumorigenicity in vivo
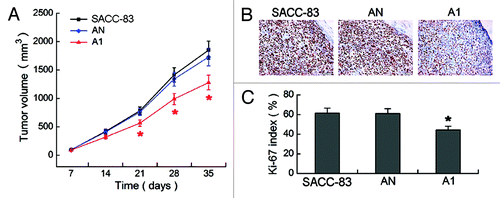
Thirty-five days after cells were inoculated into nude mice, a remarkable reduction of transplanted tumors volume was observed in A1 group as compared the SACC-83 or AN groups (p < 0.01, ). The tumor sections were immunostained with Ki-67 () to assess the Ki-67 labeled proliferation index (). The Ki-67 index was 44.23 ± 4.86% in A1 group, which was significantly lower than 61.34 ± 6.14% in SACC-83 group and 60.87 ± 5.98% in AN group (p < 0.01).
Figure 4. Effects of EMMPRIN silencing on tumorigenicity in vivo. (A) The growth curves of transplanted tumors in eight nude mice of each group. (B) Immunohistochemical staining of Ki-67 in the transplanted tumors of three groups (magnification × 400). (C) The Ki-67 labeled proliferation index. *p < 0.01.
Effects of EMMPRIN silencing on tumor perineural invasion in vivo
The in vivo PNI model was established by implanting tumor cells onto the left sciatic nerve of nude mice (). We assessed the propensity of SACC-83, AN, and A1 tumors to induce sciatic nerve paralysis in nude mice. Five weeks after tumor implantation, the sciatic nerve function (limb function and hind paw width) in A1 group was significantly better than the SACC-83 or AN groups (p < 0.01, ).
Figure 5. Effects of EMMPRIN silencing on tumor perineural invasion (PNI) in vivo. (A) The in vivo PNI models were established in 12 mice of each group. (a) The operating area on the nude mice; (b) tumor cells were microscopically injected onto the perineurium of the sciatic nerve (black arrow); (c) tumor cells (red arrow) grew around the left sciatic nerve and caused complete left hind limb paralysis (white arrow). (B) Sciatic nerve score analysis. (C) Paw span analysis. *p < 0.01.
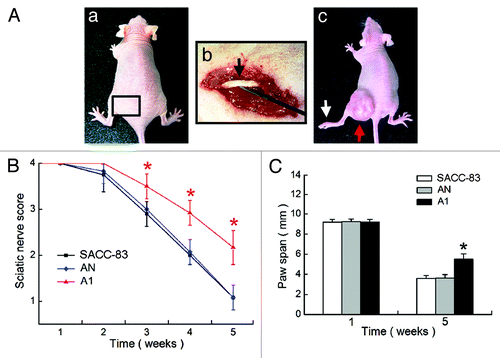
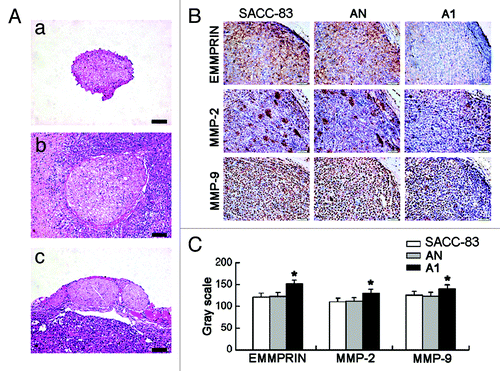
All mice were then sacrificed for histopathologic analysis. H&E staining showed the normal right sciatic nerve () and the swollen left sciatic nerves (and). PNI was observed in all of the mice with the SACC-83 or AN tumors (). However, in 12 mice with A1 tumors, only 7 mice displayed PNI; the other 5 mice just showed tumor-constricted nerves (). The swelling of the sciatic nerve at the primary tumor site in A1 group (0.91 ± 0.08 mm) was significantly weaker than SACC-83 group (1.25 ± 0.10 mm, p < 0.01) and AN group (1.23 ± 0.11 mm, p < 0.01). The immunohistochemical staining of the tumor sections () showed a significant decrease in the expression of EMMPRIN, MMP-2, and MMP-9 in A1 group as compared with the other two groups (p < 0.01, ).
