Abstract
Triple-negative breast cancer, which is negative for the estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2, represents about 15–26% of all breast cancer cases. However, because of its genotype, a triple-negative disease accounts for a remarkable metastasis and mortality. Moreover, no targeted treatment is available because the molecular mechanism of triple-negative breast cancer initiation is still unclear. Secreted clusterin (sCLU) is associated with the refractory to anti-estrogen in breast cancer cells. We investigated the sCLU expression in 384 human breast cancer cases, including 61 triple-negative cases, as well as the relationship between sCLU and clinical pathological characteristics. Triple-negative patients (75.4%) were positive for sCLU based on immunohistochemical analysis, and sCLU expression in this subtype was proven related to a larger tumor size, an axillary node status, and a higher clinical stage. Furthermore, we used a spontaneous breast cancer mouse strain with a triple-negative genotype to detect the sCLU dynamic expression in breast cancer oncogenesis using western blot and real-time polymerase chain reaction. The sCLU mRNA and protein expression in the tumor and hyperplastic epithelium were upregulated and reached a peak compared with those of a normal mammary gland. These results suggest that sCLU is involved in the initiation of triple-negative breast cancer, which is beneficial for the clinical trial design of an anti-CLU treatment for triple-negative breast cancer.
Introduction
Breast cancer is one of the most malignant threats against women worldwide. Tumor behavior and phenotype are closely associated with tumor genotype, on which the corresponding individual therapy strategy is also based. Estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (hEGFR-2) status are the most important prognostic and predictive markers in invasive breast cancer.Citation1 ER positivity predicts the sensitivity to endocrine therapy such as anti-estrogen (tamoxifen) administration or ovarian suppression.Citation2 Similarly, hEGFR-2 positivity is useful in selecting a targeted therapy with monoclonal antibody (trastuzumab) against hEGFR-2.Citation3 These markers have become standard practice in the management of this neoplasm.Citation4
A subtype of breast cancer, the so-called triple-negative (TN) breast cancer, does not express ER, PR, or hEGFR-2.Citation5 TN breast cancer accounts for about 15–26% of all breast cancer cases.Citation6-Citation9 Regardless of the stage upon diagnosis, women with TN breast cancer have poorer survival than those with other breast cancer types because of the former’s unique genotype and clinical behavior.Citation5 TN breast cancer is more likely to be aggressive and lead to visceral metastasis. Patients suffering from this neoplasm type cannot benefit from either endocrine or anti- hEGFR-2 therapy.Citation10 Clinical trials are in progress to find an effective agent or a therapy strategy for these patients;Citation11 however, no effective drug has yet been manufactured because the molecular mechanisms of TN breast cancer progression and metastasis are still unclear.Citation12 The molecules responsible for TN breast cancer initiation and metastasis need to be identified to find predictive markers and new therapy targets.
Clusterin (CLU), also known as apolipoprotein J (APOJ), sulfated glycoprotein 2 (SGP2), complement-associated protein SP-40, and complement lysis inhibitor (CLI), was first isolated in 1983 as a prostaglandin synthetase inhibitor from testicular tissue.Citation13,Citation14 CLU encodes two isoforms, namely, secreted clusterin (sCLU) and nuclear clusterin (nCLU).Citation15,Citation16 They are expressed in virtually all tissues and are found in all human body fluids. Both are involved in numerous physiological processes important for carcinogenesis and tumor growth, including apoptotic cell death, cell cycle, DNA repair, cell adhesion, tissue remodeling, lipid transport, and immune system regulation.Citation15 Previous studies have also linked CLU expression to the induction and progression of many cancers, including breast, prostate, and lung cancers, lymphoma, ovarian carcinoma, and renal cell carcinoma.Citation17-Citation21 CLU downregulation was also found in some breast cancer patients.Citation22,Citation23 CLU expression was upregulated in breast cancers refractory to hormone treatment, and downregulation of CLU in the MCF cell line increased the cancer cell sensitivity to tamoxifen and radiation.Citation24,Citation25 The possible reasons are that different protein isoforms play different roles in breast cancer development and that CLU expression is distinguished in different breast cancer subgroups. In the current study, we used 384 breast cancer cases to investigate sCLU expression in the TN breast cancer subgroup.
