Abstract
Advanced melanoma is the most virulent form of cancer and has a poor prognosis. In a previous study, myriocin, an inhibitor of serine palmitoyltransferase, was found to suppress melanoma cell proliferation by cell cycle arrest at the G2/M phase through decreased sphingolipid levels and increased p53 and p21waf1/cip1 expression.Citation1 In the present study, myriocin (1 mg/kg, every other day for 3 weeks) was administered intradermally or intraperitoneally to melanoma mice. Tumor formation was significantly inhibited by intradermal and intraperitoneal administrations of myriocin. The expression of Cdc25C, Cdc2 and cyclin B1 was decreased in tumor tissues from myriocin-treated mice, while the expression of p53 and p21waf1/cip1 was increased compared with that of the controls. The levels of sphingolipids in serum, liver and tumor tissue from myriocin-treated mice were decreased compared with those of controls. The decreased levels of sphingolipids in serum and liver of melanoma mice treated with myriocin suggest that myriocin may be accessible to tumor tissues of advanced melanoma. Taken together, the suppression of sphingolipid synthesis by myriocin inhibits the expression of Cdc25C or activates the expression of p53 and p21waf1/cip1. This is followed by Cdc2 and cyclin B1 inhibition which results in the suppression of tumor growth.
Introduction
Malignant melanoma is an aggressive form of cancer with the highest mortality among skin cancers and its incidence rate has been increased in the Caucasian population worldwide. In the United States, the number of new melanoma cases was estimated to be 68,130, and there were expected to be 8,700 deaths due to melanoma in 2010.Citation2 Although early localized melanoma can be treated with surgery, advanced melanoma has the limited efficacy to systemic therapies.Citation3 Poor survival rates among melanoma patients are mainly due to the resistance of this disease to radiation or chemotherapy through dysregulation of apoptotic pathways.Citation4,Citation5 Several postoperative adjuvant immunotherapies have been approved for treating malignant melanoma.Citation6,Citation7 Interferon-α and interleukin-2 are commonly used as immunotherapies for advanced melanoma,Citation6-Citation8 but these cytokines are associated with significant toxicity and low efficacy. Recently two targeted approaches were expected to show excellent promise for melanoma therapy. The first of these is the selective inhibitor of serine-threonine protein kinase B-RAF (BRAF) for the therapy of metastatic melanoma. Activating mutations in BRAF was identified in 50–60% of melanomas.Citation9 Thus, BRAF kinase inhibitor Vemurafenib (PLX-4032) has shown selective antitumor activities of more than 50% in patients with metastatic melanoma harboring BRAF mutation.Citation10 Also, recent clinical trial results came with novel immunologic therapy. The increased ratio of CD28:CTLA4 activates T cell, while the decreased ratio caused anergy. Thus, CTLA4-blocking antibody allows the B7/CD28 interface to continue to drive T-cell activation. Monotherapy of Yervoy (Ipilimumab), CTLA4-specific antibody, in patients with metastatic melanomas improved overall survival in a phase III trial.Citation11
New potential drugs derived from natural sources have been screened as effective anticancer treatments. Myriocin, also known as ISP-1 or thermozymocidin, is produced from Mycelia sterilia, Isaria sinclairii and Cordyceps cicadae.Citation12-Citation15 Myriocin suppresses the proliferation of lymphocytes and IL-2-dependent mouse cytotoxic T cells.Citation16 Myriocin inhibits serine palmitoyltransferase, the enzyme that catalyzes the first step in de novo sphingolipid biosynthesis.Citation17 Myriocin decreased the levels of sphingomyelin, ceramide, sphingosine-1-phosphate and sphingosine in B16F10 melanoma cells.Citation1 Myriocin administration to apoE-KO mice lowered the plasma levels of glycosphingolipid, sphingomyelin, ceramide, sphinganine, sphingosine-1-phosphate, cholesterol and triglycerides, and prevented the development of atherosclerotic lesions.Citation18-Citation21
Sphingolipids are highly bioactive and are involved in the regulation of apoptosis, cell proliferation, cell migration, senescence and inflammation.Citation22-Citation26 Human endometrial cancer is characterized by elevated levels of ceramide, dihydroceramide, sphingosine, sphinganine and sphingosine-1-phosphate as well as increased activity of serine palmitoyltransferase and sphingosine kinase 1.Citation27 Ceramide levels in malignant breast tumor tissues are significantly elevated compared with normal tissues. In malignant and benign breast tumor tissues, ceramide levels were increased by 12-fold and 4-fold, respectively, relative to normal tissues.Citation28 The levels of dihydroceramide and ceramide in tumor tissues from mice inoculated with Sarcoma 180, B16 melanoma, or Lewis lung carcinoma cells are very high compared with those in healthy tissues.Citation29
Aberration of p53 is the most frequent molecular event in human cancers. p53 is involved in the regulation of the cell cycle, DNA repair, apoptosis and senescence.Citation30,Citation31 Increased p53 expression in B16F10 melanoma cells treated with myriocin is correlated with the inhibition of the tumor cell growth.Citation1 The anti-tumor activity of SN50, an NF-κB inhibitor, is associated with p53-related activation of autophagy and apoptosis in human gastric carcinoma cells.Citation32 Expression of p53 in Ewing’s sarcoma cells treated with nutlin-3 is increased. This is followed by increased expression of p53 target genes such as p21, MDM2 and PUMA, thereby resulting in cell growth inhibition and apoptosis.Citation33 p21, the downstream signaling molecule of p53, inhibits the activity of the cyclin B1/Cdc2 complex and causes arrest in the G2 phase of the cell cycle.Citation34 The progression of cell cycle is controlled by cyclins that bind to cyclin-dependent kinases (CDKs).Citation35-Citation38 The expression of p21 in B16F10 melanoma cells treated with myriocin is increased, while levels of cyclin B1 and Cdc2 are decreased, thus leading to cell cycle arrest in the G2/M phase.Citation1
In a previous study, cell proliferation and DNA synthesis of B16F10 melanoma cells treated with myriocin was inhibited by the suppression of de novo sphingolipid pathway.Citation1 The purpose of the present study was to demonstrate that the antitumor activity of myriocin is accomplished through the inhibition of sphingolipid synthesis in a murine melanoma model. Thus, pharmacological manipulation of sphingolipid metabolism to modulate the sphingolipid levels in tumor cells emerges as a potential target for malignant skin cancer therapy.