Figure 6. EMMPRIN silencing inhibits the tumor PNI and the expression of EMMPRIN, MMP-2 and MMP-9 in vivo. (A) Tumors and affected sciatic nerves were excised for H&E analysis 5 weeks after tumor implantation. (a) Normal right sciatic nerve; (b) tumor PNI caused serious swelling of the left nerve in SACC-83 and AN groups; (c) the tumor constricted nerve caused moderate swelling of the left nerve in A1 group. Bar, 200 μm. (B) Immunohistochemical staining of EMMPRIN, MMP-2 and MMP-9 in the tumors of three groups (magnification × 400). (C) Gray scale analyses of the positive staining intensity. A lower gray scale value represents a stronger positive staining. *p < 0.01.
Discussion
SACC is a salivary gland malignancy that tends to invade the nerves. EMMPRIN, known as CD147, is a cellular adhesion molecule involved in cell-cell and cell-ECM interactions.Citation8-Citation10 EMMPRIN is highly expressed on the surface of various malignant tumor cells compared with their normal counterparts,Citation17,Citation18 and is involved in tumor invasion,Citation19-Citation21 angiogenesis,Citation22,Citation23 and metastasis.Citation21 Our previous immunohistochemical study found that EMMPRIN expression was significantly correlated with tumor progression and patients’ prognosis in SACC.Citation11 In the present study, we further demonstrated that silencing of EMMPRIN expression by RNAi significantly reduced the proliferation and PNI activity of SACC cells both in in vitro and in vivo models.
RNAi mediated by shRNA is a specific gene-silencing technology that is useful for the study of functional genomics.Citation24,Citation25 Here, we used this technology to construct the EMMPRIN shRNA lentiviral vectors for transduction into the human SACC cell line, SACC-83. Two stable cell clones, A1 and A2, were obtained. EMMPRIN expression was effectively inhibited at both the mRNA and protein levels by shRNA1 in A1, while the shRNA2 was less efficient in A2. It was suggested that vector-based EMMPRIN-specific shRNA could knockdown the expression of EMMPRIN effectively and specifically in SACC cells, and shRNA targeting at different sites of the same mRNA have different silencing efficiencies.
The proliferation of tumor cells is important for the invasive growth of malignant tumors. EMMPRIN was found to interact with cyclophilin A and promote pancreatic cancer cell proliferation through the activation of the ERK1/2 and p38 MAPK signaling pathways.Citation26 On the other hand, knockdown of EMMPRIN gene expression by RNAi could inhibit the proliferation and malignant potential of cancer cells.Citation27-Citation29 Our study found that silencing of EMMPRIN by RNAi could obviously reduce the tumor growth of SACC via inhibiting the proliferation potential of SACC cells.
Adhesion of tumor cells to the nerve is the first step for PNI and is highly dependent upon an increased production of proteases by the tumor cells. A role for EMMPRIN as an adhesion molecule has been proposed, and it has been reported to bind to a variety of cell types, including endothelial cells and fibroblasts, as well as the ECM.Citation30 Matrigel is a solubilized basement membrane preparation containing almost all of the ECM components.Citation31 Our study showed that knockdown of EMMPRIN could directly inhibit the attachment of SACC-83 cells to the Matrigel-coated plates and the monolayer of neural cells. The ECM and the neural cells are the main components of neural tissues. The special proclivity of the SACC cells to invade nerves may be related to their high chemotactic response to the ECM.Citation3,Citation32 These results indicate that EMMPRIN induces the adhesion of the SACC cells to the ECM and the neural cells. However, further study of the adhesion of SACC cells to the neural cells induced by EMMPRIN might shed more light on the mechanism of PNI.
Degradation and remodeling of the ECM are necessary steps in tumor invasion.Citation33 MMPs, a major family of enzymes that can degrade various components of the ECM, are believed to play important roles in tumor invasion.Citation7 Among MMPs, MMP-2 and MMP-9 are particularly upregulated in SACC and many other malignant tumors and contribute to the invasion of tumor cells by degrading the ECM.Citation34-Citation36 Our previous study found that the expression of MMP-2 and MMP-9 was associated with EMMPRIN expression in SACC.Citation11 In the present study, we found that knockdown of EMMPRIN expression in SACC-83 cells reduced the secretion of MMP-2 and MMP-9, thus inhibiting the PNI ability of SACC-83 cells through the reconstituted basement membrane in vitro. Using an in vivo PNI model, we found that EMMPRIN silencing in SACC-83 cells could remarkably inhibit the infiltration, swelling and functional loss of the affected sciatic nerve, as well as the expression of MMP-2 and MMP-9 in both the tumor and stromal compartments. Our results suggest that EMMPRIN is involved in the PNI of SACC cells by regulating the secretion of MMP-2 and MMP-9 to participate in the degradation of the ECM.
