Abstract
Background: The large individual variability for anticancer drugs in both outcome and toxicity risk makes the identification of pharmacogenetic markers that can be used to screen patients before therapy selection an attractive prospect.
Aims: This work aimed to evaluate the importance of genetic polymorphisms involved in drug detoxification to predict clinical outcomes of anthracycline-based neoadjuvant chemotherapy for breast cancer.
Results: GSTP1 313 AA genotype was associated with a poor clinical response relative to G allele carrier (58.4% vs 80.8%; p = 0.006), and MDR1 3435 TT genotype had a worse response compared with C allele carrier (33.3% vs 71.2% p = 0.001). Patients with both the adverse genotypes of GSTP1 314AA and MDR 3435TT showed the worst therapy efficacy in all (14.3%; p = 0.000). Kaplan-Meier survival analysis showed that the patients with no adverse genotype were associated with decreased hazard of relapse (p = 0.002), compared with those with 1 or 2 adverse genotypes. Multivariate analysis demonstrated that clinical response and no adverse genotype was independent predictors of disease-free survival (DFS).
Methods: Genotyping was performed by allele-specific oligonucleotide ligation reaction (MnSOD, CAT, GSTP1), multiplex PCR (GSTM1, GSTT1) or PCR-RFLP (MDR1). Based on 153 patients received anthracycline-based neoadjuvant chemotherapy, these genotypes or their combinations in relation to treatment-related response, hematologic toxicity and DFS were investigated.
Conclusions: These results suggest that polymorphisms in GSTP1 and MDR1 may help to predict clinical response and DFS of anthracycline-based chemotherapy, and a polygenic pathway approach should provide more useful information. The findings required independent prospective confirmation.
Introduction
Anthracycline is one of the most effective cytotoxic agents in breast cancer and forms the backbone of most regimens used in chemotherapy setting. However, its efficacy is variable in individual, with higher susceptibility to drug resistance and with greater risk of side effect such as cardiotoxicity.Citation1 Therefore, pretherapeutic predictors of response and prognosis would be of utmost interest to individualizing treatment.
The rapidly evolving field of pharmacogenomics holds great promise for assisting selection of predictive markers of drug efficacy and safety. Pharmacogenomic studies aim to elucidate the genetic bases for interindividual differences and to use such genetic information to predict drug efficacy and toxicity.Citation2,Citation3 There exist several clinically relevant examples of the utility of pharmacogenomics that associate specific genetic polymorphisms in drug metabolizing enzymes (e.g., TPMT, DPD, UGT1A1) and drug transporters (MDR1) with clinical outcomes in patients treated with commonly prescribed chemotherapy drugs, such as 6-mercaptopurine, 5-fluorouracil and irinotecan.Citation3 Clearly, the polymorphisms of genes involved in cellular detoxification of anthracycline likely also contribute to predicting treatment-related outcome.
Anthracycline, including doxorubicin and epirubicin and so on, exerts its antineoplastic effects largely by generation of reactive oxygen species (ROS), resulting in DNA damage as well as mitochondrial membrane disruption, triggering the apoptotic cascade.Citation4 This process may be affected by the enzymes involved in ROS neutralizing pathways, including manganese superoxide dismutase (MnSOD), catalase (CAT) and glutathione-S-transferases (GSTs).Citation4,Citation5 MnSOD catalyzes the formation of H2O2 from superoxide radicals generated by chemotherapeutics. It has been demonstrated that upregulated MnSOD expression can enhance resistance to doxorubicin treatment in cancer cell culture.Citation6 A common polymorphism of MnSOD (Val16Ala, 47 T→C) in the mitochondrial targeting sequence has been associated with overexpression of MnSOD, resulting in an increased production of H2O2, however, which has also been proposed to generate increased levels of ROS if not subsequently neutralized.Citation4,Citation5 By neutralizing H2O2, CAT participates in defense mechanisms against oxidative stress. A common CAT-262 C→T polymorphism has been associated with lower enzyme activity compared with wild C allele and thus, increased levels of ROS.Citation4 In addition, GSTs also regulate the cellular response to oxidative stress