Results
Myriocin inhibits tumor formation in melanoma mouse model
Myriocin was administered intradermally or intraperitoneally to the mice every other day starting from day 2–18. Body weight and tumor mass were measured after sacrificing the animals (). Tumor growth in mice treated with myriocin (1 mg/kg, n = 10) was compared with that in the saline control group (saline, n = 10).
Figure 1. Inhibitory effect of myriocin on tumor formation in melanoma mouse model. (A) B16F10 melanoma cells were planted intradermally into the flank of C57BL/6J mice on day 0. Myriocin (1 mg/kg) was intradermally (i.d.) or intraperitoneally (i.p.) administered into mice every other day for 3 weeks. (B) The tumor weight and tumor volume are expressed in grams and mm3, respectively. Tumor dimensions were measured using a caliper, and tumor volume was calculated by the equation [(width)Citation2 x (length)/2]. (C) The body weight of the mice was measured every other day during the experimental period. Values are expressed as the mean ± SE of three independent experiments (n = 10, *p < 0.05, **p < 0.01 and ***p < 0.001 vs. the saline control)
![Figure 1. Inhibitory effect of myriocin on tumor formation in melanoma mouse model. (A) B16F10 melanoma cells were planted intradermally into the flank of C57BL/6J mice on day 0. Myriocin (1 mg/kg) was intradermally (i.d.) or intraperitoneally (i.p.) administered into mice every other day for 3 weeks. (B) The tumor weight and tumor volume are expressed in grams and mm3, respectively. Tumor dimensions were measured using a caliper, and tumor volume was calculated by the equation [(width)Citation2 x (length)/2]. (C) The body weight of the mice was measured every other day during the experimental period. Values are expressed as the mean ± SE of three independent experiments (n = 10, *p < 0.05, **p < 0.01 and ***p < 0.001 vs. the saline control)](/cms/asset/52602457-55d9-4c3e-9531-b00977492afe/kcbt_a_10918870_f0001.gif)
Myriocin had a significant inhibitory effect on tumor growth in mouse model (). Tumor weight and volume were significantly decreased by intradermal and intraperitoneal administrations of myriocin compared with the saline control. Most tumor tissues disappeared in the mice that received myriocin intradermally compared with those in conrol group. The mean weights of the tumors in mice treated with myriocin by intradermal or intraperitoneal administration were 0.1 and 0.5 g, respectively, while those of the corresponding control mice were approximately 2.1 and 2.0 g (). Mean tumor volumes in the mice treated with intradermal administration of myriocin and the corresponding control group were 123 and 1,663 mm3, respectively, indicating that tumor volume was reduced by approximately 93% following myriocin treatment compared with control. Mean size of the tumors in mice intraperitoneally administered with myriocin or control was 341 or 1,582 mm3, respectively.
In the saline control group, body weight increased abnormally from day 8 of the 20-d study (). Differences in the body weights of the control vs. the groups treated with intradermal and intraperitoneal administrations of myriocin were significant at day 18, possibly due to the formation of melanoma tissues.
Myriocin inhibits sphingolipid synthesis in melanoma mice
The inhibition of sphingolipid synthesis by myriocin was thought to be associated with antitumor activity in the melanoma model. The levels of sphingolipids including ceramide, dihydroceramide, sphingosine, sphinganine, sphingosine-1-phophate and sphinganine-1-phosphate in tumor tissues, serum and liver were determined. When the mice were treated with myriocin, the levels of sphingolipids in tumor tissue, serum and liver decreased compared with the saline control ( and ).
