Abstract
Nlp (ninein-like protein), an important molecule involved in centrosome maturation and spindle formation, plays an important role in tumorigenesis and its abnormal expression was recently observed in human breast and lung cancers. In this study, the correlation between overexpression of Nlp and paclitaxel chemosensitivity was investigated to explore the mechanisms of resistance to paclitaxel and to understand the effect of Nlp upon apoptosis induced by chemotherapeutic agents. Nlp expression vector was stably transfected into breast cancer MCF-7 cells. With Nlp overexpression, the survival rates, cell cycle distributions and apoptosis were analyzed in transfected MCF-7 cells by MTT test and FCM approach. The immunofluorescent assay was employed to detect the changes of microtubule after paclitaxel treatment. Immunoblotting analysis was used to examine expression of centrosomal proteins and apoptosis associated proteins. Subsequently, Nlp expression was retrospectively examined with 55 breast cancer samples derived from paclitaxel treated patients. Interestingly, the survival rates of MCF-7 cells with Nlp overexpressing were higher than that of control after paclitaxel treatment. Nlp overexpression promoted G2-M arrest and attenuated apoptosis induced by paclitaxel, which was coupled with elevated Bcl-2 protein. Nlp expression significantly lessened the microtubule polymerization and bundling elicited by paclitaxel attributing to alteration on the structure or dynamics of β-tubulin but not on its expression. The breast cancer patients with high expression of Nlp were likely resistant to the treatment of paclitaxel, as the response rate in Nlp negative patients was 62.5%, whereas it was 58.3 and 15.8% in Nlp (+) and Nlp (++) patients respectively (p = 0.015). Nlp expression was positively correlated with those of Plk1 and PCNA. These findings provide insights into more rational chemotherapeutic regimens in clinical practice, and more effective approaches might be developed through targeting Nlp to increase chemotherapeutic sensitivity.
Introduction
Breast cancer is the most frequently diagnosed cancer in women. In the United States and Europe, women with breast cancer account for 25–30% in all kinds of female cancers. In China, the incidence of breast cancer is secondary to that of lung cancer in female cancers. Recently, many improvements were made in early diagnosis and treatment approaches, and the mortality of breast cancer began to decrease gradually. Among them, chemotherapy played a very important role in reducing the rates of recurrence and death of breast cancer patients. Although proactive treatments were given, about 30% of breast cancer patients still suffered from recurrence and metastasis. Chemotherapy is well established as one of the major treatment modalities for metastatic breast cancer patients. Response rates to first line chemotherapy with anthracyclines, taxanes, capecitabine, vinorelbine, and gemcitabine range from 25–60%, with the median time to progression averaging approximately 6 mo.Citation1 But it is disappointing that chemotherapy will fail sooner or later. One of reasons is that tumor cells may become resistant to the chemotherapy agents. Resistance could be attributed to the overexpression of MDR (multiple drug resistant protein) and MRP (multiple resistant protein),Citation2,Citation3 the changes of activities of drug metabolism enzymes,Citation4,Citation5 the upregulation of anti-apoptosis genes,Citation6 and enhancement of activities of transcription factors,Citation7 etc. The mechanisms of resistance to anticancer drugs were too complicated to be clearly understood until now. Lack of effective solutions is the most common reason of failure of chemotherapy.
Recently, abnormalities of centrosomes have been found in most human tumors and have been thought to play an important role in the initiation and development of cancers.Citation8-Citation11 A number of investigations in invasive breast tumors have shown that 60–80% of cancers are aneuploid and approximately 80% exhibit amplified centrosomes. Centrosome amplification and the associated chromosomal instability may greatly contribute to breast cancer development and progression.Citation12 Thus, centrosome abnormalities may have clinical diagnostic and/or prognostic value and centrosomes may also be a potential target for cancer therapy. Currently, the correlation between the abnormal expression of centrosome proteins and sensitivity to chemotherapeutic agents has attracted more attention in the field.Citation13-Citation15 With the abnormal expression of centrosome proteins, the tumor cells may develop resistance to the apoptosis induced by DNA-damage agents, and spindle poisons. Therefore, we assume that the abnormal expression of centrosome proteins might influence the sensitivity of cancer cells to chemotherapy agents since abnormal centrosomes would result in dyspoiesis of mitosis spindle, which disrupts the therapeutic targets of spindle poisons, such as taxane and vinblastine.
Aurora-A and Plk1 (polo-like kinase 1) are important kinases located in the centrosome. The abnormal expression of Aurora-A and Plk1 disrupts the formation of mitosis spindle.Citation16 Cells with abnormal chromosomes, namely heteroploidy can override the spindle checkpoint because of the dysfunction of the checkpoint.Citation15-Citation17 Abnormal expression of Aurora-A and Plk1 is not only related to the development of tumor, but also to the resistance of chemotherapy agents, especially taxane.Citation18 Inhibition of Aurora-A and Plk1 by interfering RNA technique or kinase inhibitors can bring cancer cells back to sensitivity to chemotherapy.Citation13,Citation19-Citation22
BRCA1 is an important tumor suppressor gene and is associated with the sensitivity of chemotherapy agents.Citation23 The upregulation of BRCA1 leads to a higher sensitivity to DNA-damaged drugs, such as cisplatin.Citation24 But dysfunction of BRCA1 is associated with decrease of sensitivity to spindle poisons, such as taxane.Citation25,Citation26 In addition, the inactivation of JNK pathway due to BRCA1 dysfunction results in disrupted apoptosis which caused by spindle poisons, thus contributing to chemoresistance.Citation27 The MCF-7 transfected with BRCA1 targeting siRNA showed great resistance to paclitaxel compared with control.
