Abstract
The inverse correlation between levels of dietary calcium and colorectal cancer (CRC) incidence has been extensively investigated. However, the impact of supplemental calcium on cancer therapy remains unknown. We used four models of CRC, Caco-2 and HCT116 human cancer cell lines and ApcMin/+ and azoxymethane carcinogen-induced mouse models, to investigate the impact of a Western-style diet low in calcium (0.05%) vs. a similar diet but supplemented with calcium (5%) on therapeutic targeting of the epidermal growth factor receptor (EGFR). We found that calcium supplementation combined with pharmacologic blockade of EGFR results in an additive effect on tumor growth inhibition in all models. Unexpectedly, the combined use of dietary calcium supplementation and EGFR inhibitors also resulted in elevated toxicity suggesting that careful consideration be given when combining dietary supplements with prescribed cancer therapies.
Introduction
Exposure to Western-style diets results in hyperproliferation in pancreatic, prostate, mammary and colonic epithelial cells.Citation1–Citation3 Some of the growth effects caused by a Western-style diet can be attributed to calcium deficiency since repletion with calcium suppresses epithelial hyperproliferation. Calcium stimulates expression of a membrane bound calcium-sensing receptor (CaR), and expression of this receptor appears to decrease with progression of human colorectal cancer (CRC).Citation4 Furthermore, extracellular calcium and its receptor promote CDH1 (E-cadherin) expression and inhibition of CTNNB (β-catenin) activation in CRC.Citation5
A review of 401 case-control studies, epidemiological studies and randomized controlled trials (RCTs) revealed just two appropriately-designed studies that provided evidence for a potential preventative effect of calcium.Citation6 Doses of calcium supplementation in the two studies were 2,000 and 1,200 mg/day (the recommended daily allowance) for 3 and 4 years, respectively.Citation7,Citation8 Both studies revealed a moderate effect for calcium in preventing the development of colonic adenomas, but only the study using 1,200 mg calcium/day reached statistical significance. In another study, calcium supplementation of 1,200 mg/day in patients previously diagnosed with colorectal adenomas had a protective effect even up to five years after the end of the study.Citation9
Several studies using mouse models for CRC have confirmed a role for calcium in preventing epithelial growth. Calcium supplementation reverses both hyperproliferation and hyperplasia of the distal colon in mice maintained on a Western-style diet and calcium combined with vitamin D prevents Western-diet induced colonic tumors.Citation3,Citation10 In another study mice maintained on a diet supplemented with 1.0% calcium had increased apoptosis but no change in proliferation in the distal colon compared with a control diet.Citation11 Similarly, low levels of calcium supplementation (40% increase above recommended dose) to rats treated with the colon carcinogen azoxymethane (AOM) resulted in inhibition of colon carcinogenesis.Citation12
Crosstalk between epidermal growth factor receptor (EGFR) and CaR has been reported suggesting a possible combinatorial treatment against CRC. Deregulation of the EGFR pathway occurs in up to 50% of epithelial cancers, an observation that lead to the development of numerous pharmacological agents to block EGFR activity.Citation13 Intestinal tumors in mice carrying ApcMin or colon tumors in mice treated with AOM, both involving the WNT/CTNNB pathway, display increased EGFR activity, and treatment of these mice with small molecule inhibitors targeting EGFR results in a significant decrease in tumor incidence.Citation14–Citation19
EGFR-inhibition using the EGFR-selective agent AG1478 inhibits calcium-mediated proliferation of rat cancer and fibroblast cells, while depletion of calcium was shown to cause increased EGFR signaling via increased CDC42 activity, which in turn led to increased CDH1 degradation in MCF-7 breast cancer cells.Citation20–Citation22 G-protein coupled receptors (GPCR) like CaR may mediate the effects of calcium on EGFR since GPCRs are known to transactivate EGFR via regulation of ligand proteolytic cleavage and shedding.Citation21 The intracellular calcium/calmodulin complex can also directly bind the juxtamembrane region of EGFR releasing the kinase domain from the membrane thereby facilitating activation of the receptor.Citation23
Based upon the similarities between calcium supplementation and EGFR inhibition, we investigated whether combinatorial treatments would have greater therapeutic efficacy than EGFR inhibitor monotherapy. Four models of CRC were used to demonstrate that calcium has the potential to enhance efficacy of EGFR-targeted therapies, but that special attention should be given to potential adverse toxicities associated with high calcium levels.
