Abstract
Current knowledge of changes in the mammary epithelium relevant to breast carcinogenesis is limited to when histological changes are already present because of a lack of biomarkers needed to identify where such molecular changes might be ongoing earlier during the decades-long latent stages of breast carcinogenesis. Breast reduction tissues from young women and teenagers, representative of the USA's high breast cancer incidence population, were studied using immunocytochemistry and a targeted PCR array in order to learn whether a marker of chronic oxidative stress [protein adducts of 4-hydroxy-2-nonenal (4HNE)] can identify where molecular changes relevant to carcinogenesis might be taking place prior to any histological changes. 4HNE-immunopositive (4HNE+) mammary epithelial cell-clusters were identified in breast tissue sections from most women and from many teenagers (ages 14–30 y) and, in tissues from women ages 17–27 y with many vs. few 4HNE+ cells, the expression of 30 of 84 oxidative stress associated genes represented in SA Bioscience RT2 Oxidative Stress and Antioxidant PCR array was decreased and only one was increased > 2-fold. This is in contrast to increased expression of many of these genes known to be elicited by acute oxidative stress. The findings validate using 4HNE-adducts to identify where molecular changes of potential relevance to carcinogenesis are taking place in histologically normal mammary epithelium and highlight differences between responses to acute vs. chronic oxidative stress. We posit that the altered gene expression in 4HNE+ tissues identified reflects adaptive responses to chronic oxidative stress that enable some cells to evade mechanisms that have evolved to prevent propagation of cells with oxidatively-damaged DNA and to accrue heritable changes needed to establish a cancer.
Introduction
Implicit in our understanding of carcinogenesis as a multistep process is that initiated cells need time to accrue a constellation of heritable changes required to establish a cancer. Studies of occupational cancers, of lung cancer in smokers, and of cancers in atom bomb survivors demonstrate that cancers attributable to carcinogenic agents appear only after about two decades and continue to surface during several decades thereafter.Citation1–Citation7 Current knowledge of molecular changes during the decades-long latent stages of carcinogenesis is limited to when histological changes are already present. The lack of biomarkers needed to identify where within histologically normal tissues such changes might be taking place has prevented learning about earlier stages of cancer-latency. This study was designed to test two interrelated hypotheses: (1) that chronic oxidative stress provides a final common path via which diverse environmental/lifestyle factors implicated in the high incidence of breast cancer in “westernized” populations contribute to breast carcinogenesis, and (2) that protein adducts of 4-hydroxy-2-nonenal (4HNE), a marker of chronic oxidative-stress, can identify where molecular changes of potential relevance to carcinogenesis are taking place prior to histological changes. 4HNE is a major, reactive end product of organic hydroperoxides generated as a consequence of free radical initiated oxidation of polyunsaturated fatty acids.Citation8–Citation10 When the rate of production of 4HNE exceeds tissues' capacity to inactivate it, 4HNE forms immunoreactive adducts that persist, which makes them valuable in studies of chronic oxidative stress-associated diseases.Citation11–Citation14
A critical role of oxidative stress in carcinogenesis is recognized. Much is known also about mechanisms by which oxidative stress can contribute to carcinogenesis.Citation15–Citation17 Most mechanistic studies, however, have been performed under conditions that can provide insight into effects of acute oxidative stress, but not into the consequences of sustained oxidative stress. More recently, several studies have focused on the differences between responses to acute vs. sustained oxidative stress, and on the phenomenon of adaptation to chronic or repeated stressors.Citation18–Citation27 Of special relevance to carcinogenesis are adaptive responses to chronic oxidative stress identified that might lead to the evolution of subpopulations of cells that can evade mechanisms that limit the propagation of cells with oxidatively-damaged DNA, notably, increased resistance to apoptosisCitation28–Citation31 and tolerance to DNA damage.Citation32–Citation34
The chronic oxidative stress final-common-path hypothesis, referred to above, is based on the following considerations. Breast cancer-incidence in the USA has increased 8-fold since the beginning of the last century and is now the highest in the world.Citation35 During this period there has been an exponential increase in the number and amount of chemicals released into the environment.Citation36 While the carcinogenic potential of most of these chemicals is not known, the ability of some prevalent chemicals to contribute to oxidative stress is known. Mammary epithelial cells from which the majority of breast cancers arise express enzymes that metabolize xenobiotics and estrogens via pathways that generate reactive oxygen species (ROS)Citation37–Citation42 and only during lactation are multidrug resistance proteins that can extrude xenobiotics from cells expressed in mammary epithelial cells.Citation43,Citation44 Many xenobiotics and their potentially mutagenic derivatives are lipophilic. Hence, they can be sequestered in adipose tissue surrounding the mammary gland.Citation45–Citation47 Industrialization of food production that has occurred concurrent with increased production of chemicals is a likely contributor to oxidative stress, e.g., by increasing calorie intake.Citation48,Citation49 The fact that one in seven (14%) women living in USA's high breast cancer risk-posing environment can be expected to develop breast cancer in their lifetime, an incidence that is only 1% shy of the percent of smokers expected to develop lung cancer, implies that foci where molecular changes relevant to carcinogenesis might be taking place are likely to be prevalent in breast tissue of healthy women representative of the US population.
Here we present immunocytochemical evidence that 4HNE immunopositive (4HNE+) mammary epithelial cells are, indeed, prevalent in reduction mammoplasty tissues from young women and teenagers representative of the USA's high breast cancer-incidence population. We also document a marked difference between the transcriptional profile of breast tissues with many 4HNE+ vs. those with few 4HNE+ mammary epithelial cells, specifically, reduced expression of genes represented in a targeted PCR array that protect against and/or are responsive to oxidative stress. Since simple macromastia, the indication for reduction mammoplasty, does not pose any risk for breast cancer over and above that posed by living in a particular environment,Citation50–Citation52 these tissues can be considered representative of breast tissue of women within a population. Together, these findings validate using 4HNE adducts to identify where in histologically-normal human breast mammary epithelium cells might be undergoing changes relevant to carcinogenesis. A conceptual framework for these findings is provided by a model of carcinogenesis that incorporates Darwinian principles, namely, that sustained oxidative stress elicits adaptive responses that enable some cells to evade mechanisms that limit replication of cells with oxidatively-damaged DNA and to accumulate the constellation of heritable changes needed to establish a cancer.Citation53–Citation56
Results
Immunopositive 4HNE+ mammary epithelial cells are present in breast parenchyma of many young women and teenagers ( and ).
Clusters of 4HNE+ mammary epithelial cells were seen in tissue sections from most of the 58 subjects studied (ages 14–30 y), and even in over half the tissues from teenagers. Clusters of 4HNE+ mammary epithelial cells were dispersed throughout different anatomical compartments of the mammary ducts and varied greatly in their number and in the intensity of immunostaining. Some 4HNE+ mammary epithelial cells occupied a luminal, others a basal position. Mammary epithelial cells in terminal end-buds (TEBs) that are likely to be particularly vulnerable to initiating actions of carcinogensCitation59–Citation62 were often 4HNE+ (). In some tissues the collagen was also strongly immunopositive. However, there was no consistent correlation between immunostaining in the stroma and mammary epithelium. Some of this diversity in immunostaining patterns is illustrated in and .
Confirmation of the presence of 4HNE adducts in breast parenchyma of teenagers by western blot analysis ().
In immunoblots of proteins extracted from tissues from seven subjects (ages 14–19 y), and from a histologically normal tissue from a breast cancer patient, there was a prominent band that corresponded closely in MW to that reported by others using an antibody also specific for 4HNE-lysine adducts.Citation63,Citation64 In one case, there was a second prominent immunopositive band of a somewhat larger MW. The only distinguishing feature of tissues from this subject was the presence of an unusually large number of growing ducts that terminated in 4HNE+ mammary epithelial cells.
Expression of many of the oxidative stress-related and “housekeeping” genes represented in the PCR arrays was reduced in 4HNE+ tissues > 2-fold (, ).