Most recently, we have found that the centrosomal protein Nlp is a BRCA1-interacting protein and plays a critical role in the control of mitotic progression and chromosomal segregation.Citation28-Citation31 Nlp is found to be overexpressed in about 80% of human breast and lung carcinomas, and its deregulated expression is associated with NLP gene amplification in human lung cancer. Overexpression of Nlp is able to induce NIH3T3 transformation, and Nlp transgenic mice displayed spontaneous tumorigenesis.Citation32 In this study, we investigated Nlp’s role in the therapeutic sensitivity to spindle poisons and found that its overexpression substantially decreases chemosensitivity to paclitaxel. Furthermore, we have also characterized the possible mechanism by which Nlp affects therapeutic sensitivity of tubulin-stabilizing agents.
Results
Establishment of Nlp overexpressing cell lines
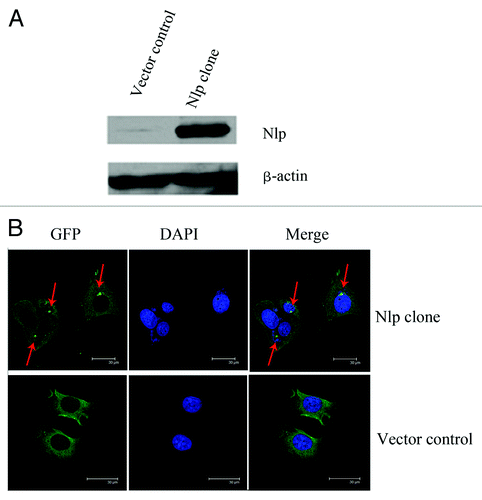
pEGFP-Nlp plasmid and pEGFP-C3 plasmid were transfected into MCF-7 cells to generate MCF7-GFP-Nlp cells and MCF7-GFP cells respectively. The expression of Nlp was examined by protein gel blot analysis. As shown in , expression of Nlp in MCF7-GFP-Nlp cells was much higher than that in control cells (MCF7-GFP) (). In addition, using immunofluoresent microscopy method, Nlp protein was observed to localize in centrosome (), which is similar to what we have reported recently.Citation28
Figure 1. Nlp locates in centrosome. (A) The identification of transfected cell lines: MCF-7 breast cancer cell line was transfected by pEGFP-C3 and pEGFP-Nlp respectively. Nlp clones and control were detected by protein gel blot analysis. β-Actin was used as an internal standard to indicate equal loading for each clone. (B) The localization of Nlp in centrosome was shown by immunofluoresent approach (red arrow marked bright green points).
Nlp overexpression confers breast cancer cells resistance to paclitaxel
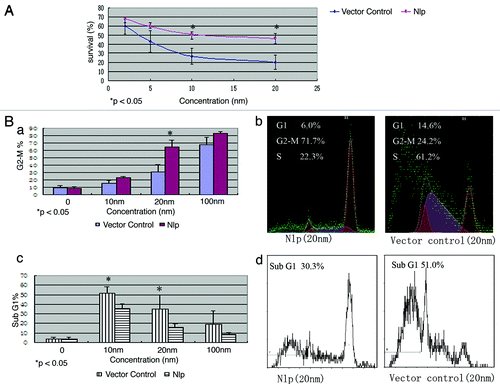
To examine whether Nlp expression affects the sensitivity of breast cancer cells to the treatment of paclitaxel, MCF7-GFP-Nlp and MCF7-GFP were treated with paclitaxel for 72 h in different concentrations (0, 2, 5, 10 and 20 nmol/L). The survival rates were examined by MTT approach. The results showed that survival rates in MCF7-GFP-Nlp cells were higher than that in MCF7-GFP cells. As indicated in , with paclitaxel treatment the survival rates of MCF7-GFP-Nlp cells and MCF7-GFP cells were 51.0 ± 5.2% and 26.7 ± 8.5% respectively at the concentration of 10 nmol/L (p < 0.05) and changed to 46.4 ± 6.1% and 20.0 ± 7.8% respectively (p < 0.05) at the 20 nmol/L. These results suggested that overexpression of Nlp might confer MCF-7 cells resistance to paclitaxel.
Figure 2. Nlp enhanced G2-M phase arresting but induced less Sub G1 phase in transfected cells. (A) Nlp overexpression facilitate resistance to paclitaxel in transfected breast cancer cells. Paclitaxel with different concentrations were administered in Nlp transfected cells and control and then analyzed by MTT approach. At least three independent experiments were done and the average values were calculated. (B) Nlp enhanced G2-M phase arresting in transfected breast cancer cells but induced less Sub G1 phase analyzed by FCM. (a-d) With different concentrations, transfected cells were treated by paclitaxel for 24 h, the percentages of G2-M phase cells and Sub G1 phase cells in two transfected cells were analyzed by FCM approach. (a) Quantitative analyses of G2-M phase cells, in which the assays were repeated at least three times. (b) A representative figure of cell cycle G2 analyses. (c) Quantitative analyses of Sub G1 phase cells, in which the assays were repeated at least three times. (d) A representative figure of Sub-G1 analyses.