and participate in the metabolism of lipids and DNA products derived from oxidative stress.Citation5 α-, π-, µ- and θ-class GSTs are active in detoxification of numerous products resulting from reactive oxidant damage to DNA and lipids. For example, GSTP1 is reported to be involved in anthracycline detoxification and is widely expressed in human breast tumors.Citation7,Citation8 The GST gene families contain several polymorphic loci. At least three of the genes that code for GSTs (GSTM1, GSTT1 and GSTP1) have been found to have functional polymorphisms that are frequently present in general populations. The polymorphism of GSTP1 A313G and null variants of GSTM1 and GSTT1 that lead to the diminished or abolished enzyme activity have been associated with increased drug treatment benefit in some cancer patients.Citation5,Citation7
In addition to these enzymes, drug transporters are increasing recognized to be important to drug disposition and response. P-glycoprotein (P-gp), the encoded product of multidrug resistance gene (MDR1), is of particular clinical relevance in that this transporter has broad substrate specificity, including a variety of structurally divergent drugs such as anthracycline and vinblastine.Citation9 Pharmacogenomics and pharmacogenetics studies have revealed that MDR1 polymorphisms have an impact on P-gp expression and function in different ethnicities and subjects.Citation2 Currently, at least 50 polymorphisms have been identified for MDR1, in which exon 26 C3435T, exon 21 G2677T/A and exon 12 C1236T are common and functionally significant.Citation9–Citation12
In this study, we examine eight functional polymorphisms in the genes (MnSOD, CAT, GSTP1, GSTM1, GSTT1 and MDR1) described above, and tested the hypothesis whether these polymorphisms, alone or in combination, may have the potential to predict clinical response and prognosis in breast cancer patients with anthracycline-based chemotherapy.
Results
Patients' characteristics.
The clinical characteristics of 153 patients (median: 48.0 y, range: 19–70 y) are shown in . After the first two cycles of neoadjuvant chemotherapy, 16 (10.5%) of the 153 patients obtained complete response (CR) and 85 (55.6%) showed a partial response (PR), with 101 patients classified as responders (66.0%, CR + PR); 50 (32.7%) had stable disease (SD) and 2 (1.31%) progressed in disease (PD), with 52 patients as nonresponders (44.0%, SD + PD). There were no significant differences in clinical characteristics between the two different response groups (CR + PR vs. SD + PD, ). There were also no significant differences by any of the genotypes for age, menopausal status, clinical TNM stage (II vs. III), ER or PR status, chemotherapy regimens (p > 0.05, data omitted).
Genotypes and MDR1 haplotypes.
The polymorphism distributions in MnSOD, CAT, GSTs and MDR1 were shown in –. The LD among the MDR1 SNPs was found and was strongest between exon 12 and exon 21 SNPs (D' = 0.731, r2 = 0.226). We also observed remains pair-wise LD between C1236T and C3435T (D' = 0.586, r2 = 0.125) as well as G2677T/A and C3435T SNPs (D' = 0.595, r2 = 0.289). Nine haplotypes were observed in the study population, of which three major haplotypes T-T-T (39.4%), T-G-C (20.6%) and C-G-C (19.1%) of MDR1 1236-2677-3435 together constituted 79.1%.
Genotypes and clinical responses.
MnSOD (T47C) or CAT (C-262T) genotypes were not statistically associated with clinical responses (p > 0.05; ). Since the MnSOD and CAT are involved in the same biological pathway, we tried to investigate a possible synergetic effect of the two SNPs. However, combining genotypes for MnSOD and CAT was somewhat non-informative due to the small numbers of women with homozygous variant MnSOD 47CC and no women with homozygous variant CAT-262TT.
The significant differences in clinical responses were observed for GSTP1 A313G, but not for GSTT1 or GSTM1 deletion polymorphisms. Patients with GSTP1 313 AA genotype had a decreased response rate relative to those with AG (58.4 vs. 77.8%) or GG (58.4 vs. 100.0%; p = 0.027; ). Moreover, the response rate of the combination of GSTP1 AA with both GSTM1- and GSTT1-present was 44.0%, being also lower comparing with the other groups (70.3%; p = 0.011). The effect of “GSTs combination” on chemotherapy response seemed to be slightly more pronounced comparing with GSTP1 AA (44 vs. 58.4%), but not statistically significant (p = 0.404).
