Abstract
Plitidepsin (Aplidin), an antitumor agent of marine origin, presently is undergoing phase II/III clinical trials, and has shown promise for the treatment of lymphoma. Here, we describe the antitumor effects of plitidepsin alone and in combination with rituximab and investigated the effects of each drug and the combination on the cell cycle and mechanism of cell death. Several Diffuse Large Cell Lymphoma (DLCL) lines and Burkitt cell lines were tested for sensitivity to plitidepsin and rituximab. All DLCL and Burkitt lymphoma cell lines were inhibited by plitidepsin in nanomolar concentrations, while rituximab sensitivity varied among different cell lines. Ramos and the RL cell lines proved sensitive to rituximab and were used to test the effects of each of the two drugs. The two agents exhibited synergism at all tested concentrations. For in vivo studies, irradiated athymic nude mice were engrafted with the Ramos lymphoma. Treatment was initiated when the tumors were ~0.5 cm in diameter, and toxic and therapeutic effects were monitored. In the in vivo study, additive effects of the combined two drugs, was demonstrated without an increase in host toxicity. The in vitro synergy and the in vivo additive antitumor effects without an increase in host toxicity with two relatively non-marrow suppressive agents encourages further development of this combination for treatment of aggressive B-cell lymphomas.
Introduction
Non-Hodgkin lymphoma (NHL) is the fifth most common cause of cancer, with the number of cases increasing every year. NHL includes a broad number of distinct lymphoid malignancies. It is characterized by monoclonal expansion of B or T lymphocytes with B-cell lymphomas representing the majority (85%) of the cases. Rituximab, a chimeric anti-CD20 monoclonal antibody mediates its antitumor activity by apoptosis, antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity.Citation1–Citation4 Rituximab, is used alone or in combination for the treatment of a variety of B-cell lymphoma types.Citation5–Citation9 Whether used alone or in combination, resistance to therapy may occur.Citation10,Citation11 The combination of rituximab and CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) remains the standard immunochemotherapy for DLCLCitation12–Citation14 with a complete response rate of 61–76%.Citation15,Citation16 This regimen has significant toxicity and patients who relapse, if not cured by autologous stem cell transplantation and high dose chemotherapy, die of this disease.
Plitidepsin is a marine derived antitumor agent currently in phase II/III clinical trials for solid and hematologic malignancies.Citation17,Citation18 Plitidepsin has strong antiproliferative activity against different human cancer cell lines and tumors.Citation19,Citation20 Importantly, little or no bone marrow toxicity has been detected in clinical trials.Citation21,Citation22 Despite the interest generated by the clinical activity of plitidepsin in various malignant diseases, the exact mechanism of its antitumor activity remains elusive.Citation23–Citation26 Recently, plitidepsin was shown to have activity with a safe toxicity profile in patients with peripheral T-cell lymphomas.Citation27 To date, clinical trials with patients with B-cell malignancies have not been reported.
We investigated the effect of plitidepsin alone in DLCL and Burkitt lymphoma cell lines and then in combination with rituximab in a Burkitt lymphoma cell line (Ramos) and a DLCL cell line (RL). Herein, we describe studies showing that plitidepsin is a potent cytotoxic agent against lymphoma cell lines, and in rituximab sensitive cell lines, the combination of plitidepsin and rituximab results in synergistic cell kill. We also evaluated the antitumor activity of plitidepsin and rituximab as single agents and their combination on Ramos lymphoma xenografts in mice and show that the combination is more effective than either agent alone without an increase in host toxicity. By analyzing the method of cell death and the effects of these agents on the cell cycle, supportive evidence for the synergistic effect of the plitidepsin-rituximab combination is presented.
Results
The effect of plitidepsin and rituximab alone and in combination on B-lymphoma cell lines.
shows the cytotoxic effects of plitidepsin alone and rituximab alone on DLCL and Burkitt lymphoma cell lines. All cell lines were highly sensitive to plitidepsin (1–9 nM), while only Ramos and RL cell lines were sensitive to rituximab. After treatment for 96 h, the IC50 of plitidepsin was 1.5 ± 0.5 nM for RL and 1.7 ± 0.7 nM for the Ramos cell line. The IC50 for rituximab was 1 ± 0.1 nM (0.15 µg/ml) for Ramos and 1.5 ± 0.1 nM (0.22 µg/ml) for the RL cell line. For plitidepsin and rituximab combination studies, we used these two rituximab sensitive cell lines, which also had high CD20 expression (, ). For combination studies, plitidepsin was combined with rituximab at a fixed ratio of doses (P:R = 0.078:0.13 nM to P:R = 40:69.5 nM). Using the Chou-Talalay analysis, marked synergy between plitidepsin and rituximab was observed in the cell lines (). Sequential and simultaneous treatment had a similar synergistic effect. The effect of plitidepsin in combination with rituximab was compared with doxorubicin (), which is part of the CHOP regimen used to treat DLCL. In the combination of doxorubicin with rituximab, at an IC90 only additive effect was seen with higher CI values in the Ramos cells (CI = 0.7). For RL cells, the combination of doxorubicin and rituximab was antagonistic (CI > 1). We observed significant rituximab-resistance in other B-cell lines (). These B cells are characterized by high surface expression of CD19 (> 90%) but with different levels of CD20 (, ). To determine whether this resistance was a result of the need for complement; we evaluated the full effect of rituximab and its synergy with plitidepsin by exposing these cell lines to the two drugs in media with 8% non-heat inactivated FBS and also added 2% human serum. In Daudi, Raji, and Jiyoyi, Burkitt lymphoma cell lines; 96 h of exposure to rituximab at 1.39 µM (200 µg/ml) did not decrease cell viability. At 0.69 µM (100 µg/ml) concentration (data not shown) rituximab had a minimal toxic effect on HT, a DLBCL cell line. The lack of correlation between rituximab sensitivity and the percentage of CD20 antigen () may indicate that even though there is binding there is no downstream signaling.Citation29–Citation31
Plitidepsin blocks Ramos and RL cells in the G1/S phase of the cell cycle.