Figure 2. Inhibitory effect of myriocin on sphingolipid synthesis in tumor tissues. (A) Ceramide, dihydroceramide, (B) sphingosine and sphinganine levels in tumor tissues from melanoma mice with or without myriocin treatment (i.d., i.p.) were measured by HPLC. Data are expressed as the mean ± SE of three independent experiments (n = 10, *p < 0.05 and **p < 0.01 vs. the saline control).
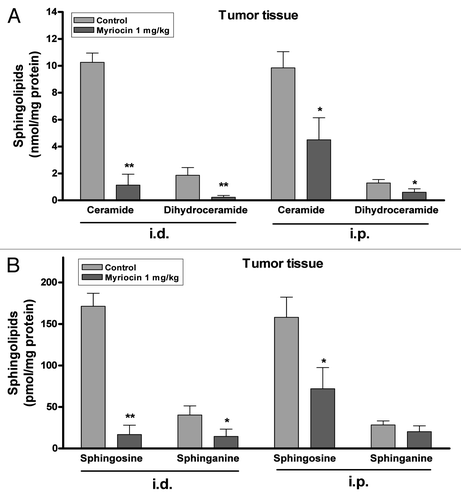
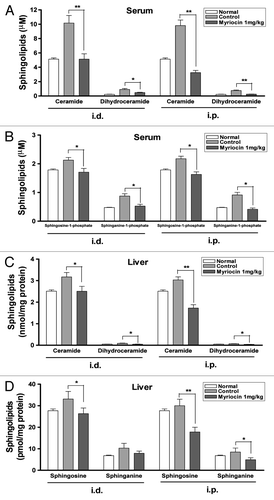
Figure 3. Inhibitory effect of myriocin on sphingolipid synthesis in mouse serum and liver. (A) Ceramide, dihydroceramide, (B) sphingosine-1-phosphate and sphinganine-1-phosphate levels in serum, and (C) ceramide, dihydroceramide, (D) sphingosine and sphinganine contents in liver from melanoma mice with or without myriocin treatment and normal mice (i.d., i.p.) were measured by HPLC. Data are expressed as the mean ± SE of three independent experiments (n = 10, *p < 0.05 and **p < 0.01 vs. the saline control).
The levels of ceramide, dihydroceramide, sphingosine and sphinganine in tumor tissues from mice treated with myriocin by intradermal administration were significantly decreased compared with those of the control (). The levels of ceramide and dihydroceramide in the tumor tissues from the control mice following intradermal administration of control were 10.3 ± 0.7 and 1.9 ± 0.6 nmol per mg protein, respectively, while the ceramide and dihydroceramide levels in the tumors from mice treated intradermally with myriocin were 1.1 ± 0.8 and 0.2 ± 0.1 nmol (). The levels of sphingosine and sphinganine in the tumor tissues from the control mice after intradermal administration were 171.4 ± 15.6 and 40.3 ± 11.1 pmol per mg protein, respectively; the sphingosine and sphinganine levels in the tumors from mice treated intradermally with myriocin were 16.8 ± 11.3 and 14.5 ± 8.8 pmol (). These results suggest that decreased sphingolipid concentrations in tumor tissues may be closely related to the inhibition of tumor growth.
Sphingolipid levels of serum and liver from melanoma mice were increased compared with those from the normal mice (). The serum levels of ceramide and dihydroceramide in normal mice were 5.1 ± 0.2 and 0.2 ± 0.0 μM, respectively; the ceramide and dihydroceramide levels in melanoma mice treated with vehicle intradermally were 10.2 ± 1.0 and 0.9 ± 0.1, respectively (). These results indicated that tumor formation may be related to the activation of sphingolipid synthesis. The serum concentrations of sphingolipids were significantly reduced after intradermal or intraperitoneal administration of myriocin. Intradermal administration of myriocin reduced the serum levels of ceramide and dihydroceramide by approximately 50 and 44%, respectively, compared with the controls. Intraperitoneal administration of myriocin decreased the serum levels of ceramide and dihydroceramide by about 66 and 63%, respectively, compared with the controls (). The serum levels of sphingosine-1-phosphate in the control mice after intradermal and intraperitoneal administrations were 2.1 ± 0.1 and 2.2 ± 0.1 μM, respectively; the serum sphingosine-1-phosphate levels in mice treated intradermally and intraperitoneally with myriocin were 1.7 ± 0.1 and 1.6 ± 0.1 μM ().
The levels of ceramide, dihydroceramide, sphingosine and sphinganine in the liver were reduced by intradermal and intraperitoneal administrations of myriocin (). The decreased sphingolipid concentrations in serum and liver suggest that myriocin may be accessible to the tumor tissues and can be useful for the treatment of advanced melanoma.