MDR1 3435 variant TT genotype had a significantly worse response compared with CT or CC genotypes (33.3 vs. 71.4 or 70.6%; p = 0.003). In addition, we analyzed the haplotypes of MDR1 1236-2677-3435. The response rates of the patients with 3435T-2677T, 3435T-1236T or 3435T-2677T-1236T haplotypes were also lower than that of the patients with the other combined haplotypes. However these haplotypes could not provide more information than 3435TT alone did ().
Genotypes and hematologic toxicities.
The hematologic toxicities were analyzed in all the 153 patients. The incidences of grade 3–4 neutropenia, grade 3 anemia and grade 3 thrombocytopenia were 24.2, 1.3 and 2.0%, respectively. So, a further analysis was performed for patients with grade 3–4 neutropenia, because it was the most frequent hematologic toxicity. However, no significant differences in grade 3–4 neutropenia were found in groups differentiated by the genotype frequencies of the genes analyzed (–).
Combination of adverse genotypes and clinical outcomes.
Based on the observations above, we performed a second analysis to elucidate whether a pattern of adverse genotypes could be used to determine clear-cut differences of clinical responses. For this, the genotypes GSTP1 313AA and MDR1 3435 TT were designated as adverse genotypes because of their direct associations with poor response.
The association of the combined adverse genotypes with clinical responses was examined (). In all, 45 patients had 0 adverse genotype, 94 patients had 1 adverse genotype, and 14 patients had 2 adverse genotypes. The combined effects of GSTP1 A313G and MDR1 C3435T were greater than those for either polymorphism alone, and an increased response with decreasing number of adverse genotypes was observed (14.3 vs. 66.0 vs. 82.2%; p = 0.000).
One hundred forty-nine patients were followed up on, except four patients lost. At the time of analysis, mean follow-up time was 51 months (range: 8–64). The recurrence rate in patients with GSTP1 313AA or/and MDR 3435TT (i.e., one or two adverse genotypes) was 39.0% (41/105), higher significantly than those with 0 adverse genotype [15.9% (7/44); x2 = 7.602, p = 0.006]. Kaplan-Meier survival analysis showed that patients with 0 adverse genotype were associated with reduced hazard of relapse in comparing with those with one or two adverse genotypes (long-rank test, p = 0.002), but GSTP1 313AA or MDR 3435TT alone did not (p > 0.05; ). Univariate Cox regression analyses showed that TNM stage, clinical response and adverse genotypes were significant factors affecting DFS (). The variables with p ≤ 0.10 in univariate analysis were selected into a stepwise multivariate Cox proportional hazards model. As listed in , clinical response and adverse genotypes were still related to DFS (p = 0.002 and p = 0.049), although this DFS advantage for patients with no adverse genotype was weakened after adjustment for potential confounding factors, including TNM stage, chemotherapy regimens and clinical response.
Discussion
In this study, we evaluated the association of polymorphisms in genes involved in the cellular detoxification of anthracycline with neoadjuvant chemotherapy-related outcomes in Chinese Han breast cancer patients. The results indicated that GSTP1 313AA and MDR1 3435TT genotypes were significantly associated with inferior response, and a combination of the two adverse genotypes was at increased risk for poor response and relapse. These suggest that the treatment outcome of anthracycline-containing regimens may be influenced by specific genetic polymorphisms that alter the drug detoxification.
MnSOD is a key mitochondrial antioxidant enzyme that can protect against oxidative damage induced by endogenetic and exogenous factors. While some studies have reported that Val16Ala (T47C) is associated with the risk to develop breast cancer,Citation13 only few studies examined the effect of this polymorphism on chemotherapy outcome for breast cancer, and their results were inconsistent.Citation4,Citation5,Citation14 Bewick et al. found an association between the MnSOD 47T allele and an increased risk of disease progression and breast cancer-specific death among 95 metastatic breast cancer patients who received high dose chemotherapy and autologous stem cell support.Citation5 Glynn et al. found that carriers of MnSOD 47CC genotype were at a significantly increased risk of poor survival when treated with specific chemotherapeutic drugs.Citation14 Ambrosone et al. did not find a significant association between MnSOD T47C and outcome based on 279 breast cancer patients receiving adjuvant chemotherapy, radiation or both.Citation4 We did not also observe a significant association of MnSOD genotypes with clinical response. Although TT genotype had an increased response trend relative to CC (non-significant, RR: 2.03, CI 95%: 0.28–14.91), only four patients had the CC genotype limiting interpretation. So, it remains to be shown whether Val16Ala has a dominant effect on MnSOD activity in vivo.