Cells were treated either with plitidepsin, rituximab, or their combination for 24 h. In both Ramos and RL cells, plitidepsin treatment caused an increase in percent of cells in G1 and a concomitant decrease in percentage of cells in S phase. In Ramos cells, rituximab alone had no effect on the cell cycle when compared with the untreated cells, indicating that this drug killed cells in all phases of the cell cycle. Addition of rituximab to plitidepsin reversed the G1/S block caused by plitidepsin alone (), whereas in the RL cell line, rituximab did not alter the G1/S block of plitidepsin ().
The role of apoptosis in cell death induced by plitidepsin, rituximab and their combination in the lymphoma cell lines.
To examine the mechanism of cell death of plitidepsin and rituximab, cells were treated with drugs for 48 h, and were stained with Annexin V-FITC and 7-AAD and analyzed by flow cytometry. Apoptosis-induced by rituximab at µg/ml in Ramos cells was 45%, where plitidepsin at nM concentrations produced 20% apoptotic cell death (). The combination of the two drugs caused apoptosis in 75% of the population. Although in RL cells, while viability assays showed complete cell death at 96 h, cell death due to apoptosis was much less with all doses and time points. Even with the highest concentration of plitidepsin (5 nM) only 12% of cells were apoptotic in RL cells; and the combination of plitidepsin and rituximab increased apoptosis only to 20% ( and B). To confirm the Annexin V results, we investigated activation of the final executioners of apoptosis: caspase-3 and caspase-7, as well as caspase-9 and PARP cleavage, in Ramos and RL cells. In Ramos cells, plitidepsin induced apoptosisCitation33–Citation35 by cleavage of caspase-3 and caspase-7 (), but rituximab triggered only cleavage of caspase-7 (). We did not observe activation of caspase-9, while PARP was completely cleaved after 8 h with all of the doses (data not shown). In RL cells, activation of these caspases by plitidepsin or rituximab did not occur, even after long exposures (96 h). At higher plitidepsin concentrations (25 nM), PARP cleavage was only modestly increased (data not shown). We conclude that in contrast to Ramos cells, apoptosis plays a minimal role in cell death induced by plitidepsin and rituximab in RL cells, a DLBC cell line. The role of necrotic cell death and autophagy as a mechanism of cell death caused by these drugs in the RL cell line is under study.
We analyzed cleavage of caspase-3 and -7 in the additional B-cell lines when treated with either plitidepsin and rituximab and cultured in 8% non-heat inactivated FBS and 2% human serum for 16, 24 and 48 h. Daudi, similarly to Raji and Jiyoyi, showed cleavage of caspase-7, but only when treated with plitidepsin, (). Similar to the RL cell line, HT did not show cleavage of caspase-3 and -7 under any treatment. Of interest is that treatment with rituximab caused an increase in intensity of the caspase bands in a time-dependent manner ( and B). In summary, we conclude that cell death in the four Burkitt lymphoma cell lines—Daudi, Jiyoyi, Raji and Ramos—is mainly due to apoptosis while apoptosis is not a cause of cell death with either rituximab or plitidepsin in the two DLCL cell lines HT and RL.
Effect of plitidepsin, rituximab and their combination on the growth of the Ramos B-cell lymphoma xenograft.
To determine if the synergistic activity in vitro of the combination of plitidepsin and rituximab could be observed in vivo, we studied the effect of the single agents and their combination in xenografts of Ramos B-cell lymphoma propagated in athymic nude mice. Based on previous studies of rituximab in mice,Citation36–Citation38 we treated mice with low doses of rituximab (200 µg/kg) given as a single agent or in combination with plitidepsin (0.2 or 0.4 mg/kg), and plitidepsin as a single agent at 0.4 mg/kg (). Low doses of rituximab were used in order to observe any additive or synergistic effect of the combination. The lower dose of plitidepsin (0.2 mg/kg) was chosen based on previous studies showing that lower doses may be more effective in combination than higher doses.Citation26 At day 29, 11 d after the therapy was terminated, significant tumor growth inhibition by plitidepsin or rituximab alone and the combination was evident. Survival analysis showed a significant prolongation of survival of mice treated with the combination as compared with either of the agents alone (p = 0.03). Untreated animals had a median survival of 31 d, and rituximab or plitidepsin treatments as single agents increased survival to 35 d, while the combination of rituximab with plitidepsin at 0.2 mg/kg provided the longest median survival of 41 d (). The combination significantly inhibited tumor growth and increasing the dose of plitidepsin from 0.2–0.4 mg/kg in combination with rituximab did not increase its antitumor effect. One animal in each of the single agent treated groups had a complete regression and two of eight animals in the combination group had complete tumor regression. All of the treatments were well tolerated as measured by weight loss (data not shown).