In conclusion, the present study indicates that silencing of EMMPRIN expression by RNAi inhibits the proliferation and PNI of SACC cells in vitro and in vivo. This is the first study to demonstrate that EMMPRIN facilitates the PNI of SACC cells via (1) inducing the adhesion of the SACC cells to the ECM and the neural cells and (2) regulating the expression of MMP-2 and MMP-9. Our findings provide new insights for the development of gene therapy technology to treat SACC patients with PNI.
Materials and Methods
Cell culture
The SACC-83Citation12 and the human embryo pulmonary fibroblast-1 cell lines were purchased from the Chinese Academy of Medical Sciences. They were maintained in DMEM (Gibco) with 10% fetal bovine serum in a 5% CO2 humidified atmosphere at 37°C. Normal human neural progenitor (NHNP) cells and neural progenitor cell maintenance medium (NPMM) were purchased from Lonza Walkersville, Inc. NHNP cells were maintained in supplemented NPMM in a 5% CO2 humidified atmosphere at 37°C until they were differentiated to neural cells.
Silencing of EMMPRIN by RNAi in SACC-83 cells
Based on the EMMPRIN sequence in GenBank (NM_001728), two pairs of template oligonucleotides (shRNA1 and shRNA2) encoding different target sequences and another pair of oligonucleotides (negative shRNA) encoding a non-specific sequence were designed and synthesized (). shRNAs were cloned into the lentiviral shRNA expression vector pRNAT-U6.2/Lenti (GenScript) and were packaged according to the manufacturer’s instructions in 293T cells (Invitrogen) by use of ViraPower Lentiviral Expression reagents (Invitrogen). Then, 1 ml of the packaged lentivirus were delivered to SACC-83 cells in the presence of 6 mg/ml polybrene. Forty-eight hours post-transduction, the transduced SACC-83 cells were selected with 400 μg/ml G418 (Invitrogen) for 10 d. The resultant stable pools were designated A1, A2 and AN, respectively.
Table 1. Oligonucleotide sequences of EMMPRIN-specific shRNAs
Semi-quantitative RT-PCR
Total RNA was isolated from cells with Trizol reagent (Invitrogen) and reverse transcribed into cDNA with Superscript First-Strand Synthesis Kit (Invitrogen). The sequences used were: EMMPRIN primer, (sense) 5′-GGCCAGAAAACGGAGTTCAA-3′ and (antisense) 5′-GCGCTTCTCGTAGATGAAGA-3′; β-actin primer, (sense) 5′-CGTGACATTAAGGAGAAGCTG-3′ and (antisense) 5′-CTAGAAGCATTTGCGGTGGAC-3′. PCR products were electrophoresed on 2% agarose gels and the gray scale ratio of EMMPRIN/β-actin was calculated.
Western blot
The cellular proteins were separated by 10% SDS-PAGE and transferred onto PVDF membranes (Pall Corporation). Subsequently, the membranes were immunoblotted with a 1:500 dilution of mouse anti-human EMMPRIN (Santa Cruz) or β-actin antibody (Sigma). Then, the proteins were visualized with enhanced chemoluminescence reagent (Santa Cruz).
Proliferation assays
Cell proliferation in vitro was analyzed with 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT). Briefly, cells were plated in 96-well plates at a density of 103 cells per well. Every 24 h over the course of 7 d, a group of cells were stained with 20 µl of MTT substrate (5 mg/ml; Sigma) at 37°C for 4 h. Then, 150 µl of DMSO was added and thoroughly mixed in for 10 min. Spectrometric absorbance at 490 nm was measured on a microplate reader (Bio-Rad).
Adhesion assays
To perform ECM adhesion assays, the 96-well plates were coated with Basement Membrane Matrigel (BD) at a concentration of 5 mg/ml. Then, tumor cells (2 × 104/well) suspended in serum-free DMEM were added to the wells and incubated at 37°C for 45 min. After removing the medium and non-attached cells, 0.2% crystal violet was added for 10 min and 5% SDS/50% ethanol was added for 20 min. Finally, the plate was read at 540 nm.