Effect of myriocin on cell cycle regulatory protein expression through downregulation of sphingolipid synthesis
Cell cycle progression is regulated through a complex network of cell cycle regulatory proteins such as CDKs and cyclins. In a previous study, myriocin was found to inhibit the proliferation of B16F10 melanoma cells and induce cell cycle arrest in the G2/M phase.Citation1 To examine the mechanisms underlying myriocin-induced G2/M phase arrest in the melanoma mouse model, the expression of Cdc2 and cyclin B1 was measured. Cdc2 expression in tumor tissues was significantly reduced after intradermal administration of myriocin compared with the saline controls (). Cyclin B1 expression was also significantly decreased after intradermal administration of myriocin (). Cdc25C expression in tumor tissues decreased after intradermal and intraperitoneal administrations of myriocin compared with control (), while the expression of p53 and p21 increased (). These results suggest that suppression of de novo sphingolipid synthesis by myriocin may inhibit tumor growth by cell cycle arrest in the G2/M phase through either the activation of the p53-p21-dependent pathway or inhibition of Cdc25C ().
Figure 4. Effect of myriocin on cell cycle regulatory protein expression in mouse melanoma tissues. Tumor tissue homogenates from control and myriocin-treated mice were lysed to measure (A) Cdc2, (B) cyclin B1, (C) Cdc25C, (D) p53 and (E) p21waf1/cip1 expression by 10% SDS-PAGE and immunoblotting. Data from densitometric quantification are expressed as the mean ± SE of three independent experiments conducted in triplicate (*p < 0.05 and **p < 0.01 vs. the saline control).
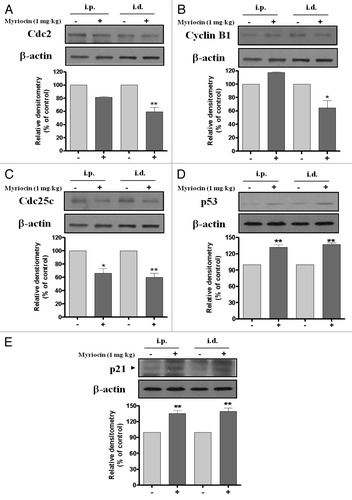
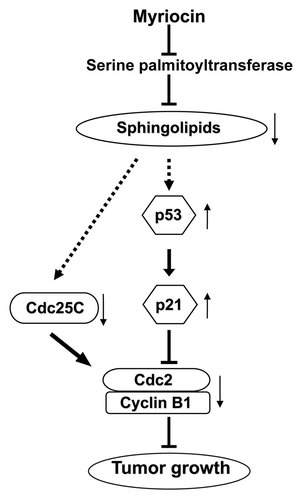
Figure 5. Proposed mechanism underlying myriocin-induced antitumor activity in melanoma mice. Myriocin is known to inhibit serine palmitoyltransferase in the de novo sphingolipid synthesis pathway. Myriocin-induced suppression of sphingolipid synthesis may upregulate the expression of p53 and p21 or downregulate Cdc25C expression. This would be followed by decreased cyclin B1 and Cdc2 expression, and lead to G2/M phase arrest resulting in tumor growth inhibition. The symbols ⊥, ↑ and ↓ represent inhibition, upregulation and downregulation, respectively.
Discussion
Malignant melanoma is an aggressive form of cancer with the highest mortality among skin cancers. In a previous study, myriocin inhibited the proliferation of B16F10 melanoma cells and induced the cell cycle arrest at the G2/M phase.Citation1 Myriocin decreased the expression of Cdc25C, cyclin B1 and Cdc2, and increased the expression of p53 and p21waf1/cip1 in malignant melanoma cells. Sphingolipid including ceramide, sphingomyelin, sphingosine and sphingosine-1-phosphate in melanoma cells was reduced by myriocin. The present study demonstrated that myriocin induced antitumor activity in melanoma mouse model. When myriocin was administered intradermally or intraperitoneally, the antitumor activity occurred.
Pharmacological manipulation of sphingolipid metabolism to modulate the sphingolipid levels in tumor cells has emerged as a new cancer chemotherapy modality. Fenretinide induces antitumor activity by increasing ceramide levels through the activation of de novo sphingolipid synthesis in ovarian, breast, and neuroblastoma tumor cells.Citation39 Resveratrol induces apoptosis by elevating ceramide levels in PC3 cells.Citation40 Thus, anticancer activities associated with these compounds appear to occur through the activation of ceramide synthesis. Combinations of C6-ceramide and paclitaxel increase anti-tumor activity in SKOV-3 human ovarian adenocarcinoma xenograft models.Citation41 Dietary sphingomyelin inhibits colonic tumorigenesis by increasing alkaline sphingomyelinase expression in ICR mice, and consumption of milk sphingomyelin reduces the number of aberrant colonic crypt foci in these animals.Citation42-Citation44
However, increased levels of sphingolipid metabolites in a diversity of cancers compared with those in normal tissues have been observed. Human endometrial cancer patients are characterized by elevated levels of ceramide, dihydroceramide, sphingosine, sphinganine and sphingosine-1-phosphate as well as increased activity of serine palmitoyltransferase and sphingosine kinase 1 in tumor tissues.Citation27 The ceramide levels in malignant and benign breast tumor biopsies are significantly elevated compared with normal tissue samples.Citation28 The levels of dihydroceramide and ceramide in tumor tissues from mice inoculated with Sarcoma 180, B16 melanoma, or Lewis lung carcinoma cells are increased compared with those in healthy tissues.Citation29
In this study, we proposed a new approach to inducing antitumor activity by downregulating de novo sphingolipid synthesis in mice with melanoma. The levels of ceramide, dihydroceramide, sphingosine, sphinganine, sphingosine-1-phosphate and sphinganine-1-phosphate in serum and liver from melanoma mice were elevated compared with those from the normal mice. These results indicated that tumor formation may be closely associated with the activation of sphingolipid synthesis. Furthermore, reducing cellular sphingolipid levels by blocking de novo ceramide synthesis can be an effective strategy for inducing anticancer activity. The levels of ceramide, dihydroceramide, sphingosine, sphinganine, sphingosine-1-phosphate and sphinganine-1-phosphate in tumor tissues, serum and liver of the mice treated with myriocin were decreased compared with those of the untreated groups. Additionally, tumor formation in the myriocin-treated mice was inhibited under these conditions. Thus, the decreased levels of sphingolipids in serum and liver of melanoma mice treated with myriocin suggest that myriocin may be accessible to tumor tissues in advanced melanoma.