Discussion
Plitidepsin has been found to be well tolerated by patients and has selective cytotoxicity in vitro toward leukemia cells compared with hematopoietic progenitors.Citation39 Clinical studies indicate that plitidepsin has potential activity for the treatment of acute lymphoblastic leukemia and melanoma.Citation40 Additionally, in vitro, and in vivo multiple myeloma experimental models have demonstrated its antiangiogenic effect.Citation41 A phase II study of plitidepsin with dexamethasone in relapse/refractory multiple myeloma reported a response rate of 13% with plitidepsin alone and 22% in the cohort of patients with the addition of dexamethasone.Citation18 In peripheral T-cell lymphoma, a response rate of 20% was recently reported.Citation27
In this study, we explored the efficacy of plitidepsin alone and in combination in two aggressive type lymphomas. Treatment of two B-cell lymphoma cell lines with the combination of plitidepsin and rituximab resulted in enhanced antitumor activity compared with either agent alone. When evaluated in Ramos xenograft bearing mice, rituximab and plitidepsin alone were able to inhibit tumor growth and the combination produced additive effects, using a low minimally effective dose of rituximab (). Of interest, the low dose of plitidepsin with rituximab was more effective than the combination with the higher plitidepsin dose. Significant clinical toxicity would be unlikely from this combination; plitidepsin doses of 0.2–5 mg/m2 every two weeks was well tolerated with minimal marrow toxicityCitation42 and lack of marrow toxicity of rituximab has already been demonstrated. Although not tested, we would also predict that cell lines like SU-DHL4, SU-DHL6 and BL60, which have been reported to be highly sensitive to rituximab, would also show synergy with plitidepsin and rituximab treatment.Citation32
Low levels of plitidepsin and rituximab when used alone induced apoptosis in Ramos, the Burkiitt's lymphoma cell line, and the individual doses that produced partial apoptosis produce almost complete apoptosis in combination. To confirm the Annexin V results, we examined expression of the executioner caspases-3 and -7. While cleavage of caspase-3 and -7 was triggered by plitidepsin treatment, only activation of caspase-7 occurred with exposure to rituximab. In Daudi, Raji, and Jiyoyi, other Burkitt lymphoma cell lines with different levels of CD20 surface expression () and resistance to rituximab (), apoptosis was only induced by plitidepsin. Therefore in agreement with a previous study,Citation31 rituximab-resistance might be a result of lack or modulation of CD20 antigen and dysregulated signaling of the B-cell receptors.
The type of cell death appears to vary for the different cell lines, since the level of apoptosis in the DLBC lines, RL and HT to plitidepsin or rituximab was low and cleavage of caspase-7 () and PARP were slightly affected but complete cleavage was not observed even after prolonged exposure to the drugs, despite induction of cell death. Thus cell death in these two cell lines maybe a result of necrosis or autophagy.
In the xenograft study only a low dose of rituximab was used. It is likely that higher doses of rituximab would be more effective indicated that the combination of plitidepsin and rituximab may be an effective combination for the treatment of B-cell malignancies, in particular for treatment of patients with DLCL and Burkitt lymphoma.
Materials and Methods
Cell lines.
BJAB, EBV-negative, Jiyoye (CCL-87 B) EBV-positive and Ramos (CRL-1596 B) EBV-negative, Burkitt lymphoma cell lines, were kindly provided by Dr. A. Levine, Cancer Institute of New Jersey. Daudi (CCL-213 B) EBV-positive, Namalwa (CCL-1432 B) EBV-positive, Raji (CCL-86 B) EBV-positive Burkitt lymphoma cell lines and HT (CRL-2260) EBV-negative DLBCL cell line were a kind gift of Dr. Ricardo Dalla Favera, Institute of Cancer Genetics, Columbia University, NY. The RL line, a transformed large B-cell (CRL-2261), EBV-negative, was obtained from the American Type Culture Collection. The cell lines were maintained in RPMI 1640 medium supplemented with 10% heat inactivated fetal bovine serum (FBS). During experimental conditions, non-heat inactivated FBS and an additional 2% human serum was used to provide complement in the culture medium. The cell lines were authenticated as expressing B-cell markers by the surface expression of CD19 and CD20 using flow cytometry.
Chemotherapeutic agents.
Rituximab, clinical grade was obtained from the Cancer Institute of New Jersey (CINJ) pharmacy. Plitidepsin was provided by Pharmamar S.A., Madrid, Spain.
Cytotoxicity assays.