Nlp expression enhances cells arrest in G2-M phase but suppresses apoptosis following treatment with paclitaxel
The cell cycle phase and apoptosis in MCF7-GFP-Nlp and MCF7-GFP were analyzed by flow cytometry after treatment by paclitaxel with different concentrations.
With different concentrations (0, 10, 20 and 100 nmol/L) MCF7-GFP-Nlp and MCF7-GFP were treated by paclitaxel for 24 h, the cell cycle analyses revealed that the populations of G2-M phase cells in MCF7-GFP-Nlp were obviously higher than that of MCF7-GFP. The percentages of G2-M phase cells in MCF7-GFP-Nlp and MCF7-GFP were 65.2 ± 9.3% and 33.2 ± 9.9% respectively at the concentration of 20 nmol/L (p < 0.05) ().
We also examined the populations of Sub G1 phase cells in MCF7-GFP-Nlp and MCF7-GFP with flow cytometry. The percentage of Sub G1 cells in MCF7-GFP was more than that in MCF7-GFP-Nlp after treatment of paclitaxel for 24 h at different concentrations. The percentages of Sub G1 phase cells in MCF7-GFP and MCF7-GFP-Nlp were 51.7 ± 6.8 vs. 35.7 ± 4.7% at the concentration of 10 nmol/L and changed to 35.0 ± 14.8 vs. 15.8 ± 3.9% at 20 nmol/L respectively (p < 0.05) ().
Nlp protects breast cancer cells from apoptosis through suppressing activity of microtubule polymerization elicited by paclitaxel
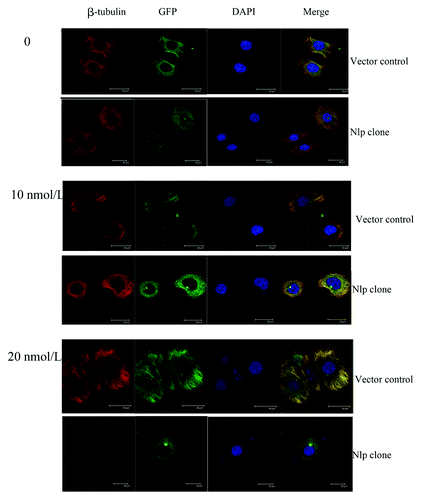
Given that the MCF7-GFP-Nlp was more resistant to paclitaxel-induced apoptosis than control, we then investigated the mechanism involved in Nlp-generated drug resistance. As shown in , after the treatment of paclitaxel with different concentrations (0, 10 and 20 nmol/L) for 24 h, the polymerization of β-tubulin polymerized to form bundles was observed. Clearly MCF7-GFP-Nlp cells exhibit much less alterations of tubulin bundling and apoptosis phenotype, suggesting Nlp might lessen the ability of paclitaxel depressing depolymerization of microtubule and resist apoptosis induced by paclitaxel.
Figure 3. Nlp protected breast cancer cells from cytotoxic apoptosis through suppressing activity of microtubule polymerization elicited by paclitaxel. Two transfected cells were treated by paclitaxel at different concentrations. Cells (attached cells plus those floating in the medium) were harvested and then were immunofluorescence stained by β-tubulin (red) antibody and DAPI (blue) to observe the changes of cytoskeleton after the paclitaxel treatment. The phenomena of microtubules polymerizing into bundles were clearer in the vector control compared with the Nlp transfected clone.
Nlp might upregulate the expressions of anti-apoptosis proteins such as Bcl-2
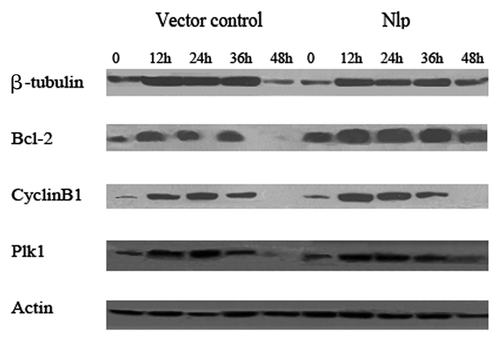
We next examined the expression of several proteins involved in cell cycle regulation and apoptosis and found that the expression levels of β-tubulin, Cyclin B1, Plk1 and Bcl-2 increased to some extent following the treatment of paclitaxel for various times (0, 12, 24, 36 and 48 h) in MCF7-GFP-Nlp and MCF7-GFP. However, the increased levels of Bcl-2 appeared to be most significant in MCF7-GFP-Nlp (), suggesting that overexpression of Nlp in MCF-7 cells might promote cellular capability of anti-apoptosis
Figure 4. Nlp might upregulate the expressions of Bcl-2 protein. Two transfected cells were treated by paclitaxel for different time followed by extracting their proteins. One hundred micrograms of whole-cell protein was used for protein gel blot analysis with anti-β-tubulin, anti-Bcl-2, anti-Plk1, anti-Cyclin B1 antibody as described in materials and methods. No differences of expressions of β-tubulin, Cyclin B1 and Plk1 in two transfected cells were shown, whereas, the expression of Bcl-2 increased significantly in Nlp transfected clone.