The oxidative stress PCR microarray revealed a striking difference between tissues encompassing many 4HNE+ mammary epithelial cells vs. those encompassing few 4HNE+ mammary epithelial cells. The expression of only a single gene (MGST3) was > 2-fold higher in 4HNE+ than in 4HNE-tissues, while the expression of 31 genes was > 2-fold lower (including 5 in the housekeeping array, ). The modified binomial test used to evaluate statistical significance described under Methods yielded a p value of < 0.016. It is likely that the modeled correlation structure used is overly conservative and that the true statistical significance is much greater than this p value. The small number of genes covered by the microarray, and because the RNA was isolated from whole breast tissue sections encompassing diverse anatomical compartments, precluded any meaningful cluster or functional enrichment analyses. Functions of some of genes expressed at a decreased level in 4HNE+ tissues are highlighted in the Discussion.
Confirmation of the microarray findings by qRT-PCR.
Relative expression values for four genes (PRG3, SOD3, PRDX3 and GPX5) obtained by qRT-PCR closely matched the microarray values (). Primer sequences used are presented in .
Discussion
We document the presence of a hallmark of chronic oxidative stress, 4HNE protein adducts, in the mammary epithelium of young women and teenagers without any known risk factors for breast cancer over and above those posed by living in the high breast cancer risk-posing USA environment. We also present evidence of marked phenotypic changes associated with the presence of such adducts. Since transcriptional profiling was performed using RNA isolated from whole tissue cryosections, these findings reflect average values for the heterogeneous cell populations within these sections. Nevertheless, because we took care to extract RNA from tissue sections that encompassed several TDLUs, where cells that ultimately give rise to a BC reside, mammary epithelial cells within TDLUs are likely to have contributed significantly to the findings.
As the data from the focused PCR array show, the presence of many 4HNE+ cells in breast tissues was associated with reduced expression of many of the 84 oxidative stress defensive and/or responsive genes represented in the array. This is in contrast to increased expression of many of these genes that acute oxidative stress is known or would be expected to elicit.Citation65–Citation67 The finding of reduced steady-state levels of transcripts for enzymes and proteins that protect against oxidative stress would seem counterintuitive (, ). However, the selection of genes represented in the array was based, no doubt, on responses of cells and tissues to acute oxidative stress, about which much is known, and not on responses of cells and tissues subjected to sustained oxidative stress, about which much less is known. The status in 4HNE+ tissues of the many other mechanisms that serve to protect tissues and cells from oxidative damage remains to be determined. It is interesting to note that MGST3, the only gene found to be expressed over 2-fold in the 4HNE+ tissues, encodes a glutathione S-transferase that has recently been shown to be inducible by a wide range of xenobiotics.Citation68 Presumably, this enzyme plays a role in protecting cells from oxidative damage caused by xenobiotics.
The novel finding of reduced expression of genes encoding proteins that might contribute to oxidative stress in 4HNE+ tissues makes sense. Of these genes, NOX5 encodes an NADPH oxidase, a source of superoxide radical (O2•-); NOS2 encodes an inducible nitric oxide synthase, a source of nitric oxide, a reactive free radical; PGR3 encodes proteoglycan 3, a homolog of the apoptosis-inducing factor AIF,Citation69 that possesses cytotoxic and cytostimulatory activities via neutrophils and macrophages; DUOX2 encodes a dual oxidase, which generates H2O2 and supports bactericidal functions of lactoperoxidase; and LPO encodes lactoperoxidase, which might contribute to oxidative stress by catalyzing metabolic activation of aromatic and heterocyclic amine, as well as of primary estrogens.Citation70–Citation72 The long-term, potentially detrimental consequences of these adaptive responses will need to be studied.
An altered status of DNA damage-response mechanisms in 4HNE+ tissues is suggested by the 2.9-fold decrease in transcripts for PNKP in 4HNE+ tissues: PNKP encodes polynucleotidekinase/phosphatase, an enzyme critical for faithful repair of DNA strand-breaks. The status in 4HNE+ tissues of the many other participants in DNA damage-response that are not represented in the array, in particular the error-prone DNA repair mechanisms, will need to be examined.Citation32–Citation34 Activation of the latter mechanisms, in conjunction with increased resistnce to apoptosis, would enable cells with oxidatively-damaged DNA to continue to replicate. While in the short run this would preserve tissue integrity, it would enable some cells to accrue the heritable changes needed to establish a cancer.
Our data also suggest an impaired ability of 4HNE+ tissues to clear misfolded proteins and products of apoptosis. Transcripts for two proteins involved in this function, UBC and MBL were, respectively, 2.2-fold and 6.9-fold lower in 4HNE+ than in 4HNE-tissues: UBC encodes polyubiquitin C, an essential participant in the clearing process, while MBL encodes mannose-binding lactin, an innate host-defense molecule now recognized to play a role not only in the elimination of pathogens by macrophages, but also of late products of apoptosis.Citation73–Citation76 Transcripts for surfactantprotein-D, with functions similar to MBL, were also decreased (1.6-fold) in 4HNE+ tissues. Impaired functioning of clearing/recycling mechanisms could explain the persistence of 4HNE adducts, as well as the increase with age in extent and intensity of 4HNE immunoreactivity observed by us in FFPE sections from older women (n = 63, ages 30–60 y) (data not shown). Impaired functioning of these mechanisms is known to be association with both aging and with diseases in which chronic oxidative stress is implicated, e.g., neurodegenerative diseases.Citation77–Citation79 The potential of oxidative stress to compromise the functioning of these essential clearing/recycling mechanisms has also been reported.Citation80 suggesting that failure to eliminate or recycle waste products creates a viscous cycle.
As indicated above, the findings represent only an average of the profile of heterogeneous populations of stromal and mammary epithelial cells. Identifying and characterizing subpopulations of mammary epithelial cells that might be on the path to carcinogenesis will require using new tools of molecular pathology that enable obtaining a molecular profile of even single cells in tissue sections. Among these, Fourier-transform infrared and Raman microspectroscopy can provide biomolecular profile of individual cells in tissue sections,Citation81–Citation84 and certain high-throughput multiplex immunocytochemical techniques can map a virtually unlimited number of proteins to individual cells in tissue sections (in situ proteome).Citation85–Citation87 These technologies will enable taking advantage of the focal nature of the 4HNE immunostaining of mammary epithelial cells seen in most subjects. Being able to compare the molecular profiles of 4HNE+ and 4HNE-cells in tissue sections from the same subject will eliminate confounding variables of inter-individual differences in genetic background, environment/lifestyle factors and hormonal status. These technologies will also enable comparing the molecular profile of 4HNE+ and 4HNE-mammary epithelial cells in TEBs where cells are likely to be especially vulnerable to cancer-initiationCitation59–Citation62 and were often 4HNE immunopositive (). Importantly, new tools of molecular pathology will make possible molecular epidemiological studies needed to learn whether and which molecular changes in 4HNE+ tissues correlate with cancer risk in individuals sharing the same environment and between populations that differ in their environment and in breast cancer incidence.
The final common path hypothesis referred to in the Introduction predicts a correlation between the prevalence of 4HNE adducts in breast tissues and breast cancer-incidence. An attempt to test this prediction was made using archived breast tissues obtained at forensic autopsy by Bartow et al. from three populations of women living in the southwestern USA that differed significantly in their breast cancer risk.Citation88 Although the poor quality of fixation precluded a comprehensive study, the findings from the few tissues that could be used support the final common path hypothesis: in tissues from Native American women, (the population with the lowest breast cancer incidence, 24.9/100,000), 4HNE+ mammary epithelial cells were rare, whereas in tissues of non-Hispanic white women, (the population with the highest BC-incidence, 89/100,00), 4HNE+ cells were at least as prevalent as in tissues used in our study from a population with a BC-incidence of > 120/100,000 (data not shown). Testing the relevance of the molecular changes identified during cancer-latency to breast cancer incidence will require molecular epidemiological studies using new, high-throughput technologies of molecular pathology, such as referred to above, taking advantage of the availability of vast stores of archived tissues from reduction mammoplasties in many countries with different breast cancer incidence and from individuals with long-term follow-up.