Lymphoma cells were seeded in 96-well plates at a density of 10,000 cells per well. Immediately after seeding, cells were treated with concentrations of plitidepsin ranging from 0.078–40 nM, rituximab ranging from 0.13–69.5 nM (0.019–10 µg/ml) for the Ramos and RL cell lines or 2.6 nM–1.3 µM (0.38–200 µg/ml) for BJAB, HT, Jiyoyi, Namalwa and Raji cell lines, or their combination. The number of surviving cells after drug exposure was determined using the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, Promega] colorimetric assay for determining the number of viable cells. SOFTmaxPRO (Molecular Devices Corporation) was used to determined cell viability and IC50s. Cell death and viability was also corroborated with the V-cell counter (Beckman Coulter). For combination studies plitidepsin (P) was combined with rituximab (R) at a fixed dose ratio ranging from P:R = 0.078:0.13 nM to P:R = 40:69.5: nM and Doxorubicin (D) from D:R = 0.24:0.13 nM to D:R = 125:69.5 nM. The combination index (CI) was calculated based on the Chou-Talalay equation, which takes into consideration the median-effect dose or potency (shown by IC50) and shape of the dose-effect curve (concentration).Citation28 CalcuSyn software (Biosoft) was used for the Chou-Talalay combination index analysis. Both sequential and simultaneous combinations were tested.
Flow cytometry.
Expression of CD19 and CD20 antigens, were measured using FITC conjugated murine anti-human antibodies. FITC anti-human IgG1 was used as an isotype control (Caltag, Invitrogen). For cell cycle analysis, cells (1 × 106) following drug exposure were fixed for 2 h in 100% ethanol and stored in 70% alcohol at −20°C. At the time of analysis, cells were stained with 0.5 ml Propidium Iodide (PI) RNase staining buffer (BD PharMingen) for 15 min at room temperature. The percentage of cells in each phase of the cell cycle was calculated using the ModFit v3.0 software (Becton Dickinson) and the histograms with the DNA content were plotted with the Cell Quest software. To quantify apoptosis, cells (0.1 × 105) were washed with cold PBS and incubated in the dark for 15 min with 5 µl Annexin V-FITC and 5 µl 7-Amino-Actinomycin D (7-AAD) in binding buffer. The percentage of total apoptosis was determined as percentage of Annexin V-FITC and 7-AAD stained cells, using the Cell Quest software. Fluorescent intensities were measured on a Becton Dickinson FACS Calibur.
Protein expression.
Protein expression was determined by western blot analysis. Cell lysates (30–40 µg) were denatured and loaded on 15% SDS acrylamide gels and electrophoresed. Membranes were blocked with 5% dry milk dissolved in 1X TBS plus 0.1% Tween, and probed with the respective antibody followed by incubation with peroxidase-conjugated secondary antibody. Monoclonal antibodies against caspase-3 and caspase-7 (C7) were purchased from Cell Signaling. In order to determine mechanism of cell death following treatment of cells with drugs, cleaved proteins such as caspase-3 and -7 and PARP were visualized by western blot analyses following treatment of cells with doses of drugs that were higher than those used for the IC50 studies.
In vivo studies.
Athymic Nu/nu female mice were purchased from Taconic at 5–6 wks of age. When the mice were 7–8 wks old (21–25 g), they were exposed to total body irradiation (400 cGy) to further suppress their residual immune system and facilitate the establishment of Ramos xenografts. After 3 d, the irradiated mice were injected s.c. in the right flank with 1 × 107 Ramos cells. When the tumor diameters reached ∼0.5 cm, the mice were randomized into five groups containing 8 mice each, to ensure that the mean tumor size would be approximately the same in all groups prior to the administration of therapy. Therapeutic agents were administered i.p. in 0.1 mL volume as follows; rituximab (200 µg/kg) as a single agent and in combination with plitidepsin at 0.2 or 0.4 mg/kg, and plitidepsin as a single agent at 0.4 mg/kg. Four doses of the drugs were administered 3 d apart. Tumor size and body weight was assessed every other day. Tumor volume was calculated as (mm3) = (length) × (width2) × 0.5. Mean (+/−SEM) tumor volume was calculated and compared with the vehicle treated group by an unpaired t-test. Survival of animals in different groups following treatment was compared using Kaplan-Meier curves.
Statistical analysis.
A standard unpaired Student t-test is applied to the data samples to investigate the significance of the results at the 95% confidence level. P < 0.05 was considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Abbreviations
| DLCL | = | diffuse large cell lymphoma |
| NHL | = | non-Hodgkin lymphoma |
| CHOP | = | cyclophosphamide, doxorubicin, vincristine, prednisone |
| EBV | = | Epstein-Barr virus |
| FBS | = | fetal bovine serum |
| CINJ | = | Cancer Institute of New Jersey |
| P | = | plitidepsin |
| R | = | rituximab |
| CI | = | combination Index |
| FITC | = | fluorescein iIsothiocyanate |
| PI | = | propidium iodide |
| BD | = | Becton Dickinson |
| 7-AAD | = | 7-amino-actinomycin |
| PARP | = | poly (ADP-ribose) polymerase |
| MFI | = | X-mean fluorescence intensity |
Figures and Tables
Figure 1 Relative expression of CD20 on lymphoma B-cell lines. B-cell lines were tested for CD20 expression and analyzed by flow cytometry. Histograms of CD20 fluorescence intensity are shown compared with the IgG1 isotype. The results presented are representative of at least three independent experiments.