The expression of Nlp is closely correlated with paclitaxel chemosensitivity
To investigate whether Nlp overexpression is correlated with therapeutic sensitivity of paclitaxel in breast cancer patients, we retrospectively analyzed Nlp expression in tissue samples of breast cancer patients. These advanced patients were administered with chemotherapy consisting of paclitaxel as first line chemotherapy. Totally 55 tumor specimens were collected and examined by immunohistochemistry approach. The results showed that Nlp mainly expressed in cytoplasma ().
Figure 5. Nlp expression in human breast cancer tissues. The samples derived from breast cancer patients were collected and subjected to immunohistochemical staining with anti-Nlp antibody, which indicates Nlp mainly expressed in cytoplasm.
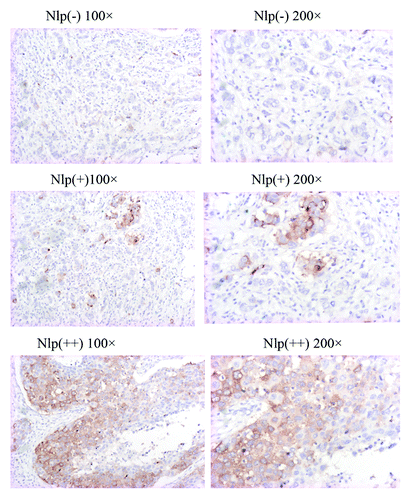
Among 55 human breast cancer patient samples, the positive cytoplasmic staining (+–++) of Nlp protein was detected in 31 tumors (56.4%). Negative staining (-) was seen in 24 tumor samples (43.6%).
We found the expression of Nlp protein was strongly related to paclitaxel chemosensitivity. The response rate in Nlp negative patients was 62.5%, but it was 58.3 and 15.8% in Nlp (+) and Nlp (++) patients respectively (p = 0.015, ).
Table 1. Correlations between expression of Nlp protein and paclitaxel sensitivity
The expression of Nlp protein is correlated with that of Plk1 and PCNA in breast cancer patients
In order to explore the correlation between expression of Nlp and other prognostic factors in breast cancer patients, tumor size (T), lymph node status (N), clinical stage, Her-2, ER, PR, P53, PCNA and Plk1 were analyzed. The results indicated that there was only correlation between expression of Nlp and that of PCNA and Plk1 (), the p value is 0.043 and 0.04.
Table 2. Correlations between expression of Nlp protein and other prognostic factors in breast cancer patients
Discussion
Deregulation of cell cycle regulators, including p53, BRCA1, Aurora-A, Plk1 and Cyclin B1, are closely associated with tumorigenesis and clinical therapeutic sensitivity as well.Citation33-Citation38 In addition, we have recently demonstrated that Nlp, a BRCA1-interacting centrosomal protein that functions in spindle formation and chromosome segregation, is upregulated in several types of human tumors and plays a role in the development of tumorigenesis.Citation28-Citation30,Citation32 In this report, we have shown that the cells expressing high level of Nlp were resistant to paclitaxel treatment compared with control cells. Using a MTT approach, we found the survival rates of MCF7-GFP-Nlp cells was higher than that of MCF7-GFP administrated with paclitaxel at different concentrations. These findings indicated that Nlp may confer resistance to the treatment of paclitaxel.
It is well-known that paclitaxel enhances the polymerization of tubulin to stabilize microtubules and also interacts directly with microtubules, stabilizing them against depolymerization, which readily depolymerize normal microtubules. Paclitaxel blocks cells in the G2-M phase of the cell cycle and such cells are unable to form a normal mitotic apparatus and then undergo apoptosis.Citation39,Citation40 Thus, we analyzed cell cycle distributions of G2-M cells in two transfected cells (control and Nlp expressing cells) after treatment of paclitaxel using Flow Cytometry (FCM), and found that the percentages of G2-M phase cells in Nlp clone were obviously higher than that in vector control, especially at the concentration of 20 nmol/L (65.2 ± 9.3 vs. 31.2 ± 9.9%, p < 0.05). These observations revealed that Nlp enhances G2-M phase arrest caused by paclitaxel. In contrast, percentages of Sub G1 phase cells in Nlp clone were predominantly lower than that in vector control especially in the concentration of 10 and 20 nmol/L (51.7 ± 6.8 vs. 35.7% and 35.0 ± 14.8 vs. 15.8 ± 3.9%, p < 0.05). These findings were in line with the results of MTT approach, and suggested that Nlp might participate in the regulation of cell cycle checkpoint and modulate proteins involved in apoptotic pathway. Likely, Nlp could arrest more cells in G2-M phase favoring the repair of those damaged cells. In support of this hypothesis, the percentages of apoptotic cells were lower in Nlp overexpressing cells than control cells after the treatment of paclitaxel. It is speculated that this phenomenon is because the arrested cells recover and enter the cell cycle again. The mechanism in depth is intriguing and is worthy of further study.
Many factors may disrupt the binding of paclitaxel to β-tubulin, including suppression of β-tubulin, changes of microtubules structure especially binding site and microtubules dynamics, and β-tubulin gene mutation.Citation41-Citation43 These factors will impair the cytotoxic effect of paclitaxel. Clearly, in the current study, after the treatment of paclitaxel, the polymerization of β-tubulin to form bundles was observed. However, MCF7-GFP-Nlp cells exhibit much less alterations of tubulin bundling. The expression of β-tubulin showed no difference in two transfected cell lines (Nlp expressing cells and vector control cells), suggesting Nlp might change microtubule structure or dynamics and then disrupt formation of spindle through demolished function of cell cycle checkpoint.