To perform neural cells adhesion assays, NHNP cells were continually cultured in 24-well plates with NPMM until they differentiated to neural cells. Then, tumor cells (1 × 105/well) suspended in serum-free DMEM were added onto the monolayer culture of neural cells and incubated at 37°C for 45 min. After washing with PBS to exclude nonspecific cell attachment, the number of attached cells was counted directly under a microscope as previously reported.Citation13
Gelatin zymography assays
Cells were cultured in serum-free DMEM for 24 h. Conditioned medium was collected and separated by 8% acrylamide gels containing 0.1% gelatin (Sigma). The gels were incubated in 2.5% Triton X-100 solution with gentle agitation to remove SDS and then were soaked in reaction buffer (50 mmol/l TRIS-HCl, 200 mmol/l NaCl, 10 mmol/l CaCl2, pH = 7.5) at 37°C for 24 h. After reaction, the gels were stained for 6 h and destained for 30 min.
In vitro perineural invasion assays
Transwell Boyden chambers containing 8-μm polycarbonate filters were coated on the upper surface with Basement Membrane Matrigel (BD). 1 × 105 tumor cells in 200 μl of serum-free DMEM were placed in the upper chamber. The lower chamber was filled with 600 μl of conditioned medium (incubating 5 × 105 fibroblasts and 5 × 105 neural cells in 600 μl serum-free DMEM medium for 24 h) to simulate the perineural surrounding environment. Cells were incubated for 24 h at 37°C. Then, cells on the upper surface of the filter were removed with a cotton swab. Cells that had invaded the Matrigel and reached the lower surface of the filter were fixed in methanol, stained with H&E, and counted as previously described.Citation14
In vivo tumorigenicity assays
All protocols involving animals were approved by the China Institutional Ethics Review Committee for Animal Experimentation. Tumor cells (4 × 106/mouse) suspended in 0.2 ml serum-free DMEM were s.c. inoculated into the right axillary fossa of 6-week-old female BALB/c nude mice (Shanghai SLAC Laboratory Animal Co. Ltd.). Tumor sizes were measured weekly and calculated using the formula V = (width)2 × length/2. For up to 5 weeks, tumors were harvested for immunohistochemical analysis. Ki-67 labeling and analysis were performed as previously described.Citation11
In vivo perineural invasion
assays. The left sciatic nerve of 6-week-old female BALB/c nude mice was exposed deep to the biceps femoris muscle. Then, tumor cells were microscopically injected into the site between the sciatic nerve perineurium and the biceps femoris muscle. Slow microinjection of 5 µl of cell suspension at a concentration of 1 × 105 cell/μl was performed using a 10 µl Hamilton syringe. After sewing up the wound, the in vivo PNI model was established.
Sciatic nerve function was measured weekly after injection. Functional measures for monitoring tumor PNI included (a) limb function-graded according to hind limb paw response to manual extension of the body, from 4 (normal) to 1 (total paw paralysis); (b) sciatic nerve function index-calculated as the spread length (in mm) between the first and fifth toes of the hind limb.Citation15,Citation16
For up to 5 weeks, tumors and the affected sciatic nerves were excised for H&E and immunohistochemical analysis. Immunohistochemical staining of EMMPRIN, MMP-2, and MMP-9 were performed as previously described.Citation11 The positive staining intensity was calculated by computer image analyzer (Image Pro Plus).
Statistical analysis
All in vitro experiments were performed in triplicate. All data was presented as mean ± standard deviation (SD). Results were analyzed with the Student t-test and the one-way ANOVA tests using SPSS16.0 software package. p < 0.05 was considered statistically significant.
Acknowledgments
We thank Yuan Liu for his excellent technical assistance and Lei Che for her secretarial assistance. This work was supported by a research grant from the Fourth Military Medical University (No. 2010D14).
References
- Li LJ, Li Y, Wen YM, Liu H, Zhao HW. Clinical analysis of salivary gland tumor cases in West China in past 50 years. Oral Oncol 2008; 44:187 - 92; http://dx.doi.org/10.1016/j.oraloncology.2007.01.016; PMID: 17418612
- Fordice J, Kershaw C, El-Naggar A, Goepfert H. Adenoid cystic carcinoma of the head and neck: predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg 1999; 125:149 - 52; PMID: 10037280
- Dardick I. Salivary Gland Tumor Pathology. Igaku-Shoin, NewYork, 1996. P. 274.