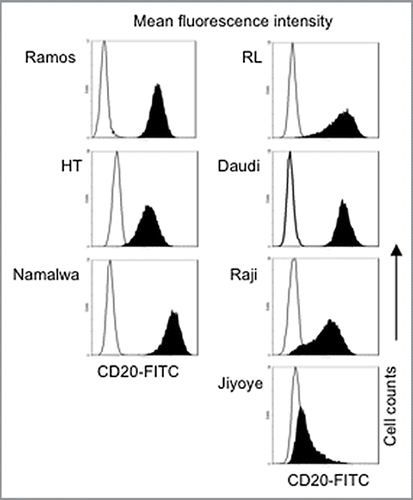
Figure 2 Combination of plitidepsin and rituximab synergistically inhibit B cells. Dose-response curves (log scale) of cells cultured in RPMI plus 10% non-heat inactivated FBS exposed to plitidepsin, rituximab and their combination (A), compared with doxorubicin, rituximab and their combination (B), after 96 h of treatment. (C) Sequence of drug concentrations at a fixed plitidepsin:rituximab dose ratio with the IC50 at 1.25 nM: 2.17 nM.
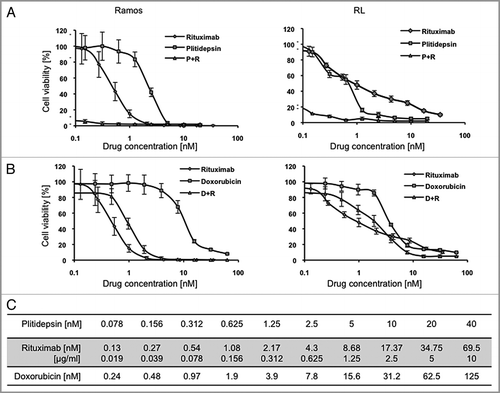
Figure 3 Plitidepsin blocks cells in the G1/S phase of the cell cycle. Ramos (A), and RL cells (B), were incubated with rituximab, plitidepsin, or their combination at 37°C for 24 h. Cells (1 á 105) were stained with 0.5 ml PI/RNase staining buffer and analyzed by flow cytometry with CellQuest software (Becton-Dickinson). Results analyzed with the Modfit software are presented as the mean and SD from three independent experiments. In Ramos cells p < 0.005 only for the plitidepsin treatments in comparison with the control. In RL cells p < 0.005 for all treatments in comparison with the control. (C) Histograms of the cell cycle fluorescence intensity after 24 h treatment with each drug alone and in combination.
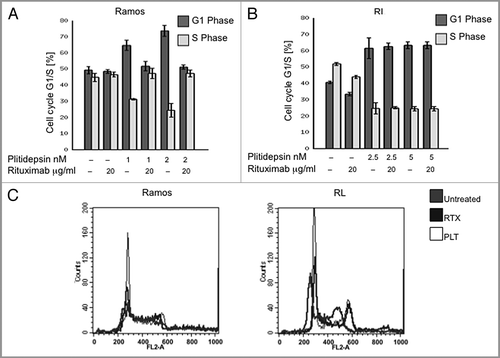
Figure 4 Apoptosis induced by plitidepsin, rituximab or their combination in rituximab-sensitive B cells. Ramos and RL cells were incubated with rituximab, plitidepsin, or their combination at 37°C for 48 h. Cells (1 × 105) were stained with Annexin V-FITC and 7AAD and analyzed by flow cytometry. Data are representative of three independent experiments. (A) Y axis represents the apoptotic cells. In Ramos and RL cells p < 0.001 for the rituximab treatments alone and in combination with plitidepsin in comparison with the control. (B) In the histograms, FL-1 (Annexin V-FITC) thresholds were determined by using cells stained without Annexin buffer per manufacturer's protocol. (C) Expression of caspase-3 and caspase-7 was analyzed by western blotting in Ramos cells treated with plitidepsin (C), plitidepsin plus rituximab (D) and rituximab (E) for the indicated times.
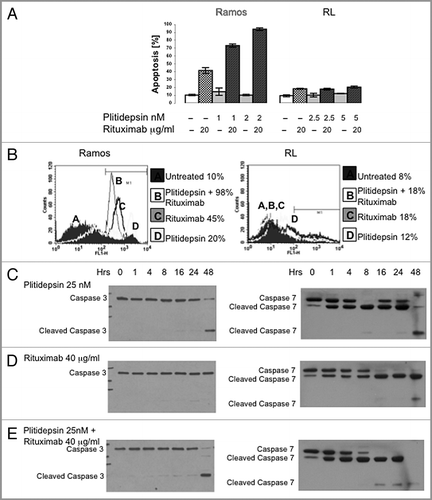
Figure 5 Western blot analysis of caspase activation in B cells expressing different levels of CD20. B cells with different levels of resistance to rituximab were treated for the indicated times with plitidepsin, plitidepsin plus rituximab and rituximab. Expression of caspase-7 (A) and caspase-3 (B) was analyzed by western blotting.