In recent years, it has become evident that GSTs not only participates in drug detoxification, but also are involved in the control of apoptosis through the inhibition of JNK signaling pathway.Citation7 As thus they have become the focus of chemotherapy resistance research. The most highly expressed GST isoenzyme in various human cancerous (especially breast cancer) tissues is GSTP1.Citation7,Citation8 Several studies have showed that GSTP1 expression may be an important predictor of early recurrence, drug resistance and bad prognosis in some cancer including breast cancer.Citation5,Citation7,Citation8 The GSTP1 gene is polymorphic, with important differences in activity according to genotypes. The GSTP1 313 A→G (Ile105Val) is located near the substrate binding site of the enzyme, and has been known to have the reduced enzyme activity in removal of chemotherapy agents.Citation15–Citation17 In our study, increasing numbers of GSTP1 G allele were observed to be associated with increased treatment benefit (), similar to the finding for colorectal cancer patients.Citation18 However, our result was different from a recent study, which found unexpectedly a better chemotherapy response for the patients with wild-type combination GSTT1/GSTP1105Ile, but not those with GSTP1 variant genotype in 40 Brazilian breast cancer women with FAC regimens.Citation19 In addition, our results did not show a significant effect of GSTM1 and/or GSTT1 null on anthracycline-based treatment. In fact, some studies also excluded a role for GSTM1 and/or GSTT1 genotypes as clinical response predictors and/or prognostic factors in breast cancer.Citation8,Citation15 Previously, the phenotype of breast cancer tissue was also investigated, and neither GSTT1 nor GSTM1 immunopositivity in tumor or non-neoplastic breast was found to correlate with therapy response.Citation20 We hypothesized that this could be related to the differences in tissue- and drug-specificity of GSTP, GSTM and GSTT isoenzymes. In breast cancer tissue, GSTP1 is predominantly expressed, and GSTM1 and GSTT1 do not seem to have a major role.Citation7,Citation8,Citation15,Citation17 Our results, in which the combination of GSTP1 with both GSTM1 and GSTT1 polymorphisms appeared to have a similar treatment effect compared with GSTP1 313AA alone (), supported this point in some degree, too.
P-gp, the MDR1 gene product, confers multidrug resistance to a variety of antineoplastic agents, e.g., anthracycline. It functions not only in protecting cells from toxic metabolites as an ATP-dependent drug efflux pump, but also in regulating cellular proliferation, differentiation and cell survival.Citation21,Citation22 Hoffmeyer et al. first described a coding synonymous 3435 C to T transition that showed a correlation with reduced P-gp levels and activity in 21 caucasians.Citation10 Kafka et al. found that the 3435TT correlated with a complete clinical response when treating breast cancer patients with anthracycline alone or in combination with taxane.Citation23 In contrast, Cizmarikova et al. recently reported 3435CC to be related to a significantly enhanced response after neoadjuvant therapy (n = 38) and longer time to progression after anthracycline-based chemotherapy (n = 102).Citation22 Our study showed that 3435 TT genotype had a significantly worse response compared with CT or CC, which was similar to most studies based on Asian populations.Citation11,Citation12,Citation24,Citation25 Contradicting results may be associated with the population studied, tumor biological characteristics, sample size and so on. Besides, the association of P-gp to drug resistance may not necessarily be through direct pumping of the active drug out of tumor cells, but also through its roles as a transporter of signals to the cell cycle and apoptosis.Citation21
Now, although there is a general consensus that a multiple SNP analysis is necessary, there is less agreement with respect to what is the proper approach. In this study, we used both a combinatorial approach and a haplotype way. Our result showed that the patients with both the adverse genotypes of GSTP1 313AA and MDR1 3435TT had the worst treatment efficacy in all, which suggested that they were responsible for the conjugation and the subsequent elimination of chemotherapeutant from the cell. Recently, some studies clearly show that a coordinated expression of efflux transporters and phase II conjugating enzymes in tumor cells is linked to the development of the multidrug resistance phenotype.Citation7,Citation16 Sun et al. reported that the polymorphic status of MRP2 C-24T and GSTP1 A313G might be the predictive markers for the treatment response of advanced NSCLC patients.Citation16 Further elucidation of the combined action would be an aid in the improvement of the chemotherapeutic treatment of cancer patients.