Bcl-2 is an anti-apoptosis protein that is related to resistance of paclitaxel.Citation44 Bcl-2 expression level is higher in Nlp clone compared with control without treatment of paclitaxel. After treatment of paclitaxel, Bcl-2 expression increased distinctively in Nlp clone compared with control. It suggests that Nlp might increase the expression level of Bcl-2 protein, thereby suppress apoptosis of Nlp overexpression cells treated with paclitaxel.
Importantly, the Nlp expression was closely linked to clinical therapeutic sensitivity. Through the retrospective study of clinical tissue samples from breast cancer patients treated by paclitaxel, we found that higher Nlp expressions were correlated with lower sensitivity for paclitaxel chemotherapy (p = 0.015, ). The response rates in Nlp negative patients were 62.5% but only 15.8% in Nlp (++) patients. The patients with Nlp overexpression were obviously resistant to paclitaxel-based chemotherapy. These findings went well along with experimental observations that dysregulated expression of Nlp attenuates paclitaxel-induced apoptosis. Interestingly, Nlp expression was positively correlated with Plk1 that is another important centrosomal kinase and an oncogenic protein as well (p = 0.04, ). It has been reported that Plk1 overexpression disrupts the formation of mitotic spindle and causes anueploidy.Citation17 Heteroploid cells with abnormal chromosome override mitotic checkpoint, which is not only related to carcinogenesis but also resistance to chemotherapy agents, especially taxane. It has been demonstrated that disruption of Plk1 through siRNA knockdown technique is able to recover sensitivity of tumor cells to chemotherapy agents. Additionally, we have found that Nlp expression was also correlated with PCNA (proliferative cell nuclear antigen), whose expression leads to short doubling time and fast proliferation.
In summary, we have demonstrated that overexpression of Nlp may confer breast cancer resistance to paclitaxel treatment. Likely, Nlp overexpression is able to alter cell cycle G2-M progression, disrupt formation of mitotic spindle, change structure and function of β-tubulin, increase Bcl-2 expression and then attenuate apoptosis induced by paclitaxel. The findings in this study have provided new insights into understanding of the molecular mechanism involved in chemotherapy resistance, and may lead to the development of new chemotherapeutic approach.
Materials and Methods
Transfection and identification of cell lines
We established Nlp overexpressing cells and control cells. pEGFP-C3 (empty plasmid)and pEGFP-Nlp (containing full length of Nlp cDNA) were transfected to breast cancer cell line MCF-7 respectively to establish control (MCF7-GFP), and Nlp overexpression clone (MCF7-GFP-Nlp). Stable transfectants were obtained by selection in the medium containing 400 ug/ml of G418 (Geneticin sulfate, GIBCO) for 14 d.
Cell culture and reagent
Human breast cancer MCF-7 cells and two stable transfected cells, MCF7-GFP and MCF7-GFP-Nlp cells were grown in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum, 100 unit/ml penicillin, 100 ug/ml streptomycin, and maintained at 37°C in humidified atmosphere of 5% CO2.
MTT assay for assessing cell viability
The cells viability was determined by the semiautomatic 3-(4,5-dimethyl-thiazol-2-yl)-2,5-Diphenyltetrazolium bromide (MTT, Sigma) assay. The dose-response curves were generated first. MCF7-GFP-Nlp and MCF-7-GFP cells (3 × 103) were seeded in a 96-well microtiter plate and incubated overnight. Paclitaxel with different concentrations (2–50 nmol/l) were added to each cell cultures. After 72 h, the cells were incubated with MTT (0.5 mg/ml) for 4 h. The formazan precipitate was dissolved in 200 µl dimethylsulfoxide (DMSO), and the Spectrometric absorbance at wavelength of 570 nm was measured on a benchmark microplate reader (Bio-Rad). Each group contained three wells. The value of [A570 (paclitaxel+)/A570 (paclitaxel–)] × 100% indicated the cell survival index. At least three independent experiments were done and the averages were calculated.
FCM analysis
In order to assess the apoptosis and distribution of cell cycle, 24 h after treatments with paclitaxel in different concentrations, cells were collected (attached and floating) and fixed with 70% ethanol at 4°C overnight. Following washing twice with phosphate buffered saline (PBS), the cells were incubated in RNase A/PBS (100 µg/ml) at 37°C for 30 min. Intracellular DNA was labeled with propidium iodide (50 µg/ml) and analyzed with a FACSCalibur fluorescence-activated cell sorter (FACS) using CELLQuest software (Becton Dickinson). The percentages of sub-G1 phase cells and distribution of cell cycle in each population were determined.
Immunofluorescence staining
MCF7-GFP-Nlp and MCF-7-GFP cells were seeded on slides in a 6-well plate and incubated overnight. Paclitaxel with different concentrations (10 and 20 nmol/l) were added to each cell cultures. After 24 h, slides were fixed in a solution containing a final concentration of 3.7% formaldehyde and incubated with anti-β-tubulin at 4°C for overnight, washed with PBS three times, incubated with tetramethyl rhodamine isothiocyanate-conjugated anti-rabbit IgG and followed by staining with 10 µg/ml 4′,6-diamidino-2-phenylindole (DAPI) in PBS. Two independent experiments were performed for each condition, and at least 300 cells were counted in each measurement.