The growth of murine melanoma B16F10 cells and tumor formation in mice with melanoma were inhibited by myriocin which caused cell cycle arrest at G2/M phase.Citation1 Cdc2 and cyclin B1, key regulatory proteins required for the transition from the G2 to M phase, are maintained in an inactive form when phosphorylated by Wee1 kinase.Citation45 Cdc2/cyclin B1 complex is activated by dephosphorylation and then triggers the initiation of mitosis. Myriocin decreased the expression of Cdc2 and cyclin B1 in B16F10 cellsCitation1 and the tumor tissues from melanoma mice. The cellular ceramide level decreased in myriocin treatment was increased by adding C8-ceramide.Citation1 Following exposure to C8-ceramide, growth and Cdc2/cyclin B1 expression of the myriocin-treated cells were similar to those of control cells. The expression of Cdc25C, an upstream regulator of the Cdc/cyclin B1 complex, was decreased in B16F10 cellsCitation1 and melanoma mice after myriocin treatment, suggesting that inhibition of sphingolipid synthesis by myriocin may downregulate Cdc25C signaling pathway.
Several studies have reported that the modulation of cellular ceramide levels triggers p53 upregulation, leading to cell growth inhibition. Exogenous ceramide induces G1 phase arrest in MCF-7 cells, which is associated with increased expression of p53 and p21.Citation46 However, the inhibition of ceramide synthesis following myriocin treatment in B16F10 melanoma cellsCitation1 and the tumor tissues from melanoma mice upregulated p53 and p21 expression. Thus, myriocin-induced antitumor activity may occur through p53 signaling pathways as a consequence of de novo sphingolipid synthesis inhibition by myriocin.
In summary, the present study demonstrated that myriocin inhibition of de novo sphingolipid synthesis in a melanoma mouse model decreases the expression of Cdc25C or activates the expression of p53 and p21waf1/cip1. This is followed by reduced cyclin B1 and Cdc2 expression which results in the inhibition of tumor growth. Thus, pharmacological modulation of sphingolipid metabolism by myriocin may be a novel strategy for treatment of advanced one as well as early-staged melanoma.
Materials and Methods
Reagents
Myriocin and o-phthalaldehyde (OPA) were purchased from Sigma Chemical Co., and sphingolipid ceramide N-deacylase (SCDase) was obtained from Takara. Primary antibodies against cyclin B1, Cdc2, Cdc25C, p53 and β-actin were purchased from Santa Cruz Biotechnology, and anti-p21waf1/cip1 was obtained from Upstate Biotechnology. Proteinase inhibitors and protease inhibitors were obtained from Roche. C17-based sphingosine and N-oleoyl-D-erythro-sphingosine (C17 base) were purchased from Avanti Polar Lipids. Other reagents were of the highest purity available.
Cell culture
B16F10 cells originating from a murine melanoma were obtained from the American Tissue Culture Collection (ATCC) and were cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 100 units/mL penicillin, 100 μg/mL streptomycin and 2 mM L-glutamine (all from Gibco).
In vivo B16F10 tumor mouse model
This study was approved by the ethical committee of animal experiment in Chungbuk National University, and all animal procedures were performed in accordance with the Public Health Service policy. Male C57BL/6J mice (20–23 g, n = 100) 6–7 weeks old were purchased from Japan SLC and acclimated for 1 week in the animal facility of Chungbuk National University (22°C, 12 h light/dark cycle). The animals had free access to drinking water and a commercial pellet diet obtained from Samyang Co. The mice were inoculated with B16F10 melanoma cells by intradermal injection (106 tumor cells in 0.2 mL PBS/mouse) with a 26-gauge needle into the flank. After 2 d, mice were intradermally or intraperitoneally administered myriocin (1 mg/kg, every other day for 3 weeks). The control group was given saline. The weight of the animals was periodically measured. The tumor dimensions were measured with vernier calipers and tumor volumes were calculated with the following formula: (A x B2)/2, where A is the larger and B is the smaller dimension of the tumor. At the end of the experiment, the animals were sacrificed by cervical dislocation. The tumors were separated from the surrounding muscles and dermis, excised and weighed. Blood was collected and liver was removed for sphingolipid analysis.