Results
Calcium supplementation enhances EGFR inhibitor efficacy.
Mice from two mouse models for CRC, ApcMin/+ and AOM, were randomly assigned to either a Western-style diet with low calcium (0.05%) or a calcium-supplemented diet (5%), each with or without the EGFR-inhibitor AG1478 (). Following treatment tumor number and size was assessed in the small intestine and colon of ApcMin/+ mice and colon of AOM-treated mice; AOM does not induce small intestinal tumors.
Contrary to previous reports using EGFR inhibitors in mice fed standard mouse chows, AG1478 did not significantly reduce tumor number in the ApcMin/+ model fed a Western-style diet ().Citation17,Citation18 However, supplementation with calcium in addition to AG1478 significantly reduced colon tumor number compared with AG1478 alone. Small intestinal tumor number was not significantly reduced by any treatment. However, all treatments (AG1478 alone, calcium alone and AG1478 + calcium) significantly reduced tumor size in the small intestine of ApcMin/+ model (). Colon tumor size was not affected by any treatment (data not shown).
Unlike the ApcMin/+ model, AG1478 alone significantly reduced tumor number in the AOM model in the context of a Western-style diet (). Calcium supplementation with AG1478 treatment resulted in a further reduction, but the additive effect was observed only in female mice. None of the treatments impacted tumor size in the AOM model (data not shown). Calcium alone did not reduce tumor number and no treatment modified the distribution of tumors along the GI tract in either model (data not shown).
Calcium enhances growth inhibition of human CRC cell lines by AG1478.
The impact of calcium on AG1478-mediated reduction in cell growth was also measured in two human CRC cell lines, Caco-2 and HCT116, to determine if calcium has a generalized effect on EGFR inhibition. Calcium alone produced a dose-dependent reduction in cell viability for both cell lines (). In order to distinguish additive from synergistic interactions with calcium, the maximal AG1478 dose producing less than a 20% reduction in cell viability was determined for each cell line (). The combined effects of AG1478 (4 mM for Caco-2 and 8 µM for HCT116) and calcium had an additive effect on cell viability in both cell lines when compared with calcium or AG1478 alone ( and D; additive model p < 0.0001). These results suggested that calcium and EGFR inhibition may impact the same cellular activity, with calcium potentially enhancing the inhibitory effects of EGFR blockade.
Calcium enhances AG1478 inhibition of EGFR signaling.
The level of EGFR activity, as determined by phosphorylated EGFR (pEGFR) was quantified in samples from mouse liver tissue and human CRC cell lines to determine if calcium supplementation can directly impact EGFR signaling. EGFR signaling was also analyzed by sex since sex-dependent effects were observed in vivo ().
In vivo, calcium alone did not significantly reduce tumor number in either ApcMin/+ or AOM mouse models, and this is reflected in absence of an effect on pEGFR. AG1478 also failed to have a detectable effect on pEGFR signaling in ApcMin/+ mice (). However, AG1478 and AG1478 + calcium did reduce levels of pEGFR in AOM-treated mice (), consistent with the observed reduction in tumor number.
The impact of calcium on AG1478-mediated EGFR inhibition was also examined in vitro. Increasing levels of calcium along with AG1478 reduced EGFR downstream signaling in a dose-dependent manner (). This inhibition was most evident in pAKT levels for Caco-2 and pERK levels for HCT116 cells. Baseline pEGFR levels were too low for detection using protein gel blot analysis, consequently cells were treated for two hours with AG1478 and/or calcium followed by EGF induction of EGFR. ELISA was performed revealing that AG1478 reduced pEGFR and calcium supplementation alone may actually enhance pEGFR, especially in the Caco-2 cell line (). In both cell lines, AG1478 effectively reduced pEGFR regardless of calcium dose. pEGFR levels in AG1478-treated cells were too low for detection of an additive effect between calcium and AG1478.
Calcium supplementation increases susceptibility to toxicity.