In the study, DFS after neoadjuvant chemotherapy was also evaluated. Based on a series of studies, von Minckwitz et al. demonstrated that nonresponse to initial neoadjuvant chemotherapy was associated with unfavorable survival in breast cancer.Citation26,Citation27 Our results also supported the point (). Thus, patients who do not benefit from inductive chemotherapy need to be identified early in the course of treatment or even better before to avoid costs, toxicity, and eventually ineffective treatment. This study found that a combination of the two adverse genotypes enhanced the predictive power before chemotherapy, because it was notably related to treatment-related response as well as recurrence risk. Apparently it would be helpful for improving risk: benefit ratio of preoperative chemotherapy for breast cancer, which deserved further study.
In conclusion, the results of this study point to the possibility of individualizing therapy based on the polymorphisms in genes involved in drug detoxification. Furthermore, we find the pattern of pathway evaluation beyond single gene polymorphisms could be more informative to predict clinical response and DFS. This exploratory study provides a direction for future larger prospective studies with the ultimate goal of more tailored therapies for breast cancer patients.
Materials and Methods
Patients.
Patients with previously untreated, primary breast ductal carcinoma could be enrolled in the study. They were all Chinese Han female patients from Jiangsu Cancer Hospital between October 2005 and December 2008. The other eligible criteria included Karnofsky performance status of at least 80, adequate hematologic parameters, normal hepatic and renal function, and normal cardiac and lung function. A total of 153 of the patients were eligible in this study. They all signed an informed consent to entering the study, which had been approved by the Ethics Review Committee at the hospital.
Treatment regimens and evaluation.
One hundred fifty-three patients with anthracycline-based neoadjuvant chemotherapy consisted of 89 patients with TA regimens, 10 patients with TAC and 54 patients with FAC [T: paclitaxel (135–175 mg/m2)/docetaxel (75 mg/m2), A: epirubicin (75 mg/m2)/doxorubicin (50 mg/m2), C: cyclophosphamide (500 mg/m2), F: 5-fluorouracil (500 mg/m2)]. All the drugs were administered intravenously, and these treatments were given once every 21 d for 2–4 cycles. During 10–14 d after completion of preoperative therapy, the patients received breast conserving or radical surgery and postoperative treatment according to standard recommendations.
All the patients had measurable lesions. Their responses to neoadjuvant chemotherapy were estimated according to World Health Organization (WHO) criteria by radiological examinations: a CR was defined as complete disappearance of all measurable lesions; a PR required at least 50% reduction in measurable lesions; a PD was assigned to patients when measurable lesions increased by more than 25% or new lesions appeared; and SD was defined as all other situations. No significant change in clinical axillary status was also classified as SD regardless of the reduced change in primary tumor diameter. For data analysis, CR and PR were combined as responders, and SD and PD were classified as nonresponders.
Hematologic toxicities (neutropenia, anemia and thrombocytopenia) were classified according to WHO criteria (grades 0, 1, 2, 3, 4) at each cycle for each patient. Grade 3/4 toxicity was considered to be a serious event and was evaluated.
Genotyping.
Before chemotherapy, blood samples from these patients were drawn into sterile tubes containing EDTA-K3 and stored at −76°C. Genomic DNA was extracted from 0.5 ml whole blood using the QiaAmp kit (Qiagen) following the manufacturer's protocol and its purity and quality were assessed using NanoDrop Spectrophotometer (ND-1000, NanoDrop Technologies) and 0.8% agarose gel-electrophoresis for the integrity assay.