Protein gel blot
MCF7-GFP-Nlp and MCF-7-GFP cells were treated with paclitaxel for different time, whole-cell protein extracts were prepared and quantified by the BCA Protein Assay Kit (Pierce). Proteins (100 µg/lane) were denatured, resolved on 12% SDS-PAGE gels and semi-dry transferred (Bio-Rad) at 12V for 3 h onto nitrocellulose membranes (Bio-Rad). The membranes were blocked and then incubated with monoclonal antibodies against Bcl-2, Plk1, Cyclin B1 (Calbiochem, PC686) overnight at 4°C, followed by incubation with secondary goat anti-rabbit or anti-rabbit IgG conjugated to horseradish peroxidase and then detected by SuperSignal ECL (Applygen Technologic Inc.). To control protein loading, membranes were stripped off and reprobed with anti-actin.
Immunohistochemical analysis of Nlp expression
The standard streptavidin peroxidase (SP) method was employed for immunostaining. Briefly, sections were cut at 4 μm from the TMA blocks. After dewaxed in xylene and rehydrated in alcohol and distilled water, antigen retrieval was performed by microwave oven heating (10 min) at middle power in 0.01 M sodium citrate buffer (pH 6.0) or incubated with protease XXIV (Biogenex) (10 min) at room temperature. Then, sections were incubated with 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity. Nonspecific staining was blocked by 10% normal goat serum (Vector Laboratories, Inc.) for 10 min. The bound antibody was detected then with biotinylated anti-mouse/rabbit IgG (H+L) (Vector Laboratories, Inc.) for 10 min and horseradish peroxidase streptavidin (Beijing Zhongshan Biotech) for 10 min. 3,3′-diaminobenzidine (Maixin Biotech, Fuzhou) was used as the chromogen. Slides were lightly counterstained with hematoxylin. In control experiments, the primary antibodies were replaced by PBS. Internal positive controls were available on the TMA itself.
Semiquantitative evaluation of immunohistochemical staining
In immunohistochemical analysis, visible brown granules in the cytoplasm were determined as positive staining. We scored protein expression with the criteria combined intensity with the rate of positive cells. First, the intensity was graded as follows: 0, negative; 1, weak; 2, moderate; 3, strong. Second, the rate of positive cells was graded: 0, < 5%; 1, 5–25%; 2, 26–50%; 3, 51–75%; 4, > 75%. A final score was achieved by multiplication of the two scores above. Scores of 0–4 were defined as “negative expression” (-); scores of 5–8 as “weakly positive expression” (+), and scores of 9–12 as “strongly positive expression” (++).
Selection of patients
We retrospectively analyzed Nlp expression in tissue samples of breast cancer patients administered with chemotherapy consisting of paclitaxel. The clinical breast cancer samples were obtained from 55 female metastatic breast cancer patients with pathologically confirmed invasive ductal carcinoma registered at Cancer Institute (Hospital), Peking Union Medical College, Chinese Academy of Medical Sciences during the period between January 1999 and March 2003 based on patient consent. The median age of those patients was 45 y old (21–72). All patients had accepted chemotherapy regimens consisted of paclitaxel at dose of 175 mg/m2 administered by intravenous infusion (IV) over 3 h followed by cisplatin 75 mg/m2 or carboplatin AUC 5 by IV infusion on day 1 as first line chemotherapy. Chemotherapy was repeated every 21 d. Standard paclitaxel pre-medication, consisting of 20 mg dexamethasone given orally 12 and 6 h before therapy, 40 mg diphenhydramine intramuscular injection and 400 mg cimetidine intravenous injection administered 30 min before paclitaxel therapy, was given to all patients. At least two cycles were administered. Efficacy was evaluated according to RECIST 1.1(response evaluation in solid tumor) criteria.Citation45
Statistics
Statistical analysis was performed using SPSS 13.0 software. The values are presented as the mean ± one standard deviation (SD).
Acknowledgments
We greatly thank Nagase of KAZUSA DNA Research Institute, Japan for KIAA0980 (NLP) cDNA clone. This work is supported by funding from the 973 National Key Fundamental Research Program of China (2009CB521801) and the National Natural Science Foundation of China (30730046, 30721001).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest are disclosed.
References
- Vincent T. DeVita J SHS(Ed): CANCER:Principles and Practice of Oncology (ed 7th). Philadelphia, PA 19106 USA: LIPPINCOTT WILLIAMS & WILKINS.
- Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci 2000; 11:265 - 83; http://dx.doi.org/10.1016/S0928-0987(00)00114-7; PMID: 11033070
- Hatanaka H, Abe Y, Naruke M, Asai S, Miyachi H, Kawakami T, et al. Modulation of multidrug resistance in a cancer cell line by anti-multidrug resistance-associated protein (MRP) ribozyme. Anticancer Res 2001; 21:879 - 85; PMID: 11396179
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol 1995; 30:445 - 600; http://dx.doi.org/10.3109/10409239509083491; PMID: 8770536
- Dingemans AC, van Ark-otte AJ, Span S, Scagliotti GV, der Valk PV, Postmus PE, et al. Topoisomerase II alpha and other drug resistance markers in advanced non-small cell lung cancer. Lung Cancer 2001; 32:117 - 28; http://dx.doi.org/10.1016/S0169-5002(00)00224-5; PMID: 11325482
- DiPaola RS, Aisner J. Overcoming bcl-2- and p53-mediated resistance in prostate cancer. Semin Oncol 1999; 26:112 - 6; PMID: 10190792
- Bentires-Alj M, Barbu V, Fillet M, Chariot A, Relic B, Jacobs N, et al. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene 2003; 22:90 - 7; http://dx.doi.org/10.1038/sj.onc.1206056; PMID: 12527911
- Salisbury JL, Whitehead CM, Lingle WL, Barrett SL. Centrosomes and cancer. Biol Cell 1999; 91:451 - 60; http://dx.doi.org/10.1016/S0248-4900(99)80086-0; PMID: 10519005
- Godinho SA, Kwon M, Pellman D. Centrosomes and cancer: how cancer cells divide with too many centrosomes. Cancer Metastasis Rev 2009; 28:85 - 98; http://dx.doi.org/10.1007/s10555-008-9163-6; PMID: 19156503
- Lingle WL, Salisbury JL. The role of the centrosome in the development of malignant tumors. Curr Top Dev Biol 2000; 49:313 - 29; http://dx.doi.org/10.1016/S0070-2153(99)49015-5; PMID: 11005025
- Kong Q. Cell brain abnormalities in cancer development. Med Hypotheses 2003; 61:120 - 5; http://dx.doi.org/10.1016/S0306-9877(03)00143-9; PMID: 12781654
- Lingle WL, Barrett SL, Negron VC, D'Assoro AB, Boeneman K, Liu W, et al. Centrosome amplification drives chromosomal instability in breast tumor development. Proc Natl Acad Sci USA 2002; 99:1978 - 83; http://dx.doi.org/10.1073/pnas.032479999; PMID: 11830638
- Liu L, Zou P, Zhang M, Tian L, Liu F. Effect of polo-like kinase 1 gene silence on cell cycle and drug resistance in K562/A02 cell. Chin Med J (Engl) 2006; 119:605 - 8; PMID: 16620704
- Ro S, Yang LX. Centrosomes–their role in tumors and cancer therapy. Anticancer Res 2004; 24:3269 - 73; PMID: 15510622
- Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell 2003; 3:51 - 62; http://dx.doi.org/10.1016/S1535-6108(02)00235-0; PMID: 12559175
- Dutertre S, Prigent C. Aurora-A overexpression leads to override of the microtubule-kinetochore attachment checkpoint. Mol Interv 2003; 3:127 - 30; http://dx.doi.org/10.1124/mi.3.3.127; PMID: 14993419
- Cogswell JP, Brown CE, Bisi JE, Neill SD. Dominant-negative polo-like kinase 1 induces mitotic catastrophe independent of cdc25C function. Cell Growth Differ 2000; 11:615 - 23; PMID: 11149596
- Yang H, He L, Kruk P, Nicosia SV, Cheng JQ. Aurora-A induces cell survival and chemoresistance by activation of Akt through a p53-dependent manner in ovarian cancer cells. Int J Cancer 2006; 119:2304 - 12; http://dx.doi.org/10.1002/ijc.22154; PMID: 16894566
- Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med 2004; 10:262 - 7; http://dx.doi.org/10.1038/nm1003; PMID: 14981513
- Peterson D, Lee J, Lei XC, Forrest WF, Davis DP, Jackson PK, et al. A chemosensitization screen identifies TP53RK, a kinase that restrains apoptosis after mitotic stress. Cancer Res 2010; 70:6325 - 35; http://dx.doi.org/10.1158/0008-5472.CAN-10-0015; PMID: 20647325
- Spänkuch B, Kurunci-Csacsko E, Kaufmann M, Strebhardt K. Rational combinations of siRNAs targeting Plk1 with breast cancer drugs. Oncogene 2007; 26:5793 - 807; http://dx.doi.org/10.1038/sj.onc.1210355; PMID: 17369857
- Payton M, Bush TL, Chung G, Ziegler B, Eden P, McElroy P, et al. Preclinical Evaluation of AMG 900, a Novel Potent and Highly Selective Pan-Aurora Kinase Inhibitor with Activity in Taxane-Resistant Tumor Cell Lines. Cancer Res 2010; 70:9846 - 54; http://dx.doi.org/10.1158/0008-5472.CAN-10-3001; PMID: 20935223
- Kennedy RD, Quinn JE, Mullan PB, Johnston PG, Harkin DP. The role of BRCA1 in the cellular response to chemotherapy. J Natl Cancer Inst 2004; 96:1659 - 68; http://dx.doi.org/10.1093/jnci/djh312; PMID: 15547178
- Husain A, He G, Venkatraman ES, Spriggs DR. BRCA1 up-regulation is associated with repair-mediated resistance to cis-diamminedichloroplatinum(II). Cancer Res 1998; 58:1120 - 3; PMID: 9515792