In most laboratory studies to date of the role of oxidative stress to carcinogenesis, cells or tissue were subjected only briefly and mostly only to pharmacological levels of pro-oxidants. Moreover, the 20% O2 concentration used in most ex vivo experiments is several fold higher than that to which most cells are exposed in vivo.Citation89 Hence, the cells used were ones likely to have already undergone adaptive changes to chronic oxidative stress. Understanding the role of chronic oxidative stress in carcinogenesis will require taking these factors into consideration, namely, exposing tissues and cells to environmentally relevant concentrations of pro-oxidants and for long enough for adaptive mechanisms to reveal themselves, and using in ex vivo experiments physiologically relevant O2 concentrations. Insights into the consequences of more sustained or repeated oxidative stress are, however, becoming available. Notably, a predominance of reduced gene expression under these conditions is suggested by a few recent in vivo studies.Citation26,Citation27,Citation90 For example, while exposing large mouthed bass to high concentration of Dieldrin acutely resulted in a significant (> 1.5-fold) increase in transcripts of 199 genes and a significant decrease in transcripts of only 28 genes, exposing the fish to environmentally relevant Dieldrin concentrations subacutely, for 57 days, resulted in a significant decrease in transcripts of 220 genes and an increase in transcripts of only 24 genes.Citation26,Citation27 A genome-wide transcriptional profiling by Illumina DASL platform of 4HNE+ vs. 4HNE-tissues, using aliquots of RNA used in the present study, also showed a predominance of reduced gene expression associated with 4HNE adducts: of the transcripts for 398 genes found to differ over 1.5-fold between 4HNE+ and 4HNE-tissues, the transcript levels were decreased for 322 genes and increased for only 76 (data not shown).
The presence of 4HNE adducts means that the tissues have been exposed also to other reactive, potentially mutagenic products of lipid peroxidation. Like 4HNE, these can form protein adducts and elevated levels of their adducts have been identified in cancer-prone tissue, including breast.Citation91–Citation94 Hence, 4HNE adducts are only the “tip of the iceberg.” Endogenously generated reactive electrophilic species act as redox-sensors that can affect the functioning of multiple molecules involved in the regulation of basic homeostatic mechanisms, including cell proliferation and apoptosis, energy metabolism and DNA damage-response.Citation8–Citation10 Actions of these electrophiles that are most likely to be relevant to progression toward a cancer are ones elicited when they are generated at elevated, albeit non-toxic levels, and over a protracted period of time, a condition that can be identified by the presence of their protein adducts. The findings presented here provide a first glimpse of the wide-ranging molecular changes that might be associated with the presence of protein adducts of lipid peroxidation.
The finding of evidence of lipid peroxidation in the mammary epithelium of girls as young as 14, living in our high breast cancer risk-posing environment, with all its functional implications, is reminiscent of the finding of atherosclerotic plaques at autopsy in young US soldiers killed in the Korean war.Citation95 It was this finding that led to the realization of the need to learn about the early, silent stages in the evolution of cardiovascular disease, and the need for early intervention if its progression to its potentially deadly ultimate manifestations were to be prevented. We posit that knowledge of molecular changes associated with the presence of markers of chronic oxidative stress in mammary epithelium already at an early age is essential for developing effective, stage-specific preventive strategies for breast cancer and for monitoring their effectiveness. This will require identifying and characterizing subpopulations of cells that have entered into and/or are further along the carcinogenic path. Some of the genes found in this study to be expressed at a decreased level in 4HNE+ tissues are known to be expressed at an elevated level in some cancers, to be associated with worse prognosis and some are even targets for cancer therapeutics.Citation96–Citation101 This implies that the changes such as noted here in 4HNE+ tissues represent phenotypic changes that are reversible. Thus, while the evidence that the mammary epithelium of those living in the USA's high breast cancer-risk posing environment might be subject to chronic oxidative stress from an early age is ominous, reversibility of associated phenotypic changes bodes well for initiatives to arrest and reverse effects chronic oxidative stress and progression toward cancer at early stages of cancer-latency.
Materials and Methods
Tissue source.
Reduction mammoplasty tissues were processed under uniform conditions by the same individuals: tissues were freed from excess fat by blunt dissection and by trimming with surgical scissors. Portions were snap-frozen under conditions that prevent mechanical and osmotic cellular damage caused by ice-crystal formationCitation57 and stored at −80°C. Other portions were fixed in 10% neutral buffered formalin for 12–24 h and embedded in paraffin. The use for research of tissues from 58 subjects (ages 14–30 y) used in this study was authorized by the Institutional Review Board.
Immunocytochemistry.
Formalin-fixed, paraffin-embedded (FFPE) tissues (n = 58, ages 14–30) were sectioned at 5 µm, transferred onto Fisher Superfrost Plus slides, deparaffinized, rehydrated and then subjected to antigen retrieval using Vector Antigen Unmasking Solution, pH 6.0 (Vector Laboratories) for 1 h at 80°C. The sections were incubated in a humidity chamber overnight at 4°C with a mouse monoclonal antibody developed against 4HNE-modified low-density lipoprotein (clone NA59) that binds to lysine adducts of 4HNE.Citation58 Immunocytochemistry was completed using Vector Alkaline Phosphatase Universal Kit with Vector Alkaline Phosphatase Substrate Kit IV chromagen. Negative controls included adjacent sections from each sample that were incubated with medium without the primary antibody. Sections of aortas with atherosclerotic plaques served as positive controls ().
Western analysis.
Flash-frozen breast tissues from seven subjects (ages 14–19 y) were homogenized for 30–60 sec with a Polytron (Brinkman) in 1:2 ratio (wt:vol) 50 mM Tris buffer, pH 7.4 containing 5 mM MgCl2, 25 mM KCl with CHAPS, BHT, EDTA and Sigma protease inhibitor cocktail (P8340) and then centrifuged for 30 min at 13,000 rpm. Protein (50 ug) was loaded onto a NuPage 4–12% Bis-Tris gel with MOPS as the running buffer and electrophoresed under reducing conditions for 50 min at 200 V constant. The separated proteins were transferred onto Immobilon PVDF membrane for 1 h at 30V constant. Immunoblotting was performed using the monoclonal antibody used for immunocytochemistry,Citation58 Vector Vectastain ABC-AmP western blotting kit and Vector Alkaline Phosphatase Substrate Kit IV.
Tissue selection for transcriptional profiling.
RNA was isolated from flash-frozen tissues selected from either end of the spectrum of 4HNE immunostaining (). Immunostained sections from multiple FFPE tissue-blocks from subjects ages 14–30 y were reviewed by three of the investigators (D.S., E.M. and J.W.) and categorized as 4HNE+ or 4HNE-based on the following criteria: the proportion and intensity of 4HNE immunostaining of mammary epithelial cell-clusters in sections from each subject were evaluated on a scale of 1 to 6. The number of terminal ductal lobular units (TDLUs) in the sections was taken into account since mammary epithelial cells in TDLUs are the presumed cells of origin of most breast cancers. Tissues with no more than three 4HNE weakly immunopositive TDLUs were placed into the 4HNE-(control) category and those with four or more immunopositive TDLUs were placed into the 4HNE+ (unknown) category (). Tissues from subjects younger than 17 y were excluded in order to minimize the effect of the confounding variable of molecular changes associated with post-puberal breast development, and tissues from subjects older than 27 y were excluded in order to minimize the effects of age-associated changes in cellular homeostatic mechanisms. Tissue from 13 subjects (ages 17–27 y) met the above criteria, 7 in the 4HNE− (control) and 6 in the 4HNE+ (unknown) category ().
Transcriptional profiling.
The relative expression of 84 genes represented in the SABioscience Human RT2 Profiler Oxidative Stress and Antioxidant PCR Array (Qiagen) was determined using RNA isolated from 10 µm cryosections from the seven 4HNE− controls and the six 4HNE+ unknowns. RNA was extracted using Qiagen RNeasy FFPE RNA Extraction Kit. The concentration and quality of the RNA extracts were measured using a NanoDrop ND-1000 (Thermo Scientific). RNA quality was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies). In addition, aliquots of the samples of RNA were analyzed using SABioscience Human RT2 RNA QC PCR array. cDNA was prepared using SABioscience RT2 First Strand Kit and RT2 Sybr/ROX qPCR Master Mix according to manufacturer's instructions (Qiagen). A minimum of two arrays/sample were run using a Stratagene MX4000 qPCR instrument. (Agilent Technologies). Data were analyzed using SABioscience RT2 Profiler analysis software, with 4HNE− and 4HNE+ samples serving as controls and unknowns, respectively. The data were normalized using HPRT1 and GAPDH, two of the housekeeping genes included in the microarray that were shown not to differ significantly between 4HNE+ vs. 4HNE− samples using the SABioscience RT2 Profiler Housekeeping array (). Statistical significance of differences between 4HNE+ vs. 4HNE− tissues was assessed using a modified two-sample binomial test.