Single nucleotide polymorphisms (SNPs) of MnSOD (T47C), CAT (C-262T), GSTP1 (A313G) and the deletion polymorphisms of GSTM1 and GSTT1 were determined using allele-specific oligonucleotide ligation reaction (ASOLR) and a multiplex PCR developed in our laboratory, respectively.28 ASOLR was a technique for multiplex genetic typing. The assay was implemented via three steps: a triplex PCR that resulted in the amplified target sequences with MnSOD T47C, CAT C-262T and GSTP1 A313G loci, a triplex allele-specific oligonucleotide probe ligation reaction that generates the ligation between perfectly matched probes at their junctions while no ligation occurred between mismatched ones and an analysis of genotype-specific ligation products by both fluorescence and size after electrophoresis on ABI PRISM 377 DNA sequencer.Citation28,Citation29 MDR1 C3435T, G2677T/A and C1236T polymorphisms were analyzed by PCR-RFLP as described by Ni et al. PCR reaction was performed on a DNA thermal cycler (Bio-Rad iCycle). Controls (no DNA template) were run to ensure there was no amplification of contaminating DNA, and Beta-actin was run as internal control. PCR or RFLP products were analyzed on 3% agarose gel. Genotyping for a 10% random sample was confirmed by DNA sequencing for quality control.
Statistical analysis.
Date analysis was performed using SPSS software, version 13.0. Linkage disequilibrium (LD) was explored based Bayesian statistical method using the PHASE v.2.1 software. Comparison of proportion among groups was performed by χ2 test. Adverse genotypes from the genetic polymorphisms that were associated with clinical response were identified. Disease-free survival (DFS) was calculated as the time from operative date to disease recurrence, metastasis or last follow-up contact. Kaplan-Meier curves for DFS were generated and the difference by the genotypes was contrasted by log-rank test. Univariate and multivariate analysis for DFS were performed by Cox proportional hazard model. All tests were two-sided and p < 0.05 was taken as statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Abbreviations
| CAT | = | catalase |
| CR | = | complete response |
| DPD | = | dihydropyrimidine dehydrogenase |
| DFS | = | disease-free survival |
| H2O2 | = | hydrogenperoxide |
| MnSOD | = | manganese superoxide dismutase |
| MDR1 | = | multidrug resistance 1 |
| GSTs | = | glutathione-S-transferases |
| PR | = | partial response |
| PCR | = | polymerase chain reaction |
| PD | = | progressive disease |
| ROS | = | reactive oxygen species |
| RFLP | = | restriction fragment length polymorphism |
| SD | = | stable disease |
| TPMT | = | thiopurine methyltransferase |
| UGT1A1 | = | uridine diphosphate glucuronosyl transferase 1A1 |
| WHO | = | World Health Organization |
Figures and Tables
Figure 1 Comparison of patients' disease-free survival between GSTP1 313 AA and GA + GG genotypes (A), and between MDR1 3435 TT and CT + CC genotypes (B), between 0 adverse genotype and 1 or 2 adverse genotypes (C).
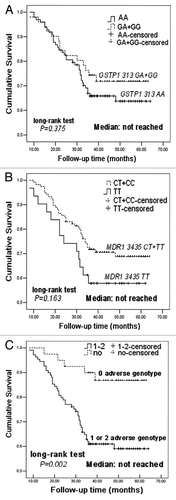
Table 1 Comparison of clinical response rate according to patient characteristics (n = 153)
Table 2 Clinical responses and hematologic toxicity to anthracycline-based chemotherapy according to MnSOD and CAT Genotypes
Table 3 Clinical responses and hematologic toxicity to anthracycline-based chemotherapy according to GSTP1, GSTM1 and GSTT1 Genotypes
Table 4 Clinical responses and hematologic toxicity to anthracycline-based chemotherapy according to genotypes and haplotypes in MDR1
Table 5 The combined adverse genotypes in GSTP1 A313G and MDR1 C3435T and clinical response to anthracycline-based chemotherapy
Table 6 Cox regression analysis for DFS in 149 neoadjuvant chemotherapy patients
Acknowledgments
This study was supported by the National Natural Science Foundation of China (30840093) and Social Development Project of Science Technology of Jiangsu Province (BS2007077).