Immunoblotting assay
To measure cyclin B1, Cdc2, Cdc25C, p53 and p21waf1/cip1 expression, homogenized tumor tissues were resuspended in lysis buffer (20 mM TRIS-HCl, 150 mM NaCl, 1 mM EDTA and 1% Triton X-100) containing proteinase inhibitors (1 mM aprotinin, 1 mM leupeptin and 1 mM PMSF) and protease inhibitors (1 mM NaOV3 and 1 mM NaF) at 4°C. The concentration of total proteins in the homogenates was measured by a BCA protein assay (Pierce). Protein samples were separated by 10% to 15% SDS-PAGE and then transferred to a polyvinylidene difluoride membrane (GE Healthcare Life Sciences). The membranes were incubated with the primary and secondary antibodies; antibody binding was viewed with enhanced chemiluminescence (Amersham Pharmacia Biotech) before the blots were exposed to X-ray film (Eastman-Kodak). The primary antibodies and dilution factors used for immunoblotting were anti-cyclin B1 (1:500); anti-Cdc2 (1:200); anti-Cdc25C (1:1,000); anti-p53 (1:1,000); anti-p21waf1/cip1 (1:1,000); and anti-β-actin (1:1,000).
Sphingolipid analysis
Quantification of ceramide and dihydroceramide was performed as previously described with modifications.Citation1 Briefly, lipids were extracted from samples of mouse serum, liver and tumor tissue for 1 h at 37°C after spiking with ceramide (C17 base, 100 pmole) as internal standard. Ceramide and dihydroceramide were separated by TLC in diisopropylether/methanol/29% NH4OH (40:10:1, v:v:v). Ceramide and dihydroceramide were deacylated by SCDase to produce sphingosine and sphinganine, respectively, followed by OPA derivatization. HPLC analysis was performed using a Shimadzu Model LC-10AT pump, SIL-10AXL auto sampler system and analytical Radial-Pak cartridge (Waters Associates, Inc.) packed with Nova-Pak C18 reversed-phase column (4 μm, 100 × 8 mm). The isocratic mobile phase composition of methanol/distilled water/triethylamine (92:8:0.1, v:v:v) and a flow rate of 1 mL/min were accurately regulated by HPLC system controller (Shimadzu SCL-10A). The Shimadzu RF-10XL fluorescence detector was set at an excitation wavelength of 340 nm and an emission wavelength of 455 nm. The resulting data and chromatographic profiles were evaluated using the Borwin system manager software (JMBS).
For the quantification of sphingoid bases and sphingoid bases-1-phosphate, lipids were extracted with 0.1 M methanolic KOH/chloroform (2:1, v:v) for 1 h at 37°C after the addition of sphingosine (C17 base) and sphingosine-1-phosphate (C17 base), respectively, as internal standards. Sphingosine-1-phosphate and sphinganine-1-phosphate were dephosphorylated by alkaline phosphatase to release sphingosine and sphinganine, respectively, which were analyzed by HPLC.
Statistical analysis
The experimental results are expressed as the mean ± SE. One-way analysis of variance was used for the Newman-Keuls Multiple Comparison test. P values less than 0.05 were considered to be statistically significant.
| Abbreviations: | ||
| CDKs | = | cyclin-dependent kinases |
| FBS | = | fetal bovine serum |
| SCDase | = | sphingolipid ceramide N-deacylase |
| OPA | = | o-phthalaldehyde |
| i.d. | = | intradermal |
| i.p. | = | intraperitoneal |
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0013320) and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (MRC, 2010-0029480).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Lee YS, Choi KM, Choi MH, Ji SY, Lee S, Sin DM, et al. Serine palmitoyltransferase inhibitor myriocin induces growth inhibition of B16F10 melanoma cells through G(2) /M phase arrest. Cell Prolif 2011; 44:320 - 9; http://dx.doi.org/10.1111/j.1365-2184.2011.00761.x; PMID: 21645154
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60:277 - 300; http://dx.doi.org/10.3322/caac.20073; PMID: 20610543
- Flaherty KT. Next generation therapies change the landscape in melanoma. F1000. Med-Rep 2011; 3:8
- Helmbach H, Rossmann E, Kern MA, Schadendorf D. Drug-resistance in human melanoma. Int J Cancer 2001; 93:617 - 22; http://dx.doi.org/10.1002/ijc.1378; PMID: 11477569
- Soengas MS, Capodieci P, Polsky D, Mora J, Esteller M, Opitz-Araya X, et al. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature 2001; 409:207 - 11; http://dx.doi.org/10.1038/35051606; PMID: 11196646
- Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature 2007; 445:851 - 7; http://dx.doi.org/10.1038/nature05661; PMID: 17314971
- Kirkwood JM, Moschos S, Wang W. Strategies for the development of more effective adjuvant therapy of melanoma: current and future explorations of antibodies, cytokines, vaccines, and combinations. Clin Cancer Res 2006; 12:2331s - 6s; http://dx.doi.org/10.1158/1078-0432.CCR-05-2538; PMID: 16609054
- Legha SS, Gianan MA, Plager C, Eton OE, Papadopoulous NE. Evaluation of interleukin-2 administered by continuous infusion in patients with metastatic melanoma. Cancer 1996; 77:89 - 96; http://dx.doi.org/10.1002/(SICI)1097-0142(19960101)77:1<89::AID-CNCR15>3.0.CO;2-4; PMID: 8630945
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417:949 - 54; http://dx.doi.org/10.1038/nature00766; PMID: 12068308
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364:2507 - 16; http://dx.doi.org/10.1056/NEJMoa1103782; PMID: 21639808
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711 - 23; http://dx.doi.org/10.1056/NEJMoa1003466; PMID: 20525992
- Kluepfel D, Bagli J, Baker H, Charest MP, Kudelski A. Myriocin, a new antifungal antibiotic from Myriococcum albomyces. J Antibiot (Tokyo) 1972; 25:109 - 15; PMID: 5034807
- Craveri R, Manachini PL, Aragozzini F. Thermozymocidin new antifungal antibiotic from a thermophilic eumycete. Experientia 1972; 28:867 - 8; http://dx.doi.org/10.1007/BF01923181; PMID: 4658889
- Fujita T, Inoue K, Yamamoto S, Ikumoto T, Sasaki S, Toyama R, et al. Fungal metabolites. Part 11. A potent immunosuppressive activity found in Isaria sinclairii metabolite. J Antibiot (Tokyo) 1994; 47:208 - 15; PMID: 8150717
- Yu J, Xu H, Mo Z, Zhu H, Mao X. Determination of myriocin in natural and cultured Cordyceps cicadae using 9-fluorenylmethyl chloroformate derivatization and high-performance liquid chromatography with UV-detection. Anal Sci 2009; 25:855 - 9; http://dx.doi.org/10.2116/analsci.25.855; PMID: 19609022
- Yoshikawa M, Yokokawa Y, Okuno Y, Yagi N, Murakami N. Synthesis of new immunosuppressive myriocin analogs, 2-epi-myriocin, 14-deoxomyriocin, Z-14-deoxomyriocin, and nor-deoxomyriocins: their structure-activity relationships. Chem Pharm Bull (Tokyo) 1994; 42:2662 - 4; PMID: 7697780
- Miyake Y, Kozutsumi Y, Nakamura S, Fujita T, Kawasaki T. Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem Biophys Res Commun 1995; 211:396 - 403; http://dx.doi.org/10.1006/bbrc.1995.1827; PMID: 7794249
- Park TS, Panek RL, Mueller SB, Hanselman JC, Rosebury WS, Robertson AW, et al. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation 2004; 110:3465 - 71; http://dx.doi.org/10.1161/01.CIR.0000148370.60535.22; PMID: 15545514
- Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, et al. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J Biol Chem 2005; 280:10284 - 9; http://dx.doi.org/10.1074/jbc.M412348200; PMID: 15590644
- Glaros EN, Kim WS, Wu BJ, Suarna C, Quinn CM, Rye KA, et al. Inhibition of atherosclerosis by the serine palmitoyl transferase inhibitor myriocin is associated with reduced plasma glycosphingolipid concentration. Biochem Pharmacol 2007; 73:1340 - 6; http://dx.doi.org/10.1016/j.bcp.2006.12.023; PMID: 17239824
- Glaros EN, Kim WS, Quinn CM, Jessup W, Rye KA, Garner B. Myriocin slows the progression of established atherosclerotic lesions in apolipoprotein E gene knockout mice. J Lipid Res 2008; 49:324 - 31; http://dx.doi.org/10.1194/jlr.M700261-JLR200; PMID: 17978313
- Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO Rep 2004; 5:777 - 82; http://dx.doi.org/10.1038/sj.embor.7400208; PMID: 15289826
- Pettus BJ, Bielawska A, Subramanian P, Wijesinghe DS, Maceyka M, Leslie CC, et al. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J Biol Chem 2004; 279:11320 - 6; http://dx.doi.org/10.1074/jbc.M309262200; PMID: 14676210
- Modrak DE, Gold DV, Goldenberg DM. Sphingolipid targets in cancer therapy. Mol Cancer Ther 2006; 5:200 - 8; http://dx.doi.org/10.1158/1535-7163.MCT-05-0420; PMID: 16505092
- Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer 2004; 4:604 - 16; http://dx.doi.org/10.1038/nrc1411; PMID: 15286740
- Kok JW, Sietsma H. Sphingolipid metabolism enzymes as targets for anticancer therapy. Curr Drug Targets 2004; 5:375 - 82; http://dx.doi.org/10.2174/1389450043345452; PMID: 15134220