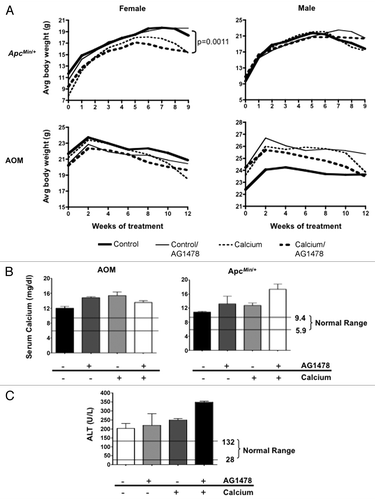
Potential adverse effects of supplemental calcium in combination with EGFR inhibitor therapy were evaluated using standard clinical parameters. Weekly and biweekly body weight was measured for ApcMin/+ and AOM-treated mice, respectively (). Female ApcMin/+ mice receiving calcium supplementation experienced weight loss during the later period of treatment; a similar albeit not significant trend was observed in females with the AOM model. Overall, considering both models and sexes, calcium supplementation did not have a significant impact on early death when compared with non-calcium supplemented diets (11.1 vs. 12.5%). However, closer examination of the group that benefited most from the combinatorial treatment, the AOM females, revealed that significantly more mice died or were euthanized before the specific experimental endpoint (24% of calcium-treated vs. 14% of no calcium treatment, p = 0.013; data not shown).
Standard clinical chemistry tests were performed to investigate the declining health of calcium treated mice. Typically calcium is tightly regulated in the body, maintaining homeostatic levels in the serum. However, both AG1478 (p = 0.011) and calcium (p = 0.014) were associated with increased serum calcium concentrations compared with control mice in both mouse models (). In addition, serum alanine aminotransferase (ALT) levels, an indicator of potential liver toxicity, were elevated in AOM-treated mice given supplemental calcium compared with the control group (p = 0.0003; ). At the end of treatment, blood glucose levels were measured. Levels for both models and all treatment groups stayed within normal ranges, though ApcMin/+ mice were at the high end of normal, while AOM mice were at the lower end. This is consistent with the increased susceptibility to diet-induced obesity generally observed in C57BL/6J mice, the strain background used for the ApcMin/+ model, compared with A/J mice, the strain background used for the AOM model. AG1478 is associated with glucose levels in both models but in opposite directions, with decreasing glucose in ApcMin/+ (p = 0.0012) and increasing glucose in AOM-treated mice (p = 0.023). When data from both models is combined, a trend toward an association between calcium intake and glucose reduction is also observed (p = 0.062). These observations are consistent with previously shown associations of both calcium and EGFR with glucose transport.
Discussion
Nutritional supplements have been on the rise in recent years as people become more health conscience. Reports in the lay press tout their benefits and even suggest that some dietary supplements may be beneficial in treating or preventing cancer. An estimated 50% of cancer patients take some form of dietary supplementation.Citation24 However, the impact of dietary supplements on established cancers and, perhaps more importantly, their potential interaction with conventional therapies is not well known.
To date literature reporting the usefulness of dietary supplements in complementing or replacing conventional treatments for cancer has been inconsistent and incomplete. While many molecular targets have been identified for these dietary supplements, their efficacy in treating or preventing cancer is unclear, in large part due to the difficulty faced when studying supplements and diets in humans. Perhaps more importantly, their impact on cancer therapies, and on general health at high doses, is not well understood.
The current study was designed to investigate the impact of supplemental calcium on molecule-targeted therapies for CRC. Calcium alone has been intensely studied and is known to have many roles in cell physiology, as well as interactions with pathways involved in cancer. It has been suggested that calcium possesses antitumor effects, but the mechanism is unclear. Some theories involving the role of calcium in colonic tumorigenesis include increasing apoptosis or inhibition of proliferation in the colon, and binding bile salts that result from diets high in fat and have been associated with colonic carcinogenesis.Citation25
In this study we observed an antitumor effect with calcium treatment in human CRC cell lines. The stronger effect observed in Caco-2 cells may reflect the higher levels of CaR reported in more differentiated tumors, since Caco-2 cells were derived from a less progressed tumor than HCT116.
In vivo mouse models for CRC did not reveal a similar effect on tumor growth. However, in both in vivo and in vitro models an additive effect is observed when combining EGFR-targeted inhibitor AG1478 with calcium. This is especially interesting in the ApcMin/+ model where mice are unresponsive to AG1478 or calcium alone, but small intestinal tumor size and colonic tumor number are reduced in the combinatorial treatment. In the AOM model this additive effect is only observed in females.
It appears that calcium may be more effective in the distal region of the intestinal tract since reduction in tumor number is only observed in the colons of ApcMin/+ and AOM-treated mice on AG1478 + calcium. However, calcium alone does appear to reduce tumor size in the small intestine of ApcMin/+ mice.