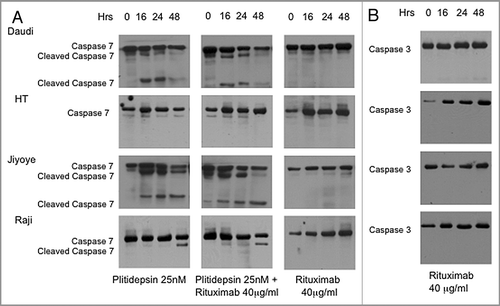
Figure 6 The effect of plitidepsin, rituximab and the combination of these drugs in Ramos tumor xenografts in mice. (A) Ramos cells (107) were inoculated subcutaneously in the flanks of irradiated athymic nude female Ncr mice. When the tumor diameter reached 0.5 cm, mice were simultaneously treated with rituximab (200 µg/kg), plitidepsin (0.2 or 0.4 mg/kg), or the combination of these drugs at four doses 3 d apart beginning on day 15 after tumor cell inoculation. Tumor measurements were taken at 2 d intervals (B) Survival of the mice treated with plitidepsin or rituximab alone, and in combination. Survival curves between control and each drug and control and combination treatment was significant (p = 0.03).
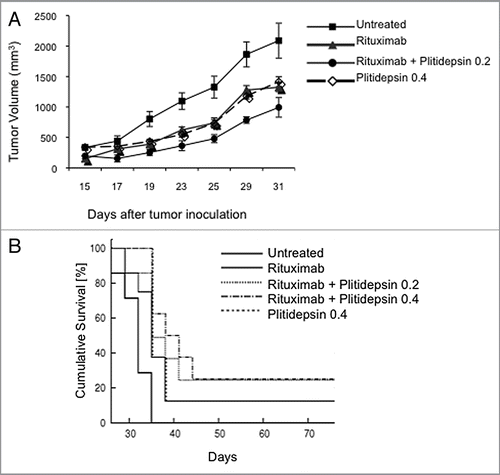
Table 1 Effect of rituximab and plitidepsin on lymphoma B-cell lines
Acknowledgments
N.B. was supported by a T32 grant CA108455 from the NIH
References
- Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994; 83:435 - 445; PMID: 7506951
- Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, et al. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med 2004; 199:1659 - 1669; PMID: 15210744; http://dx.doi.org/10.1084/jem.20040119
- Hamaguchi Y, Xiu Y, Komura K, Nimmerjahn F, Tedder TF. Antibody isotype-specific engagement of Fcgamma receptors regulates B lymphocyte depletion during CD20 immunotherapy. J Exp Med 2006; 203:743 - 753; PMID: 16520392; http://dx.doi.org/10.1084/jem.20052283
- Lefebvre ML, Krause SW, Salcedo M, Nardin A. Ex vivo-activated human macrophages kill chronic lymphocytic leukemia cells in the presence of rituximab: mechanism of antibody-dependent cellular cytotoxicity and impact of human serum. J Immunother 2006; 29:388 - 397; PMID: 16799334; http://dx.doi.org/10.1097/01.cji.0000203081.43235.d7
- Berinstein NL, Grillo-Lopez AJ, White CA, Bence-Bruckler I, Maloney D, Czuczman M, et al. Association of serum rituximab (IDEC-C2B8) concentration and antitumor response in the treatment of recurrent low-grade or follicular non-Hodgkin's lymphoma. Ann Oncol 1998; 9:995 - 1001; PMID: 9818074; http://dx.doi.org/10.1023/A:1008416911099
- Marcus R, Imrie K, Belch A, Cunningham D, Flores E, Catalano J, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood 2005; 105:1417 - 1423; PMID: 15494430; http://dx.doi.org/10.1182/blood-2004-08-3175
- Hainsworth JD, Litchy S, Morrissey LH, Andrews MB, Grimaldi M, McCarty M, et al. Rituximab plus short-duration chemotherapy as first-line treatment for follicular non-Hodgkin's lymphoma: a phase II trial of the minnie pearl cancer research network. J Clin Oncol 2005; 23:1500 - 1506; PMID: 15632411; http://dx.doi.org/10.1200/JCO.2005.05.004
- Economopoulos T, Fountzilas G, Pavlidis N, Kalantzis D, Papageorgiou E, Christodouloe C, et al. Rituximab in combination with CNOP chemotherapy in patients with previously untreated indolent non-Hodgkin's lymphoma. Hematol J 2003; 4:110 - 115; PMID: 12750729; http://dx.doi.org/10.1038/sj.thj.6200229
- Hagemeister F, Cabanillas F, Coleman M, Gregory SA, Zinzani PL. The role of mitoxantrone in the treatment of indolent lymphomas. Oncologist 2005; 10:150 - 159; PMID: 15709217; http://dx.doi.org/10.1634/theoncologist.10-2-150
- Jonathan W, Friedberg JW. Unique Toxicities and resistance mechanisms associated with monoclonal antibody therapy. Hematology (Am Soc Hematol Educ Program) 2005; 1:329 - 334
- Davis TA, Grillo-López AJ, White CA, McLaughlin P, Czuczman MS, Link BK, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin's lymphoma: safety and efficacy of re-treatment. J Clin Oncol 2000; 18:3135 - 3143; PMID: 10963642
- Czuczman MS, Grillo-Lopez AJ, White CA, Saleh M, Gordon L, LoBuglio AF, et al. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol 1999; 17:268 - 276; PMID: 10458242