- van der Wal JE, Snow GB, van der Waal I. Intraoral adenoid cystic carcinoma. The presence of perineural spread in relation to site, size, local extension, and metastatic spread in 22 cases. Cancer 1990; 66:2031 - 3; http://dx.doi.org/10.1002/1097-0142(19901101)66:9<2031::AID-CNCR2820660931>3.0.CO;2-7; PMID: 2171754
- Vrielinck LJ, Ostyn F, van Damme B, van den Boqaert W, Fossion E. The significance of perineural spread in adenoid cystic carcinoma of the major and minor salivary glands. Int J Oral Maxillofac Surg 1988; 17:190 - 3; http://dx.doi.org/10.1016/S0901-5027(88)80030-4; PMID: 2840472
- Barrett AW, Speight PM. Perineural invasion in adenoid cystic carcinoma of the salivary glands: a valid prognostic indicator?. Oral Oncol 2009; 45:936 - 40; http://dx.doi.org/10.1016/j.oraloncology.2009.07.001; PMID: 19692291
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002; 2:161 - 74; http://dx.doi.org/10.1038/nrc745; PMID: 11990853
- Toole BP. Emmprin (CD147), a cell surface regulator of matrix metalloproteinase production and function. Curr Top Dev Biol 2003; 54:371 - 89; http://dx.doi.org/10.1016/S0070-2153(03)54015-7; PMID: 12696756
- Sun J, Hemler ME. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res 2001; 61:2276 - 81; PMID: 11280798
- Tang Y, Kesavan P, Nakada MT, Yan L. Tumor-stroma interaction: positive feedback regulation of extracellular matrix metalloproteinase inducer (EMMPRIN) expression and matrix metalloproteinase-dependent generation of soluble EMMPRIN. Mol Cancer Res 2004; 2:73 - 80; PMID: 14985463
- Yang X, Dai J, Li T, Zhang P, Ma Q, Li Y, et al. Expression of EMMPRIN in adenoid cystic carcinoma of salivary glands: correlation with tumor progression and patients' prognosis. Oral Oncol 2010; 46:755 - 60; http://dx.doi.org/10.1016/j.oraloncology.2010.08.008; PMID: 20850374
- Li SL. Establishment of a human cancer cell line from adenoid cystic carcinoma of the minor salivary gland. Zhonghua Kou Qiang Yi Xue Za Zhi 1990; 25:29 - 31; PMID: 2163817
- Fukuda M, Kusama K, Sakashita H. Cimetidine inhibits salivary gland tumor cell adhesion to neural cells and induces apoptosis by blocking NCAM expression. BMC Cancer 2008; 8:376; http://dx.doi.org/10.1186/1471-2407-8-376; PMID: 19091137
- Chen W, Zhang HL, Jiang YG, Li JH, Liu BL, Sun MY. Inhibition of CD146 gene expression via RNA interference reduces in vitro perineural invasion on ACC-M cell. J Oral Pathol Med 2009; 38:198 - 205; http://dx.doi.org/10.1111/j.1600-0714.2008.00706.x; PMID: 19200179
- Gil Z, Rein A, Brader P, Li S, Shah JP, Fong Y, et al. Nerve-sparing therapy with oncolytic herpesvirus for cancers with neural invasion. Clin Cancer Res 2007; 13:6479 - 85; http://dx.doi.org/10.1158/1078-0432.CCR-07-1639; PMID: 17975160
- Gil Z, Kelly KJ, Brader P, Shah JP, Fong Y, Wong RJ. Utility of a Herpes Oncolytic Virus for the Detection of Neural Invasion By Cancer. Neoplasia 2008; 10:347 - 53; PMID: 18392138
- Zheng HC, Takahashi H, Murai Y, Cui ZG, Nomoto K, Miwa S, et al. Upregulated EMMPRIN/CD147 might contribute to growth and angiogenesis of gastric carcinoma: a good marker for local invasion and prognosis. Br J Cancer 2006; 95:1371 - 8; http://dx.doi.org/10.1038/sj.bjc.6603425; PMID: 17088917
- Zhang W, Erkan M, Abiatari I, Giese NA, Felix K, Kayed H, et al. Expression of extracellular matrix metalloproteinase inducer (EMMPRIN/CD147) in pancreatic neoplasm and pancreatic stellate cells. Cancer Biol Ther 2007; 6:218 - 27; http://dx.doi.org/10.4161/cbt.6.2.3623; PMID: 17224648
- Wang L, Wu G, Yu L, Yuan J, Fang F, Zhai Z, et al. Inhibition of CD147 Expression reduces tumor cell invasion in human prostate cancer cell line via RNA interference. Cancer Biol Ther 2006; 5:608 - 14; http://dx.doi.org/10.4161/cbt.5.6.2661; PMID: 16627983