- Zhou C, Smith JL, Liu J. Role of BRCA1 in cellular resistance to paclitaxel and ionizing radiation in an ovarian cancer cell line carrying a defective BRCA1. Oncogene 2003; 22:2396 - 404; http://dx.doi.org/10.1038/sj.onc.1206319; PMID: 12717416
- Chabalier C, Lamare C, Racca C, Privat M, Valette A, Larminat F. BRCA1 downregulation leads to premature inactivation of spindle checkpoint and confers paclitaxel resistance. Cell Cycle 2006; 5:1001 - 7; http://dx.doi.org/10.4161/cc.5.9.2726; PMID: 16639080
- Harkin DP, Bean JM, Miklos D, Song YH, Truong VB, Englert C, et al. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell 1999; 97:575 - 86; http://dx.doi.org/10.1016/S0092-8674(00)80769-2; PMID: 10367887
- Wang Y, Zhan Q. Cell cycle-dependent expression of centrosomal ninein-like protein in human cells is regulated by the anaphase-promoting complex. J Biol Chem 2007; 282:17712 - 9; http://dx.doi.org/10.1074/jbc.M701350200; PMID: 17403670
- Yu L, Song Y, Zhang Q, Zhan Q. Ninein-like protein is overexpressed in head and neck squamous cell carcinoma and contributes to cancer growth and resistance to apoptosis. Oncol Rep 2009; 22:789 - 98; PMID: 19724857
- Qu D, Qu H, Fu M, Zhao X, Liu R, Sui L, et al. Increased expression of Nlp, a potential oncogene in ovarian cancer, and its implication in carcinogenesis. Gynecol Oncol 2008; 110:230 - 6; http://dx.doi.org/10.1016/j.ygyno.2008.04.015; PMID: 18538832
- Jin S, Gao H, Mazzacurati L, Wang Y, Fan W, Chen Q, et al. BRCA1 interaction of centrosomal protein Nlp is required for successful mitotic progression. J Biol Chem 2009; 284:22970 - 7; http://dx.doi.org/10.1074/jbc.M109.009134; PMID: 19509300
- Shao S, Liu R, Wang Y, Song Y, Zuo L, Xue L, et al. Centrosomal Nlp is an oncogenic protein that is gene-amplified in human tumors and causes spontaneous tumorigenesis in transgenic mice. J Clin Invest 2010; 120:498 - 507; http://dx.doi.org/10.1172/JCI39447; PMID: 20093778
- Chesler L, Goldenberg DD, Collins R, Grimmer M, Kim GE, Tihan T, et al. Chemotherapy-induced apoptosis in a transgenic model of neuroblastoma proceeds through p53 induction. Neoplasia 2008; 10:1268 - 74; PMID: 18953436
- Quinn JE, Kennedy RD, Mullan PB, Gilmore PM, Carty M, Johnston PG, et al. BRCA1 functions as a differential modulator of chemotherapy-induced apoptosis. Cancer Res 2003; 63:6221 - 8; PMID: 14559807
- Lentini L, Amato A, Schillaci T, Insalaco L, Di LA. Aurora-A transcriptional silencing and vincristine treatment show a synergistic effect in human tumor cells. Oncol Res 2008; 17:115 - 25; PMID: 18669163
- Spänkuch B, Heim S, Kurunci-Csacsko E, Lindenau C, Yuan J, Kaufmann M, et al. Down-regulation of Polo-like kinase 1 elevates drug sensitivity of breast cancer cells in vitro and in vivo. Cancer Res 2006; 66:5836 - 46; http://dx.doi.org/10.1158/0008-5472.CAN-06-0343; PMID: 16740723
- Gomez LA, de Las Pozas A, Reiner T, Burnstein K, Perez-Stable C. Increased expression of cyclin B1 sensitizes prostate cancer cells to apoptosis induced by chemotherapy. Mol Cancer Ther 2007; 6:1534 - 43; http://dx.doi.org/10.1158/1535-7163.MCT-06-0727; PMID: 17513602
- Song Y, Zhao C, Dong L, Fu M, Xue L, Huang Z, et al. Overexpression of cyclin B1 in human esophageal squamous cell carcinoma cells induces tumor cell invasive growth and metastasis. Carcinogenesis 2008; 29:307 - 15; http://dx.doi.org/10.1093/carcin/bgm269; PMID: 18048386
- Horwitz SB. Taxol (paclitaxel): mechanisms of action. Ann Oncol 1994; 5:Suppl 6 S3 - 6; PMID: 7865431
- Abal M, Andreu JM, Barasoain I. Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr Cancer Drug Targets 2003; 3:193 - 203; http://dx.doi.org/10.2174/1568009033481967; PMID: 12769688
- Wang Y, O'Brate A, Zhou W, Giannakakou P. Resistance to microtubule-stabilizing drugs involves two events: beta-tubulin mutation in one allele followed by loss of the second allele. Cell Cycle 2005; 4:1847 - 53; http://dx.doi.org/10.4161/cc.4.12.2264; PMID: 16294009
- Sève P, Reiman T, Lai R, Hanson J, Santos C, Johnson L, et al. Class III beta-tubulin is a marker of paclitaxel resistance in carcinomas of unknown primary site. Cancer Chemother Pharmacol 2007; 60:27 - 34; http://dx.doi.org/10.1007/s00280-006-0343-1; PMID: 17021819
- Wang Y, Cabral F.. Paclitaxel resistance in cells with reduced beta-tubulin. Biochim Biophys Acta 2005; 1744:245 - 55; http://dx.doi.org/10.1016/j.bbamcr.2004.12.003; PMID: 15950754
- Ferlini C, Raspaglio G, Mozzetti S, Distefano M, Filippetti F, Martinelli E, et al. Bcl-2 down-regulation is a novel mechanism of paclitaxel resistance. Mol Pharmacol 2003; 64:51 - 8; http://dx.doi.org/10.1124/mol.64.1.51; PMID: 12815160
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45:228 - 47; http://dx.doi.org/10.1016/j.ejca.2008.10.026; PMID: 19097774