Confirmation of microarray findings by qRT-PCR.
Relative transcript levels of PRG3, SOD3, PRDX3 and GPX5 genes were evaluated by qRT-PCR in pooled aliquots of RNA from 4HNE− and 4HNE+ tissues used for transcriptional profiling. The primers and Taqman probes were designed using Primzer Express 1.5 software and sequences obtained from GenBank, qRT-PCR was performed using the one-step method: cDNA synthesis and PCR amplification were performed in one tube using Stratagene Brilliant III Ultra-Fast QRT-PCR Master Mix. RNA was added at a final concentration 40 ng/well. The primers and probes were added to the reaction mixture to a final concentration of 400 and 160 nM, respectively. The pooled RNA samples and β-actin internal controls were multiplexed and run on Stratagene MX3005P QPCR instrument (Agilent Technologies). Relative mRNA levels were calculated as fold-change in the four genes normalized to β-actin by the Efficiency Corrected Comparative Quantification Method using MX Pro QPCR software (Agilent Technologies).
Statistics.
In evaluating the significance of the finding that the expression of 32 genes was downregulated and that the transcript of only a single gene was upregulated, we needed to take into consideration that expression of some of the genes is correlated. To allow for dependency among the gene expressions we used a simple correlation structure in which each pair of genes is correlated with a correlation coefficient of 0.25 and used a modified binomial test derived through simulation to derive a p value. The modified binomial test was derived through a simulation in which a set of 31 correlated Bernoulli random variables are simulated by first drawing from a multivariate normal distribution with the above defined correlation structure. The individual components of the normal vector are then converted to a 0–1 Bernoulli random variable by thresholding at zero; values larger than zero are converted to 1, and values less that zero are converted to 0. This generates a sequence of pair-wise correlated Bernoulli random variables each with an expected value of 0.5. Such a set of 31 correlated random variables is generated 100,000 times. The number of times either 30 or more ones or zeros are present is recorded, and this total is then divided 100,000. This quantity yields the empirically derived p value calculation for the two-sided modified binomial test. It is expected that most pairs of genes are uncorrelated, and therefore this imposed correlation structure can be viewed as statistically conservative.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interests were disclosed.
Figures and Tables
Figure 1 Photomicrographs of 4HNE immunostained breast tissue sections from nine subjects ranging in age from 14–19 illustrating some of the diversity of staining patterns. Sections were not counterstained. Images were captured via a Nikon Eclipse E600 microscope with Nomarski optics using Spot Digital Camera (Diagnostic Instruments, Inc.) and Image Pro Plus software (version 2). (A1 and A2) Sections from a 14-y-old girl with only a few weakly 4HNE immunopositive mammary epithelial cells at low power in A1 and high power in A2. Insert in lower panel shows a blood vessel with no immunostaining of the endothelium, i.e., no evidence of atherosclerosis. (B1 and B2) Two areas from a section from another 14-y-old girl with intense immunostaining throughout the mammary epithelium: Upper panel shows growing ducts and the lower panel shows at high power a cross section of a duct with 4HNE+ mammary epithelial cells localized mainly in the basal epithelium. (C1 and C2) Upper panel shows at low power a terminal lobular ductal unit (TDLU) from a 19-y-old subject in which mammary epithelial cells are relatively weakly 4HNE immunopositive. Lower panel, the area marked by an arrow in the upper panel, photographed at high power shows localization of immunostaining in luminal epithelial cells. (D1 and D2) Two areas in a tissue section from a 17-y-old girl in which there are only a few intensely 4HNE immunopositive cells. The random localization of these 4HNE+ cells and the granularity of the immunostaining suggest that these might be macrophages, a supposition that needs to be confirmed using a macrophage marker, such as CD68. (E) An essentially immunonegative TDLU surrounded by intensely immunopositive collagenous stroma in a tissue section from a 16-y-old girl. (F1 and F2) 4HNE+ mammary epithelial cells in two growing ducts in a section from a 19-y-old subject. The immunostaining is more intense and widespread in (F1) and less intense and restricted to the tip of the growing duct in (F2). Insert in (F1) shows localization of immunostaining in basal epithelial cells. (G) A portion of a TDLU in a section from an 18-y-old subject in which intense immunostaining is localized in the luminal epithelial cells. (H1 and H2) From a section from an 18-y-old subject in which intensely immunopositive stroma surrounds a TDLU in the upper panel and a blood vessel in the lower panel: the upper panel shows patchy immunostaining of luminal mammary epithelial cells while the lower panel shows immunostaining of the endothelium of the blood vessel indicating atherosclerosis. (I) A section of an aorta, used as a positive control, showing streaks of 4HNE immunostaining indicating atherosclerotic lesions. Control sections incubated with medium from which the primary 4HNE antibody was omitted were uniformly immunonegative.
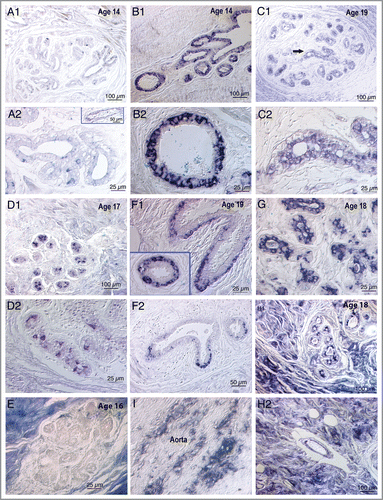
Figure 2 Photomicrographs of representative 4HNE immunostained breast tissue sections from nine subjects between the ages 17–27 y whose tissues were used for transcriptional profiling. Sections were not counterstained. Images were captured via a Nikon Eclipse E600 microscope with Nomarski optics using Spot Digital Camera (Diagnostic Instruments, Inc.) and Image Pro Plus software (version 2). Upper six panels (A–E), tissues with many strongly 4HNE+ terminal lobular ductal units (TDLU), (G–I) tissues with only few at most weakly 4HNE+ TDLUs. (A–E) Upper and lower portion of each of the panels show images captured from the same tissue section at high and low magnification, respectively. (F1) and (F2) images from sections from different tissue blocks from the same subject: In (F1) the mammary ducts are surrounded by lipocytes and in (F2) by strongly immunopositive collagenous stroma. Note: (1) in (C2), localization of 4HNE immunostaining in luminal epithelial cells in; (2) in (E2), some immunopositive nuclei in mammary epithelial cells and; (3) in (G), immunonegative TDLU is surrounded by strongly 4HNE immunopositive collagenous stroma and fat cells. Control sections incubated with medium from which the primary 4HNE antibody was omitted were uniformly immunonegative.
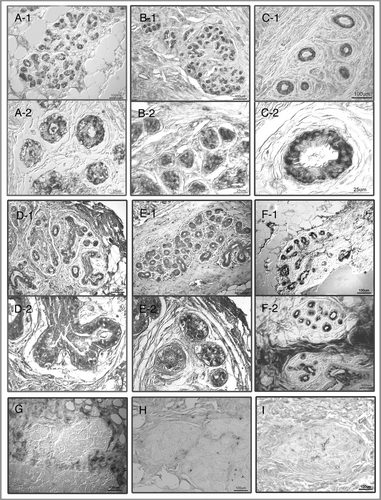
Figure 3 Immunoblot of protein isolated from breast parenchyma from reduction mammoplasty surgical specimens from seven subjects ages 14–19 y (lanes 2, 4–9) and from an invasive breast cancer (lane 3). Lane 1, molecular weight (MW) marker. Ages of subjects indicated below the image. Proteins were isolated, separated under reducing conditions and visualized by immunostaining with an antibody specific for 4HNE lysine adducts as described under Methods. The one prominent immunopositive band identified in all samples corresponds closely in size to that identified in the laboratory of Esterbauer in immunoblots of plasma from children with Systemic Lupus Erythematosus and from atherosclerotic plaques.Citation61,Citation62 In the sample from one 19-y-old subject there is a second prominent immunopositive band of a slightly higher MW. The only distinguishing immunocytochemical feature of sections from this subject was the presence of an unusually large number of growing ducts with immunopositive termini. The variable amount of 4HNE+ and 4HNE− adducts in cells in the mammary epithelium and stroma contributing to the 50 µg protein loaded onto the gels, and the difficulty of extracting proteins from the collagenous stroma, precluded being able to correlate the intensity of the protein bands with extent of immunostaining in tissue sections.