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002; 346:235 - 242; PMID: 11807147; http://dx.doi.org/10.1056/NEJMoa011795
- Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdalla R, Fermé C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 2005; 23:4117 - 4126; PMID: 15867204; http://dx.doi.org/10.1200/JCO.2005.09.131
- Vose JM, Link BK, Grossbard ML, Czuczman M, Grillo-Lopez A, Fisher RI. Long-term update of a phase II study of rituximab in combination with CHOP chemotherapy in patients with previously untreated, aggressive Non-Hodgkin's lymphoma. Leuk Lymphoma 2005; 46:1569 - 1573; PMID: 16236611; http://dx.doi.org/10.1080/10428190500217312
- Plosker GL, Figgitt DP. Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia. Drugs 2003; 63:803 - 843; PMID: 12662126; http://dx.doi.org/10.2165/00003495-200363080-00005
- Schöffski P, Guillem V, Garcia M, Rivera F, Tabernero J, Cullell M, et al. Phase II randomized study of plitidepsin (Aplidin), alone or in association with L-carnitine, in patients with unresectable advanced renal cell carcinoma. Marine Drugs 2009; 7:57 - 70; PMID: 19370171; http://dx.doi.org/10.3390/md7010057
- Mateos MV, Cibeira T, Richardson PG, Prosper F, Oriol A, de la Rubia J, et al. Phase II clinical and pharmacokinetic study of plitidepsin 3-hours infusion every two weeks alone or with dexamethasone in relapsed and refractory multiple myeloma. Clin Cancer Res 2010; 16:3260 - 3269; PMID: 20530693; http://dx.doi.org/10.1158/1078-0432.CCR-10-0469
- Urdiales JL, Morata P, DeCastro IN, Sánches-Jiménez F. Antiproliferative effect of dehydrodidemnin B (DDB), a depsipeptide isolated from Mediterranean tunicates. Cancer Lett 1996; 102:31 - 37; PMID: 8603376; http://dx.doi.org/10.1016/0304-3835(96)04151-1
- Caers J, Menu E, De Raeve H, Lepage D, Van Valchenborgh E, Van Camp B, et al. Antitumour and antiangiogenic effects of Aplidin(R) in the 5TMM syngeneic models of multiple myeloma. Br J Cancer 2008; 98:1966 - 1974; PMID: 18521088; http://dx.doi.org/10.1038/sj.bjc.6604388
- Faivre S, Chièze S, Delbaldo C, Ady-Vago N, Guzman C, Lopez-Lazaro E, et al. Phase I and Pharmacokinetic Study of Aplidine, a New Marine Cyclodepsipeptide, in Patients With Advanced Malignancies. J Clin Oncol 2005; 23:7780 - 7782; PMID: 16204006; http://dx.doi.org/10.1200/JCO.2005.09.357
- Maroun JA, Belanger K, Seymour L, Matthews S, Roach J, Dionne J, et al. Phase I study of Aplidine in a dailyx5 one-hour infusion every 3 weeks in patients with solid tumors refractory to standard therapy. A National Cancer Institute of Canada Clinical Trials Group study: NCIC CTG IND 115. Ann Oncol 2006; 17:1371 - 1378; PMID: 16966366; http://dx.doi.org/10.1093/annonc/mdl165
- Erba E, Bassano L, Di Liberti G, Muradore I, Chiorino G, Ubezio P, et al. Cell cycle phase perturbations and apoptosis in tumour cells induced by plitidepsine. Br J Cancer 2002; 86:1510 - 1517; PMID: 11986788; http://dx.doi.org/10.1038/sj.bjc.6600265
- Erba E, Serafini M, Gaipa G, Tognon G, Marchini S, Celli N, et al. Effect of Plitidepsin in acute lymphoblastic leukaemia cells. Br J Cancer 2003; 89:763 - 773; PMID: 12915891; http://dx.doi.org/10.1038/sj.bjc.6601130
- González-Santiago L, Suarez Y, Zarich N, Muñoz-Alonso MJ, Cuadrado A, Martínez T, et al. Plitidepsin induces JNK-dependent apoptosis in human breast cancer cells via alteration of glutathione homeostasis, Rac1 GTPase activation, and MKP-1 phosphatase downregulation. Cell Death Differ 2006; 13:1968 - 1981; PMID: 16543941; http://dx.doi.org/10.1038/sj.cdd.4401898
- Humeniuk R, Menon LG, Mishra PJ, Saydam G, Longo-Sorbello GS, Elisseyeff Y, et al. Aplidin synergizes with cytosine arabinoside: functional relevance of mitochondria in Aplidin-induced cytotoxicity. Leukemia 2007; 21:2399 - 2405; PMID: 17713546; http://dx.doi.org/10.1038/sj.leu.2404911