Finally, a sex-dependent effect on both EGFR signaling and tumor reduction was observed in the mouse models. Among the pathways that crosstalk with calcium and its receptor CaR is EGFR, a pathway commonly deregulated in CRC. Calcium has been shown to influence EGFR signaling through G-protein coupled receptors and PKC. Due to sex differences observed here, it is also possible that an interaction between CaR, EGFR and estrogen receptors (ER) is contributing to the variable sex results with regards to AOM tumor reduction and EGFR signaling revealed through protein gel blot analysis.
In addition to the benefits of calcium supplementation, potential negative effects on overall health must be considered. Treatment with a high dose of calcium appeared to cause hypercalcemia, indicated by increased serum calcium and decreased body weight. Liver toxicity also appeared to be higher according to ALT levels.
Materials and Methods
Mice and treatments.
Equal numbers of male and female A/J mice were obtained from The Jackson Laboratory and maintained on standard mouse chow (LabDiet 5058). At 4 months of age mice were injected weekly with AOM (10 mg/kg body weight) for 6 weeks. One week after the last AOM injection mice were randomly assigned to control (0.05% calcium) or calcium supplemented (5% calcium) Western-style diet (based on Research Diets D12450B) with or without 144 mg/kg chow of EGFR-inhibitor AG1478 (LC Labs) for 12 weeks. At the end of treatment mice were euthanized by CO2 asphyxiation and colons were flushed with phosphate buffered saline (PBS), splayed on bibulous paper, and scored for tumor number, size and location.
C57BL/6J-ApcMin/+ males were mated to wild-type B6 females, and progeny containing the ApcMin/+ allele were weaned at three weeks of age and assigned to one of the same four diets used for the AOM model. An equal number of males and females were used in each group. After nine weeks of treatment mice were euthanized by CO2 asphyxiation and the entire intestinal tract was flushed with PBS and splayed on bibulous paper. The intestine was divided into four sections: small intestine (duodenum, jejunum and ileum) and large intestine (colon) and scored for tumor number and size.
Prior to euthanasia mice were injected with 10 ml/g body weight of 5 mM phosphatase inhibitor sodium orthovanadate activated with 50 mM hydrogen peroxide 15 min prior to use.Citation26 Five minutes after injection, livers were removed and snap frozen in liquid nitrogen. For protein gel blot analysis, total protein was extracted with lysis buffer containing 10 mM TRIS-HCl (pH 8), 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 1% NP-40, 10% glycerol, 0.1% SDS, 0.5% sodium deoxycholate, 1 mM PMSF, 10 µg/ml leupeptin, 10 'g/ml aprotinin, 1 mM Na3VO4, and 1 mM NaF. A Bradford protein assay was used to determine protein concentration (Bio-Rad).
Body weight was monitored biweekly for the AOM model and weekly for the ApcMin/+ model. Food weight was recorded at the beginning and end of treatment for ApcMin/+ mice and biweekly for AOM mice to estimate consumption. At euthanasia blood glucose was measured using a FreeStyle Glucose Meter (Abbott) and serum was collected for measurement of alanine aminotransferase (ALT) and calcium levels by the UNC Animal Clinical Chemistry Core Facility.
All mice were housed in an AAALAC approved-facility and provided chow and water ad libitum. All experiments were approved by the Institutional Animal Care and Use Committee.
Cell lines and treatments.
Human colorectal adenocarcinoma cells Caco-2 and carcinoma cells HCT116 were obtained from the American Type Culture Collection (ATCC). Cell lines were maintained in RPMI-1640+L-glutamine medium (Invitrogen) supplemented with 10% fetal bovine serum, 2.0 mM L-glutamine, penicillin (10,000 U/mL), and streptomycin (10,000 U/mL). Cells were grown at 37°C in 5% CO2.
Cells were treated with a dose range of 0.424 mM (base concentration in media) to 5.5 mM calcium chloride (Sigma-Aldrich) dissolved in media immediately before use. For EGFR inhibitor treatment, AG1478 (LC Labs) was dissolved in DMSO and cells were treated with a dose range of 0–15 µM with a final DMSO concentration of 0.1%.
Cell viability was measured by seeding cells on 48-well plates (4.0 x 104 Caco-2 cells/well or 2.5 x 104 HCT116 cells/well) in triplicates 24 h before treatment. Cells were treated daily and viability data was obtained at t = 72 h after plating for Caco-2 cells, and t = 96 h after plating for HCT-116 cells.