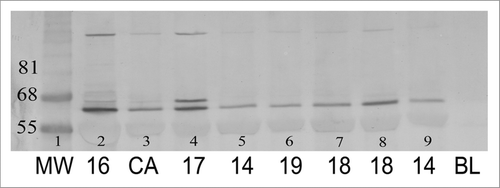
Figure 4 Fold-difference in relative expression of 84 genes represented in the Oxidative Stress Antioxidant PCR Array (upper six panels) and of 12 genes represented in the Housekeeping PCR Array (bottom left panel) in breast tissues from subjects at either end of the spectrum of immunostaining for 4HNE protein adducts [designated 4HNE-(controls) and 4HNE+ (unknown), respectively]. The average age of the 4HNE− and 4HNE+ subjects was 21.6 and 20.2 y, respectively. Tissues in the 4HNE− and 4HNE+ categories were selected based on criteria described in Material and Methods and illustrated in . The assays were carried out using SABioscience RT2 Profiler Human Oxidative Stress, Antioxidant Defense (panels A–E) and Housekeeping PCR Arrays on a minimum of two (duplicate) samples from each subject. Results from the Oxidative Stress Array are show with the genes grouped according to the broad categories suggested by the manufacturer. The two genes used for data-normalization, GAPDH and HTRP1, were ones identified in the Housekeeping Array not to differ significantly in their relative expression between the 4HNE− and 4HNE+ tissues. Vertical axis, fold differences in average gene expression in 4HNE+ (unknown) tissues compared to 4HNE− (control) tissues. The numbers above or below the bars correspond to the rank-order of fold differences in genes listed in the side panels. Black bars show the genes that differed 2-fold or more in 4HNE+ tissues vs. 4HNE− tissues. Bottom right panel shows comparison of values obtained by qRT-PCR and by Oxidative Stress and Antioxidant PCR Array for four genes (SOD3, PRDX3, PRG3 and GPX5).
![Figure 4 Fold-difference in relative expression of 84 genes represented in the Oxidative Stress Antioxidant PCR Array (upper six panels) and of 12 genes represented in the Housekeeping PCR Array (bottom left panel) in breast tissues from subjects at either end of the spectrum of immunostaining for 4HNE protein adducts [designated 4HNE-(controls) and 4HNE+ (unknown), respectively]. The average age of the 4HNE− and 4HNE+ subjects was 21.6 and 20.2 y, respectively. Tissues in the 4HNE− and 4HNE+ categories were selected based on criteria described in Material and Methods and illustrated in Figure 2. The assays were carried out using SABioscience RT2 Profiler Human Oxidative Stress, Antioxidant Defense (panels A–E) and Housekeeping PCR Arrays on a minimum of two (duplicate) samples from each subject. Results from the Oxidative Stress Array are show with the genes grouped according to the broad categories suggested by the manufacturer. The two genes used for data-normalization, GAPDH and HTRP1, were ones identified in the Housekeeping Array not to differ significantly in their relative expression between the 4HNE− and 4HNE+ tissues. Vertical axis, fold differences in average gene expression in 4HNE+ (unknown) tissues compared to 4HNE− (control) tissues. The numbers above or below the bars correspond to the rank-order of fold differences in genes listed in the side panels. Black bars show the genes that differed 2-fold or more in 4HNE+ tissues vs. 4HNE− tissues. Bottom right panel shows comparison of values obtained by qRT-PCR and by Oxidative Stress and Antioxidant PCR Array for four genes (SOD3, PRDX3, PRG3 and GPX5).](/cms/asset/d8ec14a4-f5d2-48f9-a983-023142a3e07c/kcbt_a_10918873_f0004.gif)
Table 1 List of genes represented in the SABioscience Human RT2 Profiler Oxidative Stress and Antioxidant PCR Array the expression of which differed 1.5-fold or more between 4HNE+ (experimental) and 4HNE− (control) tissues, listed in rank order
Table 2 Sequences of PCR primers and probes used in Quantitative PCR
Acknowledgments
This project was funded in part by a contract with the Pennsylvania Breast Cancer Coalition, which takes no part and is in no way responsible for any analyses, interpretations or conclusions contained herein, and in part under a grant from the Pennsylvania Department of Health using Tobacco Settlement Funds. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. We thank Dr. Linda Curtiss, Scripps Research Institute, La Jolla, CA, for the antibody against 4-hydroxynonenal lysine adducts used in this study.
References
- Selikoff IJ, Hammond EC, Seidman H. Latency of asbestos disease among insulation workers in the United States and Canada. Cancer 1980; 46:2736 - 2740; PMID: 7448712; http://dx.doi.org/10.1002/1097-0142(19801215)46:12<2736::AID-CNCR2820461233>3.0.CO;2-L
- Selikoff IJ. Lessons for living in a chemical world. Bull Environ Contam Toxicol 1984; 33:682 - 695; PMID: 6394072; http://dx.doi.org/10.1007/BF01625600
- Weiss W. Cigarette smoking and lung cancer trends. A light at the end of the tunnel?. Chest 1997; 111:1414 - 1416; PMID: 9149602; http://dx.doi.org/10.1378/chest.111.5.1414
- Miyakawa M, Tachibana M, Miyakawa A, Yoshida K, Shimada N, Murai M, et al. Re-evaluation of the latent period of bladder cancer in dyestuff-plant workers in Japan. Int J Urol 2001; 8:423 - 430; PMID: 11555006; http://dx.doi.org/10.1046/j.1442-2042.2001.00342.x
- Archer VE, Coons T, Saccomanno G, Hong DY. Latency and the lung cancer epidemic among United States uranium miners. Health Phys 2004; 87:480 - 489; PMID: 15551786; http://dx.doi.org/10.1097/01.HP.0000133216.72557.ab
- Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 2007; 168:1 - 64; PMID: 17722996; http://dx.doi.org/10.1667/RR0763.1
- Finkelstein MM. Absence of radiographic asbestosis and the risk of lung cancer among asbestos-cement workers: Extended follow-up of a cohort. Am J Ind Med 2010; 53:1065 - 1069; PMID: 20672325; http://dx.doi.org/10.1002/ajim.20881
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 1991; 11:81 - 128; PMID: 1937131; http://dx.doi.org/10.1016/0891-5849(91)90192-6
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res 2003; 42:318 - 343; PMID: 12689622; http://dx.doi.org/10.1016/S0163-7827(03)00014-6
- Dubinina EE, Dadali VA. Role of 4-hydroxy-trans-2-nonenal in cell functions. Biochemistry (Mosc) 2010; 75:1069 - 1087; PMID: 21077827; http://dx.doi.org/10.1134/S0006297910090014
- Jürgens G, Chen Q, Esterbauer H, Mair S, Ledinski G, Dinges HP. Immunostaining of human autopsy aortas with antibodies to modified apolipoprotein B and apoprotein(a). Arterioscler Thromb 1993; 13:1689 - 1699; PMID: 7692957; http://dx.doi.org/10.1161/01.ATV.13.11.1689
- Uchida K, Toyokuni S, Nishikawa K, Kawakishi S, Oda H, Hiai H, et al. Michael addition-type 4-hydroxy-2-nonenal adducts in modified low-density lipoproteins: markers for atherosclerosis. Biochemistry 1994; 33:12487 - 12494; PMID: 7918471; http://dx.doi.org/10.1021/bi00207a016
- Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, et al. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes 1999; 48:927 - 932; PMID: 10102716; http://dx.doi.org/10.2337/diabetes.48.4.927
- Guéraud F, Atalay M, Bresgen N, Cipak A, Eckl PM, Huc L, et al. Chemistry and biochemistry of lipid peroxidation products. Free Radic Res 2010; 44:1098 - 1124; PMID: 20836659; http://dx.doi.org/10.3109/10715762.2010.498477
- Halliwell B. Oxidative stress and cancer: have we moved forward?. Biochem J 2007; 401:1 - 11; PMID: 17150040; http://dx.doi.org/10.1042/BJ20061131
- Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol 2010; 38:96 - 109; PMID: 20019356; http://dx.doi.org/10.1177/0192623309356453
- Voulgaridou GP, Anestopoulos I, Franco R, Panayiotidis MI, Pappa A. DNA damage induced by endogenous aldehydes: current state of knowledge. Mutat Res 2011; 711:13 - 27
- Maulik N, Yoshida T, Engelman RM, Deaton D, Flack JE 3rd, Rousou JA, et al. Ischemic preconditioning attenuates apoptotic cell death associated with ischemia/reperfusion. Mol Cell Biochem 1998; 186:139 - 145; PMID: 9774195; http://dx.doi.org/10.1023/A:1006883717174
- Ravati A, Ahlemeyer B, Becker A, Krieglstein J. Preconditioning-induced neuroprotection is mediated by reactive oxygen species. Brain Res 2000; 866:23 - 32; PMID: 10825477; http://dx.doi.org/10.1016/S0006-8993(00)02210-1
- Pohlman TH, Harlan JM. Adaptive responses of the endothelium to stress. J Surg Res 2000; 89:85 - 119; PMID: 10720457; http://dx.doi.org/10.1006/jsre.1999.5801
- Eneman JD, Potts RJ, Osier M, Shukla GS, Lee CH, Chiu JF, et al. Suppressed oxidant-induced apoptosis in cadmium adapted alveolar epithelial cells and its potential involvement in cadmium carcinogenesis. Toxicology 2000; 147:215 - 228; PMID: 10924803; http://dx.doi.org/10.1016/S0300-483X(00)00215-8
- Rüdiger HA, Graf R, Clavien PA. Sub-lethal oxidative stress triggers the protective effects of ischemic preconditioning in the mouse liver. J Hepatol 2003; 39:972 - 977; PMID: 14642614; http://dx.doi.org/10.1016/S0168-8278(03)00415-X
- Wu HH, Hsiao TY, Chien CT, Lai MK. Ischemic conditioning by short periods of reperfusion attenuates renal ischemia/reperfusion induced apoptosis and autophagy in the rat. J Biomed Sci 2009; 16:19; PMID: 19272187; http://dx.doi.org/10.1186/1423-0127-16-19
- Wang L, Li W, Kang Z, Liu Y, Deng X, Tao H, et al. Hyperbaric oxygen preconditioning attenuates early apoptosis after spinal cord ischemia in rats. J Neurotrauma 2009; 26:55 - 66; PMID: 19196076; http://dx.doi.org/10.1089/neu.2008.0538
- Sharma RK, Netland PA, Kedrov MA, Johnson DA. Preconditioning protects the retinal pigment epithelium cells from oxidative stress-induced cell death. Acta Ophthalmol 2009; 87:82 - 88; PMID: 18494742; http://dx.doi.org/10.1111/j.1755-3768.2008.01170.x
- Martyniuk CJ, Feswick A, Spade DJ, Kroll KJ, Barber DS, Denslow ND. Effects of acute dieldrin exposure on neurotransmitters and global gene transcription in largemouth bass (Micropterus salmoides) hypothalamus. Neurotoxicology 2010; 31:356 - 366; PMID: 20438755; http://dx.doi.org/10.1016/j.neuro.2010.04.008
- Martyniuk CJ, Kroll KJ, Doperalski NJ, Barber DS, Denslow ND. Genomic and proteomic responses to environmentally relevant exposures to dieldrin: indicators of neurodegeneration?. Toxicol Sci 2010; 117:190 - 199; PMID: 20584760; http://dx.doi.org/10.1093/toxsci/kfq192
- Yu HJ, Chien CT, Lai YJ, Lai MK, Chen CF, Levin RM, et al. Hypoxia preconditioning attenuates bladder overdistension-induced oxidative injury by up-regulation of Bcl-2 in the rat. J Physiol 2004; 554:815 - 828; PMID: 14608004; http://dx.doi.org/10.1113/jphysiol.2003.056002
- Tang XQ, Feng JQ, Chen J, Chen PX, Zhi JL, Cui Y, et al. Protection of oxidative preconditioning against apoptosis induced by H2O2 in PC12 cells: mechanisms via MMP, ROS, and Bcl-2. Brain Res 2005; 1057:57 - 64; PMID: 16129420; http://dx.doi.org/10.1016/j.brainres.2005.07.072
- Halestrap AP, Clarke SJ, Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta 2007; 1767:1007 - 1031; PMID: 17631856; http://dx.doi.org/10.1016/j.bbabio.2007.05.008
- Choi H, Kim SH, Chun YS, Cho YS, Park JW, Kim MS. In vivo hyperoxic preconditioning prevents myocardial infarction by expressing bcl-2. Exp Biol Med (Maywood) 2006; 231:463 - 472; PMID: 16565442
- Goodman MF. Error-prone repair DNA polymerases in prokaryotes and eukaryotes. Annu Rev Biochem 2002; 71:17 - 50; PMID: 12045089; http://dx.doi.org/10.1146/annurev.biochem.71.083101.124707
- Clémenson C, Marsolier-Kergoat MC. DNA damage checkpoint inactivation: adaptation and recovery. DNA Repair (Amst) 2009; 8:1101 - 1109; PMID: 19464963; http://dx.doi.org/10.1016/j.dnarep.2009.04.008
- Chang DJ, Cimprich KA. DNA damage tolerance: when it's OK to make mistakes. Nat Chem Biol 2009; 5:82 - 90; PMID: 19148176; http://dx.doi.org/10.1038/nchembio.139
- Muir CS. Epidemiology of cancer in ethnic groups. Br J Cancer Suppl 1996; 29:S12 - S16; PMID: 8782793
- President's Cancer Panel 2008–2009 Annual Report: April 2010
- Hellmold H, Rylander T, Magnusson M, Reihner E, Warner M, Gustafsson JA. Characterization of cytochrome P450 enzymes in human breast tissue from reduction mammaplasties. J Clin Endocrinol Metab 1998; 83:886 - 895; PMID: 9506744; http://dx.doi.org/10.1210/jc.83.3.886
- Tannheimer SL, Barton SL, Ethier SP, Burchiel SW. Carcinogenic polycyclic aromatic hydrocarbons increase intracellular Ca2+ and cell proliferation in primary human mammary epithelial cells. Carcinogenesis 1997; 18:1177 - 1182; PMID: 9214600; http://dx.doi.org/10.1093/carcin/18.6.1177
- Williams JA, Stone EM, Millar BC, Gusterson BA, Grover PL, Phillips DH. Determination of the enzymes responsible for activation of the heterocyclic amine 2-amino-3-methylimidazo[4,5-f]quinoline in the human breast. Pharmacogenetics 1998; 8:519 - 528; PMID: 9918136; http://dx.doi.org/10.1097/00008571-199812000-00009
- Jefcoate CR, Liehr JG, Santen RJ, Sutter TR, Yager JD, Yue W, et al. Tissue-specific synthesis and oxidative metabolism of estrogens. J Natl Cancer Inst Monogr 2000; 27:95 - 112; PMID: 10963622
- Haas S, Pierl C, Harth V, Pesch B, Rabstein S, Bruning T, et al. Expression of xenobiotic and steroid hormone metabolizing enzymes in human breast carcinomas. Int J Cancer 2006; 119:1785 - 1791; PMID: 16721811; http://dx.doi.org/10.1002/ijc.21915
- Triano EA, Slusher LB, Atkins TA, Beneski JT, Gestl SA, Zolfaghari R, et al. Class I alcohol dehydrogenase is highly expressed in normal human mammary epithelium but not in invasive breast cancer: implications for breast carcinogenesis. Cancer Res 2003; 63:3092 - 3100; PMID: 12810634