Mouse model is the most common tool used in cancer research and preclinical drug tests. TA2 mouse strain has high incidence of spontaneous breast cancer without any chemical induction.Citation26 Furthermore, breast cancers from TA2 mice are negative for ER, PR, and hEGFR-2, and have high incidence of metastasis in the lung, liver, and spleen. TA2 breast cancer is extremely similar to human TN breast cancer in genotype background and behavior.Citation26 Hence, the TA2 mouse model is perfect as a means to gain a deeper insight into TN breast cancer progression and to find new therapeutic approaches. We collected normal mammary glands, mammary glands with hyperplasia, and breast cancer cells to investigate the dynamic expression of sCLU in TN breast cancer initiation.
Results
Pathological and clinical features of TN breast cancer
Based on the IHC results, 15.8% (61/384) of the cancer cases was triple-negative, and the remaining 323 cases were classified as other breast cancers via immunohistochemistry for ER, PR, and hEGFR-2 expression. TN breast cancer was composed of poorly differentiated small tumor cells. Necrosis occurred in the center of a tumor nest. Abundant mitoses were characteristics of human TN breast cancer (). TN tumor cells were negative for ER, PR, and hEGFR-2, but strong expressions of p53 and CK5/6 were detected (). shows the pathological and clinical features of TN and non-TN breast cancer cases. The median ages upon diagnosis for the TN and non-TN groups were 50 and 48 y, respectively. The tumor diameters of TN breast cancer were over 2 cm when they were diagnosed. Approximately 5.1% (16/323) of the cases were other breasts cancers, whose tumor size was less than 2 cm. Similarly, all patients suffering from TN breast cancer were diagnosed in clinical stage II or III. The axillary node metastasis in both groups showed no statistical difference, and was about 40%. The number of patients in the TN group undergoing chemotherapy before surgery was higher than in the non-TN group, showing a significant difference. The trend was similar in chemotherapy frequency after surgery. Approximately 67.6% of the patients with TN breast cancer underwent chemotherapy after surgery more than six times, whereas only 40.0% in the non-TN group were treated in the same stage. During the follow-up, 8.1% of the TN breast cancer and 4.6% of the non-TN breast cancer reoccurred. About 30% of the TN breast cancer spread to distant organs, especially in the lung, liver, and bone, and is significantly higher than the 19.4% in the non-TN group.
Figure 1. Morphological characteristics of human triple-negative breast cancer (TNBC) and non-TNBC. (A) H&E staining of a TNBC and a non-TNBC. Tumor nests are composed of poorly differentiated small tumor cells. Necrosis in the center of a tumor nest is observed (black arrow). A number of tumor cells are undergoing mitosis (red arrows). IHC for ER, PR and hEGFR-2 indicate that the TNBC is negative for them, while the non-TNBC expresses PR and hEGFR-2. (B) IHC for p53, CK5/6 and CLU. CLU expression is localized in the cytoplasm of breast cancer cells, showing that the CLU identified in this case is sCLU. There is no expression of CLU in the non-TNBC. Ruler is 100 μm.