- Ferme C, Mateos MV, Szyldergemajn S, Zucca E, Extremera S, Briones J, et al. Aplidin (Plitidepsin) Activity in Peripheral T-cell Lymphoma (PTCL): Final results Abstract 1767. 2010 ASH Annual meeting Abstracts, volume 116, number 21 Nov 19, 2010
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984; 22:27 - 55; PMID: 6382953; http://dx.doi.org/10.1016/0065-2571(84)90007-4
- Flieger D, Renoth S, Beier I, Sauerbruch T, Schmidt-Wolf I. Mechanism of cytotoxicity induced by chimeric mouse human monoclonal antibody IDEC-C2B8 in CD20-expressing lymphoma cell lines. Cell Immunol 2000; 204:55 - 63; PMID: 11006018; http://dx.doi.org/10.1006/cimm.2000.1693
- Golay J, Zaffaroni L, Vaccari T, Lazzari M, Borleri GM, Bernasconi S, et al. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood 2000; 95:3900 - 3908; PMID: 10845926
- Mathas S, Rickers A, Bommert K, Do:rken B, Mapara MY. Anti-CD20-and B-cell receptor-mediated apoptosis: evidence for shared intracellular signaling pathways. Cancer Res 2000; 60:7170 - 7176; PMID: 11156427
- Deans JP, Li H, Polyak MJ. CD20-mediated apoptosis: signalling through lipid rafts. Immunology 2002; 107:176 - 182; PMID: 12383196; http://dx.doi.org/10.1046/j.1365-2567.2002.01495.x
- Cuadrado A, González L, Suárez Y, Teresa Martínez T, Muñoz A. JNK activation is critical for Aplidin™-induced apoptosis. Oncogene 2004; 23:4673 - 4680; PMID: 15122339; http://dx.doi.org/10.1038/sj.onc.1207636
- García-Fernández LF, Losada A, Alcaide V, Alvarez AM, Cuadrado A, Gonzáles L, et al. Aplidin induces the mitochondrial apoptotic pathway via oxidative stress-mediated JNK and p38 activation and protein kinase C delta. Oncogene 2002; 21:7533 - 7544; PMID: 12386816; http://dx.doi.org/10.1038/sj.onc.1205972
- Gajate C, An F, Mollinedo F. Rapid and selective apoptosis in human leukemic cells induced by Aplidine through a Fas/CD95-and mitochondrial-mediated mechanism. Clin Cancer Res 2003; 9:1535 - 1545; PMID: 12684430
- Bertolini F, Fusetti L, Mancuso P, Gobbi A, Corsini C, Ferrucci PF, et al. Endostatin, an antiangiogenic drug, induces tumor stabilization after chemotherapy or anti-CD20 therapy in a NOD/SCID mouse model of human high-grade non-Hodgkin lymphoma. Blood 2000; 96:282 - 287; PMID: 10891463
- Dijoseph JF, Dougher MM, Armellino DC, Kalyandrug L, Kunz A, Boghaert ER, et al. CD20-specific antibody-targeted chemotherapy of non-Hodgkin's B-cell lymphoma using calicheamicin-conjugated rituximab. Cancer Immunol Immunother 2007; 56:1107 - 1117; PMID: 17160682; http://dx.doi.org/10.1007/s00262-006-0260-5
- Gopal AK, Press OW, Wilbur SM, Maloney DG, Pagel JM. Rituximab blocks binding of radiolabeled anti-CD20 antibodies (Ab) but not radiolabeled anti-CD45 Ab. Blood 2008; 112:830 - 835; PMID: 18502830; http://dx.doi.org/10.1182/blood-2008-01-132142
- Gómez SG, Bueren JA, Faircloth GT, Jimeno J, Albella B. In vitro toxicity of three new antitumoral drugs (trabectedin, Aplidin, and kahalalide F) on hematopoietic progenitors and stem cells. Exp Hematol 2003; 31:1104 - 1111; PMID: 14585376
- Maier A, Korrat A, Fiebig H, Doreen L, Guillén MJ, Cuevas C, et al. Evaluation of antitumor efficacy of Plitidepsin in vitro in 72 patient derived tumor xenografts using a clonogenic assay and determination of a predictive gene signature. AACR-NCI-EORTC 2007;
- Mitsiades CS, Ocio EM, Pandiella A, Maiso P, Gajate C, Garayoa M, et al. Aplidin, a marine organism-derived compound with potent antimyeloma activity in vitro and in vivo. Cancer Res 2008; 68:5216 - 5225; PMID: 18593922; http://dx.doi.org/10.1158/00085472.CAN-07-5725
- Faivre S, Chièze S, Delbaldo C, Ady-Vago N, Guzman C, Lopez-Lazaro L, et al. Phase I and pharmacokinetic study of Aplidine, a new marine cyclodepsipeptide in patients with advanced malignancies. J Clin Oncol 2005; 23:7871 - 7880; PMID: 16172454; http://dx.doi.org/10.1200/JCO.2005.09.357