Relative cell growth was assayed with an MTT Cell Growth Determination Kit (Sigma-Aldrich) according to manufacturer's instructions, and measured with a DU800 UV/Vis spectrophotometer (Beckman-Coulter). Relative cell viability was determined by normalizing to the control (0.1% DMSO treated) cells.
To collect protein lysates, cells were seeded at 5 x 105 cells/60 mm plate and 24 h later treated as described above with increasing doses of CaCl2 (0.424–5.5 mM) and with or without 4 µM (Caco-2) or 8 µM (HCT116) AG1478. Following 24 h of treatment protein was extracted with buffer containing 20 mM HEPES (pH 7.4), 150 mM NaCl, 10% Glycerol, 1% Triton X-100, 1 mM PMSF, 10 µg/ml leupeptin, 10 µg/ml aprotinin, 1 mM Na3VO4, and 1 mM NaF. A Bradford protein assay was used to determine protein concentration (Bio-Rad).
Protein gel blot analysis.
Thirty micrograms of liver protein for the in vivo models or 15 mg of human CRC cell lysate for the in vitro model was loaded onto 4–15% gradient TRIS-HCl gels (Bio-Rad). After transfer to PVDF membranes for one hour, membranes were blocked for 1 h in 5% BSA/TBST (containing 0.1% Tween) for rabbit anti-pEGFR and pMAPK1/3 (p42/44) (Cell Signaling) or 5% milk/TBST for sheep anti-EGFR (Millipore) and mouse anti-ACTB (β-actin) (Sigma-Aldrich). Membranes were incubated with primary antibody overnight at 4°C, anti-EGFR and pMAPK1/3 antibodies were diluted 1:2,000 in 1% BSA/TBST, EGFR diluted 1:5,000 and ACTB diluted 1:10,000 in 5% milk/TBST. After washing, membranes were incubated in secondary antibodies for one hour at room temperature. Secondary antibodies (Jackson ImmunoResearch) were diluted 1:20,000 in the same solution as the primary antibody. SuperSignal West Dura Extended Duration ECL (Pierce) was used for visualization of proteins. Three replicates were performed per treatment and model (and sex for mouse models).
ELISA.
Cells were plated onto 60 mm plates and treated with AG1478 and/or calcium chloride as described above. Following two hours of calcium treatment, 100 ng/ml EGF (R&D Systems) was added. After a 20 min EGF treatment, protein was isolated as described above. The STAR phospho-EGFR (Tyr1173) ELISA Kit (Millipore) was used according to manufacturer's instructions. Two replicates of 50 mg each were used.
Statistical analysis.
Tumor number and size was analyzed using a non-parametric Mann-Whitney test (Prism 4.0, GraphPad Software). Linear mixed models were performed to assess whether calcium levels and inhibitor on absorbance levels were independent (additive). Finally, stratified analyses were performed by cell type to assess whether evidence for any associations between calcium levels/inhibitor and absorbance levels was present in either cell type.
Analysis of covariance (ANCOVA) models was performed to test whether model (AOM, ApcMin/+), sex, calcium intake and inhibitor were associated with glucose levels, serum calcium levels and ALT. Tests of interactions were also performed.
A non-parametric Mann-Whitney test was performed on final body weight for mice in each treatment group (calcium and and non-calcium), separated by sex. Only comparisons that were significant after correcting for multiple tests within each experiment are shown.
Conclusion
We conclude that the impact of dietary supplementation on conventional cancer therapies should be given consideration in future preclinical and clinical trials. We observed using both human CRC cell lines and mouse models of CRC that calcium appears to enhance EGFR inhibitor therapy. However, this comes at an elevated incidence of toxicity indicating that combinations of conventional therapies with dietary supplements should be closely monitored.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author Contributions
E.S.R. and D.W.T. designed all experiments, and wrote edited the manuscript. E.S.R. and E.B. performed all experiments.
Abbreviations
| AOM | = | azoxymethane |
| CRC | = | colorectal cancer |
| EGFR | = | epidermal growth factor receptor |
| pEGFR | = | phosphorylated EGFR |
| CaR | = | calcium-sensing receptor |
| CDH1 | = | E-cadherin |
| CTNNB | = | β-catenin |
| RCT | = | randomized controlled trials |
| GPCR | = | G-protein coupled receptors |
| ALR | = | alanine aminotransferase |
| ER | = | estrogen receptors |
Figures and Tables
Figure 1 Experimental design. At weaning (ApcMin/+) or 1 week following the last carcinogen treatment (AOM) mice were randomly assigned to a control (0.05% calcium) or calcium (5% calcium) supplemented diet, with or without small-molecule EGFR inhibitor AG1478. ApcMin/+ mice were treated for nine weeks and body weight and food consumption was measured at the beginning and end of treatment. AOM mice were treated for four months and body weight and food were monitored biweekly throughout the treatment period.