- Knapp P, Baranowski M, Knapp M, Zabielski P, Blachnio-Zabielska AU, Gorski J. Altered sphingolipid metabolism in human endometrial cancer. Prostaglandins Other Lipid Mediat 2010; 92:62 - 6; http://dx.doi.org/10.1016/j.prostaglandins.2010.03.002; PMID: 20226264
- Schiffmann S, Sandner J, Birod K, Wobst I, Angioni C, Ruckhaberle E, et al. Ceramide synthases and ceramide levels are increased in breast cancer tissue. Carcinogenesis 2009; 30:745 - 52; http://dx.doi.org/10.1093/carcin/bgp061; PMID: 19279183
- Koyanagi S, Kuga M, Soeda S, Hosoda Y, Yokomatsu T, Takechi H, et al. Elevation of de novo ceramide synthesis in tumor masses and the role of microsomal dihydroceramide synthase. Int J Cancer 2003; 105:1 - 6; http://dx.doi.org/10.1002/ijc.11024; PMID: 12672022
- Bensaad K, Vousden KH. p53: new roles in metabolism. Trends Cell Biol 2007; 17:286 - 91; http://dx.doi.org/10.1016/j.tcb.2007.04.004; PMID: 17481900
- Hussain SP, Harris CC. p53 biological network: at the crossroads of the cellular-stress response pathway and molecular carcinogenesis. J Nihon Med Sch 2006; 73:54 - 64; http://dx.doi.org/10.1272/jnms.73.54; PMID: 16641528
- Zhu BS, Xing CG, Lin F, Fan XQ, Zhao K, Qin ZH. Blocking NF-kappaB nuclear translocation leads to p53-related autophagy activation and cell apoptosis. World J Gastroenterol 2011; 17:478 - 87; http://dx.doi.org/10.3748/wjg.v17.i4.478; PMID: 21274377
- Sonnemann J, Palani CD, Wittig S, Becker S, Eichhorn F, Voigt A, et al. Anticancer effects of the p53 activator nutlin-3 in Ewing's sarcoma cells. Eur J Cancer 2011; In press http://dx.doi.org/10.1016/j.ejca.2011.01.015; PMID: 21334198
- Schwartz GK. Development of cell cycle active drugs for the treatment of gastrointestinal cancers: a new approach to cancer therapy. J Clin Oncol 2005; 23:4499 - 508; http://dx.doi.org/10.1200/JCO.2005.18.341; PMID: 16002840
- Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 2004; 23:3151 - 71; http://dx.doi.org/10.1038/sj.onc.1207542; PMID: 15094765
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 1999; 13:1501 - 12; http://dx.doi.org/10.1101/gad.13.12.1501; PMID: 10385618
- Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene 2001; 20:1803 - 15; http://dx.doi.org/10.1038/sj.onc.1204252; PMID: 11313928
- Pines J, Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell 1989; 58:833 - 46; http://dx.doi.org/10.1016/0092-8674(89)90936-7; PMID: 2570636
- Villani MG, Appierto V, Cavadini E, Bettiga A, Prinetti A, Clagett-Dame M, et al. 4-oxo-fenretinide, a recently identified fenretinide metabolite, induces marked G2-M cell cycle arrest and apoptosis in fenretinide-sensitive and fenretinide-resistant cell lines. Cancer Res 2006; 66:3238 - 47; http://dx.doi.org/10.1158/0008-5472.CAN-05-3362; PMID: 16540676
- Sala G, Minutolo F, Macchia M, Sacchi N, Ghidoni R. Resveratrol structure and ceramide-associated growth inhibition in prostate cancer cells. Drugs Exp Clin Res 2003; 29:263 - 9; PMID: 15134383
- Devalapally H, Duan Z, Seiden MV, Amiji MM. Paclitaxel and ceramide co-administration in biodegradable polymeric nanoparticulate delivery system to overcome drug resistance in ovarian cancer. Int J Cancer 2007; 121:1830 - 8; http://dx.doi.org/10.1002/ijc.22886; PMID: 17557285
- Schmelz EM, Dillehay DL, Webb SK, Reiter A, Adams J, Merrill AH Jr.. Sphingomyelin consumption suppresses aberrant colonic crypt foci and increases the proportion of adenomas versus adenocarcinomas in CF1 mice treated with 1,2-dimethylhydrazine: implications for dietary sphingolipids and colon carcinogenesis. Cancer Res 1996; 56:4936 - 41; PMID: 8895747
- Dillehay DL, Webb SK, Schmelz EM, Merrill AH Jr.. Dietary sphingomyelin inhibits 1,2-dimethylhydrazine-induced colon cancer in CF1 mice. J Nutr 1994; 124:615 - 20; PMID: 8169652
- Zhang P, Li B, Gao S, Duan RD. Dietary sphingomyelin inhibits colonic tumorigenesis with an up-regulation of alkaline sphingomyelinase expression in ICR mice. Anticancer Res 2008; 28:3631 - 5; PMID: 19189644
- McGowan CH, Russell P. Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. EMBO J 1993; 12:75 - 85; PMID: 8428596
- Struckhoff AP, Patel B, Beckman BS. Inhibition of p53 sensitizes MCF-7 cells to ceramide treatment. Int J Oncol 2010; 37:21 - 30; PMID: 20514393