References
- Davidson A, Gelmon K. Do anthracyclines still have a role in adjuvant chemotherapy of breast cancer?. Future Oncol 2011; 7:37 - 55; PMID: 21174537; http://dx.doi.org/10.2217/fon.10.163
- Blakey JD, Hall IP. Current progress in pharmacogenetics. Br J Clin Pharmacol 2011; 71:824 - 831; PMID: 21235621; http://dx.doi.org/10.1111/j.1365-2125.2011.03912.x
- Lee W, Lockhart AC, Kim RB, Rothenberg ML. Cancer pharmacogenomics: powerful tools in cancer chemotherapy and drug development. Oncologist 2005; 10:104 - 111; PMID: 15709212; http://dx.doi.org/10.1634/theoncologist.10-2-104
- Ambrosone CB, Ahn J, Singh KK, Rezaishiraz H, Furberg H, Sweeney C, et al. Polymorphisms in genes related to oxidative stress (MPO, MnSOD, CAT) and survival after treatment for breast cancer. Cancer Res 2005; 65:1105 - 1111; PMID: 15705913
- Bewick MA, Conlon MSC, Lafrenie RM. Polymorphisms in manganese superoxide dismutase, myeloperoxidase and glutathione-S-transferase and survival after treatment for metastatic breast cancer. Breast Cancer Res Treat 2008; 111:93 - 101; PMID: 17922231; http://dx.doi.org/10.1007/s10549-007-9764-8
- Suresh A, Guedez L, Moreb J, Zucali J. Overexpression of manganese superoxide dismutase promotes survival in cell lines after doxorubicin treatment. Br J Haematol 2003; 120:457 - 463; PMID: 12580960; http://dx.doi.org/10.1046/j.1365-2141.2003.04074.x
- Sau A, Tregno FP, Valentino F, Federici G, Caccuri AM. Glutathione transferases and development of new principles to overcome drug resistance. Arch Biochem Biophys 2010; 500:116 - 122; PMID: 20494652; http://dx.doi.org/10.1016/j.abb.2010.05.012
- Arun BK, Granville LA, Yin G, Middleton LP, Dawood S, Kau SW, et al. Glutathione-s-transferase-pi expression in early breast cancer: association with outcome and response to chemotherapy. Cancer Invest 2010; 28:554 - 559; PMID: 20210524; http://dx.doi.org/10.3109/07357900903286925
- Hamidovic A, Hahn K, Kolesar J. Clinical Significance of ABCB1 Genotyping in Oncology. J Oncol Pharm Pract 2010; 16:39 - 44; PMID: 19401306; http://dx.doi.org/10.1177/1078155209104380
- Hoffimeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA 2000; 97:3473 - 3478; PMID: 10716719; http://dx.doi.org/10.1073/pnas.050585397
- Pan JH, Han JX, Wu JM, Huang HN, Yu QZ, Sheng LJ. MDR1 single nucleotide polymorphism G2677T/A and haplotype are correlated with response to docetaxel-cisplatin chemotherapy in patients with non-small-cell lung cancer. Respiration 2009; 78:49 - 55; PMID: 18812689; http://dx.doi.org/10.1159/000158454
- Ni LN, Li JY, Miao KR, Qiao C, Zhang SJ, Qiu HR, et al. Multidrug resistance gene (MDR1) polymorphisms correlate with imatinib response in chronic myeloid leukemia. Med Oncol 2011; 28:265 - 269; PMID: 20204543; http://dx.doi.org/10.1007/s12032-010-9456-9
- Mitrunen K, Sillanpaa P, Kataja V, Eskelinen M, Kosma VM, Benhamou S, et al. Association between manganese superoxide dismutase (MnSOD) gene polymorphism and breast cancer risk. Carcinogenesis 2001; 22:827 - 829; PMID: 11323405; http://dx.doi.org/10.1093/carcin/22.5.827
- Glynn SA, Boersma BJ, Howe TM, Edvardsen H, Geisler SB, Goodman JE, et al. A mitochondrial target sequence polymorphism in MnSOD predicts inferior survival in breast cancer patients treated with Cyclophosphamide. Clin Cancer Res 2009; 15:4165 - 4173; PMID: 19509150; http://dx.doi.org/10.1158/1078-0432.CCR-09-0119
- Yang G, Shu XO, Ruan ZX, Cai QY, Jin F, Gao YT, et al. Genetic polymorphisms in glutathione-S-transferase genes (GSTM1, GSTT1, GSTP1) and survival after chemotherapy for invasive breast carcinoma. Cancer 2005; 103:52 - 58; PMID: 15565566; http://dx.doi.org/10.1002/cncr.20729