- Quemener C, Gabison EE, Naïmi B, Lescaille G, Bougatef F, Podgorniak MP, et al. Extracellular matrix metalloproteinase inducer up-regulates the urokinase-type plasminogen activator system promoting tumor cell invasion. Cancer Res 2007; 67:9 - 15; http://dx.doi.org/10.1158/0008-5472.CAN-06-2448; PMID: 17210677
- Xu J, Xu HY, Zhang Q, Song F, Jiang JL, Yang XM, et al. HAb18G/CD147 functions in invasion and metastasis of hepatocellular carcinoma. Mol Cancer Res 2007; 5:605 - 14; http://dx.doi.org/10.1158/1541-7786.MCR-06-0286; PMID: 17579119
- Liang Q, Xiong H, Gao G, Xiong K, Wang X, Zhao Z, et al. Inhibition of basigin expression in glioblastoma cell line via antisense RNA reduces tumor cell invasion and angiogenesis. Cancer Biol Ther 2005; 4:759 - 62; http://dx.doi.org/10.4161/cbt.4.7.1828; PMID: 15970688
- Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, et al. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res 2005; 65:3193 - 9; PMID: 15833850
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998; 391:806 - 11; http://dx.doi.org/10.1038/35888; PMID: 9486653
- Tuschl T. Expanding small RNA interference. Nat Biotechnol 2002; 20:446 - 8; http://dx.doi.org/10.1038/nbt0502-446; PMID: 11981553
- Li M, Zhai Q, Bharadwaj U, Wang H, Li F, Fisher WE, et al. Cyclophilin A is overexpressed in human pancreatic cancer cells and stimulates cell proliferation through CD147. Cancer 2006; 106:2284 - 94; http://dx.doi.org/10.1002/cncr.21862; PMID: 16604531
- Chen X, Lin J, Kanekura T, Su J, Lin W, Xie H, et al. A small interfering CD147-targeting RNA inhibited the proliferation, invasiveness, and metastatic activity of malignant melanoma. Cancer Res 2006; 66:11323 - 30; http://dx.doi.org/10.1158/0008-5472.CAN-06-1536; PMID: 17145878
- Su J, Chen X, Kanekura TA. CD147-targeting siRNA inhibits the proliferation, invasiveness, and VEGF production of human malignant melanoma cells by down-regulating glycolysis. Cancer Lett 2009; 273:140 - 7; http://dx.doi.org/10.1016/j.canlet.2008.07.034; PMID: 18778892
- Schneiderhan W, Scheler M, Holzmann KH, Marx M, Gschwend JE, Bucholz M, et al. CD147 silencing inhibits lactate transport and reduces malignant potential of pancreatic cancer cells in in vivo and in vitro models. Gut 2009; 58:1391 - 8; http://dx.doi.org/10.1136/gut.2009.181412; PMID: 19505879
- Stockinger H, Ebel T, Hansmann C. EC/16/760 neurothelin/basigin/M6/EMMPRIN Workshop Panel Report. In: Kishimoto T, Kikutani H, editors. Leukocyte Typing VI, Garland Publishing, New York, 1997. p. 760–3.
- Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, et al. Basement membrane complexes with biological activity. Biochemistry 1986; 25:312 - 8; http://dx.doi.org/10.1021/bi00350a005; PMID: 2937447
- Shintani S, Alcalde RE, Matsumura T, Terakado N. Extracellular matrices expression in invasion area of adenoid cystic carcinoma of salivary glands. Cancer Lett 1997; 116:9 - 14; http://dx.doi.org/10.1016/S0304-3835(97)04730-7; PMID: 9177451
- Liotta LA, Kohn EC. The microenvironment of the tumor-host interface. Nature 2001; 411:375 - 9; http://dx.doi.org/10.1038/35077241; PMID: 11357145
- Shirasuna K, Saka M, Hayashido Y, Yoshioka H, Sugiura T, Matsuya T. Extracellular matrix production and degradation by adenoid cystic carcinoma cells: participation of plasminogen activator and its inhibitor in matrix degradation. Cancer Res 1993; 53:147 - 52; PMID: 8380125
- Vihinen P, Kähäri VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer 2002; 99:157 - 66; http://dx.doi.org/10.1002/ijc.10329; PMID: 11979428
- Freitas VM, Vilas-Boas VF, Pimenta DC, Loureiro V, Juliano MA, Carvalho MR, et al. SIKVAV, a laminin alpha1-derived peptide, interacts with integrins and increases protease activity of a human salivary gland adenoid cystic carcinoma cell line through the ERK 1/2 signaling pathway. Am J Pathol 2007; 171:124 - 38; http://dx.doi.org/10.2353/ajpath.2007.051264; PMID: 17591960