- Jonker JW, Merino G, Musters S, van Herwaarden AE, Bolscher E, Wagenaar E, et al. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat Med 2005; 11:127 - 129; PMID: 15685169; http://dx.doi.org/10.1038/nm1186
- van Herwaarden AE, Wagenaar E, Merino G, Jonker JW, Rosing H, Beijnen JH, et al. Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Mol Cell Biol 2007; 27:1247 - 1253; PMID: 17145775; http://dx.doi.org/10.1128/MCB.01621-06
- Martin FL, Carmichael PL, Crofton-Sleigh C, Venitt S, Phillips DH, Grover PL. Genotoxicity of human mammary lipid. Cancer Res 1996; 56:5342 - 5346; PMID: 8968081
- Phillips DH, Martin FL, Williams JA, Wheat LM, Nolan L, Cole KJ, et al. Mutagens in human breast lipid and milk: the search for environmental agents that initiate breast cancer. Environ Mol Mutagen 2002; 39:143 - 149; PMID: 11921182; http://dx.doi.org/10.1002/em.10049
- Martin FL. Genotoxins and the initiation of sporadic breast cancer. Mutagenesis 2001; 16:155 - 161; PMID: 11230559; http://dx.doi.org/10.1093/mutage/16.2.155
- Mattson MP. Dietary factors, hormesis and health. Ageing Res Rev 2008; 7:43 - 48; PMID: 17913594; http://dx.doi.org/10.1016/j.arr.2007.08.004
- Hulbert AJ. Metabolism and longevity: is there a role for membrane fatty acids?. Integr Comp Biol 2010; 50:808 - 817; PMID: 21558243; http://dx.doi.org/10.1093/icb/icq007
- Boice JD Jr, Friis S, McLaughlin JK, Mellemkjaer L, Blot WJ, Fraumeni JF, et al. Cancer following breast reduction surgery in Denmark. Cancer Causes Control 1997; 8:253 - 258; PMID: 9134250; http://dx.doi.org/10.1023/A:1018484616598
- Colwell AS, Kukreja J, Breuing KH, Lester S, Orgill DP. Occult breast carcinoma in reduction mammaplasty specimens: 14-year experience. Plast Reconstr Surg 2004; 113:1984 - 1988; PMID: 15253187; http://dx.doi.org/10.1097/01.PRS.0000122212.37703.6E
- Dotto J, Kluk M, Geramizadeh B, Tavassoli FA. Frequency of clinically occult intraepithelial and invasive neoplasia in reduction mammoplasty specimens: a study of 516 cases. Int J Surg Pathol 2008; 16:25 - 30; PMID: 18203780; http://dx.doi.org/10.1177/1066896907307176
- Karpinets TV, Foy BD. Tumorigenesis: the adaptation of mammalian cells to sustained stress environment by epigenetic alterations and succeeding matched mutations. Carcinogenesis 2005; 26:1323 - 1334; PMID: 15802302; http://dx.doi.org/10.1093/carcin/bgi079
- Heng HH, Stevens JB, Bremer SW, Ye KJ, Liu G, Ye CJ. The evolutionary mechanism of cancer. J Cell Biochem 2010; 109:1072 - 1084; PMID: 20213744
- Hart BA, Potts RJ, Watkin RD. **Cadmium adaptation in the lung -a double-edged sword?. Toxicology 2001; 160:65 - 70; PMID: 11246125; http://dx.doi.org/10.1016/S0300-483X(00)00436-4
- Thijssen S, Cuypers A, Maringwa J, Smeets K, Horemans N, Lambrichts I, et al. Low cadmium exposure triggers a biphasic oxidative stress response in mice kidneys. Toxicology 2007; 236:29 - 41; PMID: 17499415; http://dx.doi.org/10.1016/j.tox.2007.03.022
- Zoller LC, Weisz J. A demonstration of regional differences in lysosome membrane permeability in the membrana granulosa of Graafian follicles in cryostat sections of the rat ovary: a quantitative cytochemical study. Endocrinology 1980; 106:871 - 877; PMID: 7353547; http://dx.doi.org/10.1210/endo-106-3-871
- Palinski W, Yla-Herttuala S, Rosenfeld ME, Butler SW, Socher SA, Parthasarathy S, et al. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis 1990; 10:325 - 335; PMID: 1693068; http://dx.doi.org/10.1161/01.ATV.10.3.325
- Russo J, Russo IH. Influence of differentiation and cell kinetics on the susceptibility of the rat mammary gland to carcinogenesis. Cancer Res 1980; 40:2677 - 2687; PMID: 7388818
- Kenney NJ, Smith GH, Lawrence E, Barrett JC, Salomon DS. Identification of stem cell units in the terminal end bud and duct of the mouse mammary gland. J Biomed Biotechnol 2001; 1:133 - 143; PMID: 12488607; http://dx.doi.org/10.1155/S1110724301000304
- Bai L, Rohrschneider LR. s-SHIP promoter expression marks activated stem cells in developing mouse mammary tissue. Genes Dev 2010; 24:1882 - 1892; PMID: 20810647; http://dx.doi.org/10.1101/gad.1932810
- Wood CE, Hester JM, Cline JM. Mammary gland development in early pubertal female macaques. Toxicol Pathol 2007; 35:795 - 805; PMID: 17943653; http://dx.doi.org/10.1080/01926230701584213
- Grune T, Michel P, Sitte N, Eggert W, Albrecht-Nebe H, Esterbauer H, et al. Increased levels of 4-hydroxynonenal modified proteins in plasma of children with autoimmune diseases. Free Radic Biol Med 1997; 23:357 - 360; PMID: 9214570; http://dx.doi.org/10.1016/S0891-5849(96)00586-2
- Waeg G, Dimsity G, Esterbauer H. Monoclonal antibodies for detection of 4-hydroxynonenal modified proteins. Free Radic Res 1996; 25:149 - 159; PMID: 8885333; http://dx.doi.org/10.3109/10715769609149920
- Chua PJ, Yip GW, Bay BH. Cell cycle arrest induced by hydrogen peroxide is associated with modulation of oxidative stress related genes in breast cancer cells. Exp Biol Med (Maywood) 2009; 234:1086 - 1094; PMID: 19596828; http://dx.doi.org/10.3181/0903-RM-98
- Feltens R, Mogel I, Roder-Stolinski C, Simon JC, Herberth G, Lehmann I. Chlorobenzene induces oxidative stress in human lung epithelial cells in vitro. Toxicol Appl Pharmacol 2010; 242:100 - 108; PMID: 19800902; http://dx.doi.org/10.1016/j.taap.2009.09.020
- Huang CC, Aronstam RS, Chen DR, Huang YW. Oxidative stress, calcium homeostasis, and altered gene expression in human lung epithelial cells exposed to ZnO nanoparticles. Toxicol In Vitro 2010; 24:45 - 55; PMID: 19755143; http://dx.doi.org/10.1016/j.tiv.2009.09.007
- Higgins LG, Hayes JD. Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab Rev 2011; 43:92 - 137; PMID: 21495793; http://dx.doi.org/10.3109/03602532.2011.567391
- Ohiro Y, Garkavtsev I, Kobayashi S, Sreekumar KR, Nantz R, Higashikubo BT, et al. A novel p53-inducible apoptogenic gene, PRG3, encodes a homologue of the apoptosis-inducing factor (AIF). FEBS Lett 2002; 524:163 - 171; PMID: 12135761; http://dx.doi.org/10.1016/S0014-5793(02)03049-1
- Josephy PD. The role of peroxidase-catalyzed activation of aromatic amines in breast cancer. Mutagenesis 1996; 11:3 - 7; PMID: 8671708; http://dx.doi.org/10.1093/mutage/11.1.3
- Gorlewska-Roberts KM, Teitel CH, Lay JO Jr, Roberts DW, Kadlubar FF. Lactoperoxidase-catalyzed activation of carcinogenic aromatic and heterocyclic amines. Chem Res Toxicol 2004; 17:1659 - 1666; PMID: 15606142; http://dx.doi.org/10.1021/tx049787n
- Ghibaudi EM, Laurenti E, Beltramo P, Ferrari RP. Can estrogenic radicals, generated by lactoperoxidase, be involved in the molecular mechanism of breast carcinogenesis?. Redox Rep 2000; 5:229 - 235; PMID: 10994878; http://dx.doi.org/10.1179/135100000101535672
- Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med 2001; 194:781 - 795; PMID: 11560994; http://dx.doi.org/10.1084/jem.194.6.781
- Nauta AJ, Raaschou-Jensen N, Roos A, Daha MR, Madsen HO, Borrias-Essers MC, et al. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol 2003; 33:2853 - 2863; PMID: 14515269; http://dx.doi.org/10.1002/eji.200323888
- Palaniyar N, Clark H, Nadesalingam J, Shih MJ, Hawgood S, Reid KB. Innate immune collectin surfactant protein D enhances the clearance of DNA by macrophages and minimizes anti-DNA antibody generation. J Immunol 2005; 174:7352 - 7358; PMID: 15905582
- Litvack ML, Palaniyar N. Review: Soluble innate immune pattern-recognition proteins for clearing dying cells and cellular components: implications on exacerbating or resolving inflammation. Innate Immun 2010; 16:191 - 200; PMID: 20529971; http://dx.doi.org/10.1177/1753425910369271
- Rajawat YS, Hilioti Z, Bossis I. Aging: central role for autophagy and the lysosomal degradative system. Ageing Res Rev 2009; 8:199 - 213; PMID: 19427410; http://dx.doi.org/10.1016/j.arr.2009.05.001
- Kaarniranta K, Salminen A, Eskelinen EL, Kopitz J. Heat shock proteins as gatekeepers of proteolytic pathways-Implications for age-related macular degeneration (AMD). Ageing Res Rev 2009; 8:128 - 139; PMID: 19274853; http://dx.doi.org/10.1016/j.arr.2009.01.001
- Pivtoraiko VN, Stone SL, Roth KA, Shacka JJ. Oxidative stress and autophagy in the regulation of lysosome-dependent neuron death. Antioxid Redox Signal 2009; 11:481 - 496; PMID: 18764739; http://dx.doi.org/10.1089/ars.2008.2263
- Jung T, Hohn A, Catalgol B, Grune T. Age-related differences in oxidative protein-damage in young and senescent fibroblasts. Arch Biochem Biophys 2009; 483:127 - 135; PMID: 19135972; http://dx.doi.org/10.1016/j.abb.2008.12.007
- Miller LM, Dumas P. Chemical imaging of biological tissue with synchrotron infrared light. Biochim Biophys Acta 2006; 1758:846 - 857; PMID: 16781664; http://dx.doi.org/10.1016/j.bbamem.2006.04.010
- Krishna CM, Sockalingum GD, Bhat RA, Venteo L, Kushtagi P, Pluot M, et al. FTIR and Raman microspectroscopy of normal, benign, and malignant formalin-fixed ovarian tissues. Anal Bioanal Chem 2007; 387:1649 - 1656; PMID: 17043798; http://dx.doi.org/10.1007/s00216-006-0827-1
- Walsh MJ, Hammiche A, Fellous TG, Nicholson JM, Cotte M, Susini J, et al. Tracking the cell hierarchy in the human intestine using biochemical signatures derived by mid-infrared microspectroscopy. Stem Cell Res 2009; In press PMID: 19393589; http://dx.doi.org/10.1016/j.scr.2009.02.003
- Nakamura T, Kelly JG, Trevisan J, Cooper LJ, Bentley AJ, Carmichael PL, et al. Microspectroscopy of spectral biomarkers associated with human corneal stem cells. Mol Vis 2010; 16:359 - 368; PMID: 20520745
- Schubert W, Gieseler A, Krusche A, Hillert R. Toponome mapping in prostate cancer: detection of 2000 cell surface protein clusters in a single tissue section and cell type specific annotation by using a three symbol code. J Proteome Res 2009; 8:2696 - 2707; PMID: 19275201; http://dx.doi.org/10.1021/pr800944f
- Schubert W, Friedenberger M, Bode M, Krusche A, Hillert R. Functional architecture of the cell nucleus: towards comprehensive toponome reference maps of apoptosis. Biochim Biophys Acta 2008; 1783:2080 - 2088; PMID: 18718492; http://dx.doi.org/10.1016/j.bbamcr.2008.07.019
- Schubert W, Bode M, Hillert R, Krusche A, Friedenberger M. Toponomics and neurotoponomics: a new way to medical systems biology. Expert Rev Proteomics 2008; 5:361 - 369; PMID: 18466063; http://dx.doi.org/10.1586/14789450.5.2.361
- Bartow SA, Pathak DR, Black WC, Key CR, Teaf SR. Prevalence of benign, atypical, and malignant breast lesions in populations at different risk for breast cancer. A forensic autopsy study. Cancer 1987; 60:2751 - 2760; PMID: 3677009; http://dx.doi.org/10.1002/1097-0142(19871201)60:11<2751::AID-CNCR2820601127>3.0.CO;2-M
- Ivanovic Z. Hypoxia or in situ normoxia: The stem cell paradigm. J Cell Physiol 2009; 219:271 - 275; PMID: 19160417; http://dx.doi.org/10.1002/jcp.21690
- Freeman TA, Parvizi J, Della Valle CJ, Steinbeck MJ. Reactive oxygen and nitrogen species induce protein and DNA modifications driving arthrofibrosis following total knee arthroplasty. Fibrogenesis Tissue Repair 2009; 2:5; PMID: 19912645; http://dx.doi.org/10.1186/1755-1536-2-5
- Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, et al. Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci USA 1998; 95:4882 - 4887; PMID: 9560197; http://dx.doi.org/10.1073/pnas.95.9.4882
- Wang M, Dhingra K, Hittelman WN, Liehr JG, de Andrade M, Li D. Lipid peroxidation-induced putative malondialdehyde-DNA adducts in human breast tissues. Cancer Epidemiol Biomarkers Prev 1996; 5:705 - 710; PMID: 8877062
- Ishii T, Kumazawa S, Sakurai T, Nakayama T, Uchida K. Mass spectroscopic characterization of protein modification by malondialdehyde. Chem Res Toxicol 2006; 19:122 - 129; PMID: 16411665; http://dx.doi.org/10.1021/tx050231p
- Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radic Biol Med 2007; 43:1109 - 1120; PMID: 17854706; http://dx.doi.org/10.1016/j.freeradbiomed.2007.07.012
- Strong JP. Landmark perspective: Coronary atherosclerosis in soldiers. A clue to the natural history of atherosclerosis in the young. JAMA 1986; 256:2863 - 2866; PMID: 3534337; http://dx.doi.org/10.1001/jama.1986.03380200101029
- Sens MA, Somji S, Garrett SH, Beall CL, Sens DA. Metallothionein isoform 3 overexpression is associated with breast cancers having a poor prognosis. Am J Pathol 2001; 159:21 - 26; PMID: 11438449; http://dx.doi.org/10.1016/S0002-9440(10)61668-9
- Bindoli A, Fukuto JM, Forman HJ. Thiol chemistry in peroxidase catalysis and redox signaling. Antioxid Redox Signal 2008; 10:1549 - 1564; PMID: 18479206; http://dx.doi.org/10.1089/ars.2008.2063
- Wonsey DR, Zeller KI, Dang CV. The c-Myc target gene PRDX3 is required for mitochondrial homeostasis and neoplastic transformation. Proc Natl Acad Sci USA 2002; 99:6649 - 6654; PMID: 12011429; http://dx.doi.org/10.1073/pnas.102523299
- Nonn L, Berggren M, Powis G. Increased expression of mitochondrial peroxiredoxin-3 (thioredoxin peroxidase-2) protects cancer cells against hypoxia and drug-induced hydrogen peroxide-dependent apoptosis. Mol Cancer Res 2003; 1:682 - 689; PMID: 12861054
- Chua PJ, Lee EH, Yu Y, Yip GW, Tan PH, Bay BH. Silencing the Peroxiredoxin III gene inhibits cell proliferation in breast cancer. Int J Oncol 2010; 36:359 - 364; PMID: 20043069
- Glynn SA, Boersma BJ, Dorsey TH, Yi M, Yfantis HG, Ridnour LA, et al. Increased NOS2 predicts poor survival in estrogen receptor-negative breast cancer patients. J Clin Invest 2010; 120:3843 - 3854; PMID: 20978357; http://dx.doi.org/10.1172/JCI42059