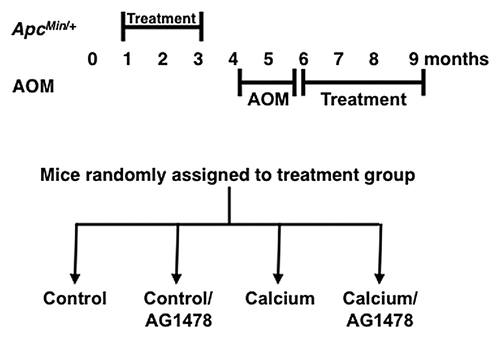
Figure 2 Effect of calcium on AG1478-mediated tumor (A) number and (B) size in ApcMin/+ and (C) tumor number in AOM models. Values are expressed as a percent of control treatment. Number of mice (A and C) and number of tumors (B) are displayed in bars of graph. Equal numbers of males and females were used and data for both sexes are shown. (C) A significant difference in tumor number is observed in AG1478 compared with Calcium/AG1478 treated mice only in females, but data for both sexes is shown. *p < 0.001; **p < 0.0001. Error bars show SEM.
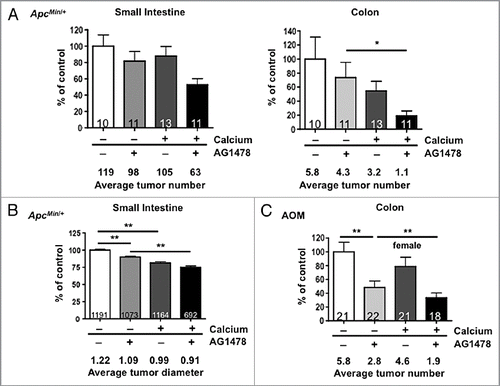
Figure 3 MTT assays of Caco-2 and HCT116 cell viability treated with AG1478 and/or calcium. (A) Calcium chloride and (B) AG1478 decrease cell viability compared with the control (no treatment) in a dose dependent manner. Supplementing a constant dose of AG1478 with increasing doses of calcium chloride results in reduced cell viability in an additive manner in both (C) Caco-2 and (D) HCT116 cells. Numbers within the bars indicate percent reduction when compared with control untreated cells. Error bars show SD.
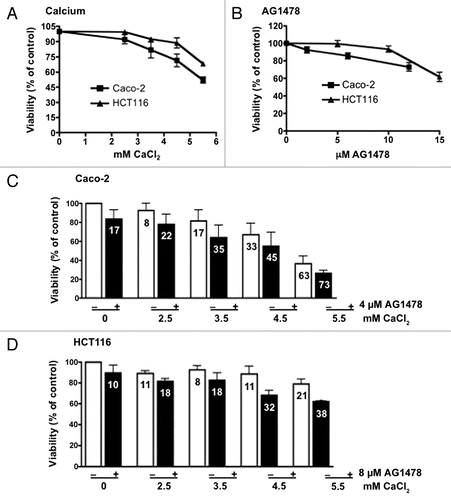
Figure 4 Effect on signaling after AG1478 and calcium treatment. (A) In vivo: mice maintained on a control or calcium supplemented diet, with or without AG1478, were injected with phosphatase inhibitor prior to euthanasia. EGFR activation was measured in liver protein lysates from ApcMin/+ and AOM mice, separately for each strain and sex. (B) In vitro: protein was extracted from human CRC cells lines after a 4 h treatment with or without 4 µM (Caco-2) or 8 µM AG1478 (HCT116) with 3.5 mM (“+”) and 4.5 mM (“++”) calcium chloride. (C) pEGFR ELISA was performed using protein extracted from cells lines after 2 h treatment with or without 4 µM (Caco-2) or 8 µM AG1478 (HCT116) with 3.5 mM (“+”) and 4.5 mM (“++”) calcium chloride followed by 20 min treatment with EGF.