- Sun N, Sun X, Chen B, Cheng H, Feng J, Cheng L, et al. MRP2 and GSTP1 polymorphisms and chemotherapy response in advanced non-small cell lung cancer. Cancer Chemother Pharmacol 2010; 65:437 - 446; PMID: 19568750; http://dx.doi.org/10.1007/s00280-009-1046-1
- Kadouri L, Kote-Jarai Z, Hubert A, Baras M, Abeliovich D, Hamburger T, et al. Glutathione-S-transferase M1, T1 and P1 polymorphisms, and breast cancer risk, in BRCA1/2 mutation carriers. Br J Cancer 2008; 98:2006 - 2010; PMID: 18542066; http://dx.doi.org/10.1038/sj.bjc.6604394
- Stoehlmacher J, Park DJ, Zhang W, Groshen S, Tsao WD, Yu M, et al. Association between glutathione S-transferase P1, T1 and M1 genetic polymorphism and survival of patients with metastatic colorectal cancer. J Natl Cancer Inst 2002; 94:936 - 942; PMID: 12072547
- Oliveira AL, Rodrigues FFO, Santos RE, Aoki T, Rocha MN, Longui CA, et al. GSTT1, GSTM1 and GSTP1 polymorphisms and chemotherapy response in locally advanced breast cancer. Genet Mol Res 2010; 9:1045 - 1053; PMID: 20568049; http://dx.doi.org/10.4238/vol9-2gmr726
- Alpert LC, Schecter RL, Berry DA, Melnychuk D, Peters WP, Caruso JA, et al. Relation of glutathione S-transferase alpha and mu isoforms to response to therapy in human breast cancer. Clin Cancer Res 1997; 3:661 - 667; PMID: 9815734
- Johnstone RW, Ruefli AA, Smyth MJ. Multiple physiological functions for multidrug transporter P-glycoprotein?. Trends Biochem Sci 2000; 25:1 - 6; PMID: 10637601; http://dx.doi.org/10.1016/S0968-0004(99)01493-0
- Cizmarikova M, Wagnerova M, Schonova L, Habalova V, Kohut A, Linkova A, et al. MDR1 (C3435T) polymorphism: relation to the risk of breast cancer and therapeutic outcome. Pharmacogenomics J 2010; 10:62 - 69; PMID: 19752884; http://dx.doi.org/10.1038/tpj.2009.41
- Kafka A, Sauer G, Jaeger C, Grundmann R, Kreienberg R, Zeillnger R, et al. Polymorphism C3435T of the MDR-1 gene predicts response to preoperative chemotherapy in locally advanced breast cancer. Int J Oncol 2003; 22:1117 - 1121; PMID: 12684679
- Nakamura T, Sakaeda T, Horinouchi M, Tamura T, Aoyama N, Shirakawa T, et al. Effect of the mutation (C3435T) at exon 26 of the MDR1 gene on expression level of MDR1 messenger ribonucleic acid in duodenal enterocytes of healthy Japanese subjects. Clin Pharmacol Ther 2002; 71:297 - 303; PMID: 11956513; http://dx.doi.org/10.1067/mcp.2002.122055
- Sohn JW, Lee SY, Lee SJ, Kim EJ, Cha SI, Kim CH, et al. MDR1 polymorphisms predict the response to Etoposide-Cisplatin combination chemotherapy in small cell lung cancer. Jpn J Clin Oncol 2006; 36:137 - 141; PMID: 16478794; http://dx.doi.org/10.1093/jjco/hyi231
- von Minckwitz G, Blohmer JU, Raab G, Lohr A, Gerber B, Heinrich G, et al. In vivo chemosensitivityadapted preoperative chemotherapy in patients with early-stage breast cancer: the GEPARTRIO pilot study. Ann Oncol 2005; 16:56 - 63; PMID: 15598939; http://dx.doi.org/10.1093/annonc/mdi001
- von Minckwitz G, Kümmel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, et al. Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: phase III randomized GeparTrio trial. J Natl Cancer Inst 2008; 100:542 - 551; PMID: 18398097; http://dx.doi.org/10.1093/jnci/djn085
- Tang JH, Zhao JH, Wu JZ, Lu JW, Pan LQ, Xu ZY. Multiplex ligation-dependent SNPs genotyping method and its application in the detection of genes related to chemotherapy drugs in breast cancer. Chin J Oncol 2009; 31:108 - 113
- Eggerding FA. Fluorescent oligonucleotide ligation technology for identification of ras oncogene mutations. Mol Biotechnol 2000; 14:223 - 233; PMID: 10890013; http://dx.doi.org/10.1385/MB:14:3:223