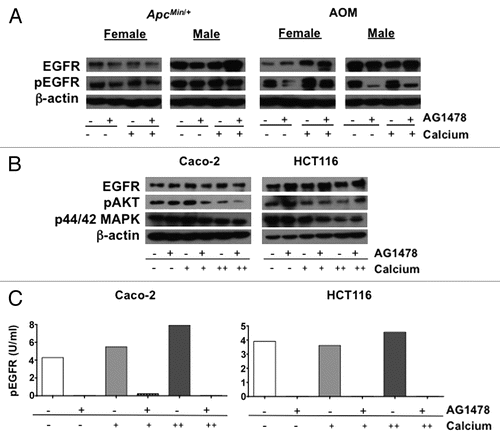
Figure 5 General health of mice. (A) Comparison of body weight change for ApcMin/+ and AOM mice maintained on a control (solid lines) or calcium (dashed lines) supplemented diets, with or without AG1478. Blood collected at the end of treatment was used for measurement of (B) serum calcium and (C) alanine aminotransferase (ALT) levels. The normal lower and upper levels are indicated for each test. Error bars show SEM.
Acknowledgments
Support was provided by National Institutes of Health grants CA105417 and CA092479 (DWT) and fellowship AT002835 (ESR). Infrastructure was supported by center grants CA016086 and DK034987. We would like to extend our gratitude to Dr. Ethan Lange of the Department of Genetics at UNC for consultations regarding the statistical analysis for this study.
References
- Xue L, Lipkin M, Newmark H, Wang J. Influence of dietary calcium and vitamin D on diet-induced epithelial cell hyperproliferation in mice. J Natl Cancer Inst 1999; 91:176 - 181; PMID: 9923860; http://dx.doi.org/10.1093/jnci/91.2.176
- Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, et al. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis 2001; 22:1871 - 1875; PMID: 11698351; http://dx.doi.org/10.1093/carcin/22.11.1871
- Yang K, Kurihara N, Fan K, Newmark H, Rigas B, Bancroft L, et al. Dietary induction of colonic tumors in a mouse model of sporadic colon cancer. Cancer Res 2008; 68:7803 - 7810; PMID: 18829535; http://dx.doi.org/10.1158/0008-5472.CAN-08-1209
- Chakrabarty S, Wang H, Canaff L, Hendy GN, Appelman H, Varani J. Calcium sensing receptor in human colon carcinoma: interaction with Ca(2+) and 1,25-dihydroxyvitamin D(3). Cancer Res 2005; 65:493 - 498; PMID: 15695391
- Chakrabarty S, Radjendirane V, Appelman H, Varani J. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of beta-catenin/TCF activation. Cancer Res 2003; 63:67 - 71; PMID: 12517779
- Weingarten MA, Zalmanovici A, Yaphe J. Dietary calcium supplementation for preventing colorectal cancer and adenomatous polyps. Cochrane Database Syst Rev 2005; CD003548; PMID: 16034903
- Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, et al. Calcium Polyp Prevention Study Group. Calcium supplements for the prevention of colorectal adenomas. N Engl J Med 1999; 340:101 - 107; PMID: 9887161; http://dx.doi.org/10.1056/NEJM199901143400204
- Bonithon-Kopp C, Kronborg O, Giacosa A, Rath U, Faivre J. European Cancer Prevention Organisation Study Group. Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: a randomised intervention trial. Lancet 2000; 356:1300 - 1306; PMID: 11073017; http://dx.doi.org/10.1016/S01406736(00)02813-0
- Grau MV, Baron JA, Sandler RS, Wallace K, Haile RW, Church TR, et al. Prolonged effect of calcium supplementation on risk of colorectal adenomas in a randomized trial. J Natl Cancer Inst 2007; 99:129 - 136; PMID: 17227996; http://dx.doi.org/10.1093/jnci/djk016
- Richter F, Newmark HL, Richter A, Leung D, Lipkin M. Inhibition of Western-diet induced hyperproliferation and hyperplasia in mouse colon by two sources of calcium. Carcinogenesis 1995; 16:2685 - 2689; PMID: 7586187; http://dx.doi.org/10.1093/carcin/16.11.2685
- Penman ID, Liang QL, Bode J, Eastwood MA, Arends MJ. Dietary calcium supplementation increases apoptosis in the distal murine colonic epithelium. J Clin Pathol 2000; 53:302 - 307; PMID: 10823127; http://dx.doi.org/10.1136/jcp.53.4.302
- Viñas-Salas J, Biendicho-Palau P, Pinol-Felis C, Miguelsanz-Garcia S, Perez-Holanda S. Calcium inhibits colon carcinogenesis in an experimental model in the rat. Eur J Cancer 1998; 34:1941 - 1945; PMID: 10023319; http://dx.doi.org/10.1016/S0959-8049(98)00197-X
- Harari PM, Allen GW, Bonner JA. Biology of interactions: antiepidermal growth factor receptor agents. J Clin Oncol 2007; 25:4057 - 4065; PMID: 17827454; http://dx.doi.org/10.1200/JCO.2007.11.8984
- Takahashi M, Wakabayashi K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci 2004; 95:475 - 480; PMID: 15182426; http://dx.doi.org/10.1111/j.13497006.2004.tb03235.x
- Kaiser S, Park YK, Franklin JL, Halberg RB, Yu M, Jessen WJ, et al. Transcriptional recapitulation and subversion of embryonic colon development by mouse colon tumor models and human colon cancer. Genome Biol 2007; 8:R131; PMID: 17615082; http://dx.doi.org/10.1186/gb-2007-8-7-r131
- Malecka-Panas E, Fligiel SE, Relan NK, Dutta S, Majumdar AP. Azoxymethane enhances ligand-induced activation of EGF receptor tyrosine kinase in the colonic mucosa of rats. Carcinogenesis 1996; 17:233 - 237; PMID: 8625444; http://dx.doi.org/10.1093/carcin/17.2.233
- Torrance CJ, Jackson PE, Montgomery E, Kinzler KW, Vogelstein B, Wissner A, et al. Combinatorial chemoprevention of intestinal neoplasia. Nat Med 2000; 6:1024 - 1028; PMID: 10973323; http://dx.doi.org/10.1038/79534
- Roberts RB, Min L, Washington MK, Olsen SJ, Settle SH, Coffey RJ, et al. Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis. Proc Natl Acad Sci USA 2002; 99:1521 - 1526; PMID: 11818567; http://dx.doi.org/10.1073/pnas.032678499
- Moran AE, Hunt DH, Javid SH, Redston M, Carothers AM, Bertagnolli MM. Apc deficiency is associated with increased Egfr activity in the intestinal enterocytes and adenomas of C57BL/6J-Min/+ mice. J Biol Chem 2004; 279:43261 - 43272; PMID: 15294912; http://dx.doi.org/10.1074/jbc.M404276200
- Tfelt-Hansen J, Yano S, John Macleod R, Smajilovic S, Chattopadhyay N, Brown EM. High calcium activates the EGF receptor potentially through the calcium-sensing receptor in Leydig cancer cells. Growth Factors 2005; 23:117 - 123; PMID: 16019433; http://dx.doi.org/10.1080/08977190500126272
- Tomlins SA, Bolllinger N, Creim J, Rodland KD. Crosstalk between the calcium-sensing receptor and the epidermal growth factor receptor in Rat-1 fibroblasts. Exp Cell Res 2005; 308:439 - 445; PMID: 15950968; http://dx.doi.org/10.1016/j.yexcr.2005.04.032
- Shen Y, Hirsch DS, Sasiela CA, Wu WJ. CDC42 regulates E-cadherin ubiquitination and degradation through an EGF receptor to SRC-mediated pathway. J Biol Chem 2008; 283:5127 - 5137; PMID: 18057010; http://dx.doi.org/10.1074/jbc.M703300200
- Sato T, Pallavi P, Golebiewska U, McLaughlin S, Smith SO. Structure of the membrane reconstituted transmembrane-juxtamembrane peptide EGFR(622–660) and its interaction with Ca2+/calmodulin. Biochemistry 2006; 45:12704 - 12714; PMID: 17042488; http://dx.doi.org/10.1021/bi061264m
- Werneke U, Earl J, Seydel C, Horn O, Crichton P, Fannon D. Potential health risks of complementary alternative medicines in cancer patients. Br J Cancer 2004; 90:408 - 413; PMID: 14735185; http://dx.doi.org/10.1038/sj.bjc.6601560
- Holt PR. Studies of calcium in food supplements in humans. Ann N Y Acad Sci 1999; 889:128 - 137; PMID: 10668489; http://dx.doi.org/10.1111/j.1749-6632.1999.tb08730.x
- Ruff SJ, Chen K, Cohen S. Peroxovanadate induces tyrosine phosphorylation of multiple signaling proteins in mouse liver and kidney. J Biol Chem 1997; 272:1263 - 1267; PMID: 8995430; http://dx.doi.org/10.1074/jbc.272.2.1263