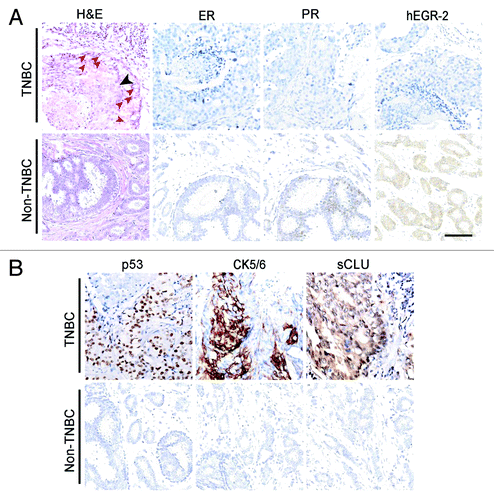
Table 2. Comparison of pathological and clinical features between triple-negative and non-triple-negative breast cancer cases
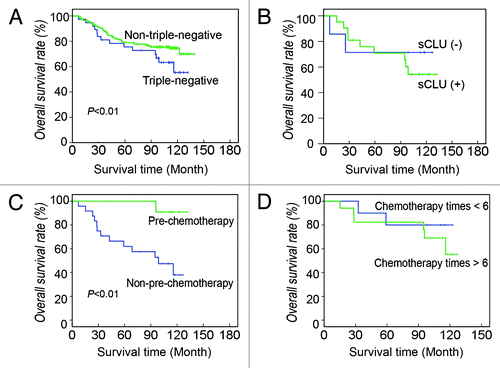
At the end of the follow-up, the survival rate of non-TN breast cancer patients was 73.0% (236/323), whereas only 62.2% (38/61) of TN breast cancer patients survived. The mean survival times of the two groups were 100.36 ± 7.26 and 113.76 ± 3.25 mo, respectively. The Kaplan–Meier analysis indicates that the prognosis of the TN group was poorer than that of the non-TN group (, χ2 = 4.595, p = 0.032).
Figure 2. Survival of TN and non-TN breast cancer patients. (A) Overall survival rate of TN and non-TN breast cancer patients. At the end of the follow-up, 62.2% of TN patients survive, which is significantly less than that of non-TN patients. Kaplan-Meier survival analysis shows that the outcome of TN breast cancer is poorer than that of non-TN breast cancer (χ2 = 4.595, p = 0.032). (B) Effect of sCLU expression on TN breast cancer survival. In TN breast cancer patients, 70% of the sCLU-negative patients survive, whereas it is only 50% in the sCLU-positive group. (C) Effect of prechemotherapy on TN breast cancer survival. The survival rate of TN breast cancer patients pretreated with chemotherapy is nearly 90%, whereas it is less than 40% in the non-pretreatment group. Kaplan-Meier survival analysis shows that the difference is statistically significant (χ2 = 6.716, p = 0.010). (D) Effect of chemotherapy frequency after surgery on TN breast cancer survival. Approximately 80% of the patients that underwent less chemotherapy survive, whereas the survival rate of patients that underwent more chemotherapy is about 50%.
sCLU expression and TN breast cancer clinical and pathological factors
IHC shows yellow grains in the cytoplasm of breast cancer cells but not in the nuclei, which demonstrates that breast cancer cells express sCLU (). sCLU-positive cases accounted for 75.4% (46/61) in the TN group, which is remarkably higher than the 53.9% (174/323) in the non-TN group (, χ² = 3.328, p = 0.050). The effects of sCLU on the pathological and clinical factors in the TN group were also determined. The tumor size of the sCLU-positive TN breast cancer was significantly larger than that of sCLU-negative cases. Approximately 41.3% of sCLU-positive cases and 6.7% of sCLU-negative cases were stage III upon diagnosis. Approximately 67.4% of sCLU-positive breast cancer invaded the axillary node, whereas the value was less than 40% in the sCLU-negative group. However, no significant difference in age, reccurrence, and distant metastasis was observed between the sCLU-positive and sCLU-negative cases (). The Kaplan–Meier analysis indicates no difference in the clinical outcome between the sCLU-positive and sCLU-negative women in the TN group (χ2 = 0.495, p = 0.482, ). The survival of the TN breast cancer patients pretreated with chemotherapy was longer than those without pretreatment (χ2 = 6.716, p = 0.010, ), whereas chemotherapy frequency after surgery did not improve the outcome of TN breast cancer (χ2 = 0.480, p = 0.489, ).
Table 3. Comparison of pathological and clinical features between sCLU-negative and positive triple-negative breast cancer cases
Histological characteristics and genotype of spontaneous breast cancer in TA2 mice
The spontaneous breast cancers of 10 TA2 mice were collected. Foci of gross necrosis and hemorrhage were commonly found in the tumor centers. Visceral metastasis was frequent, but metastatic lesion was rarely detected in the lymph nodes. Eight mice exhibited lung metastasis, and five showed simultaneous liver and spleen metastases.
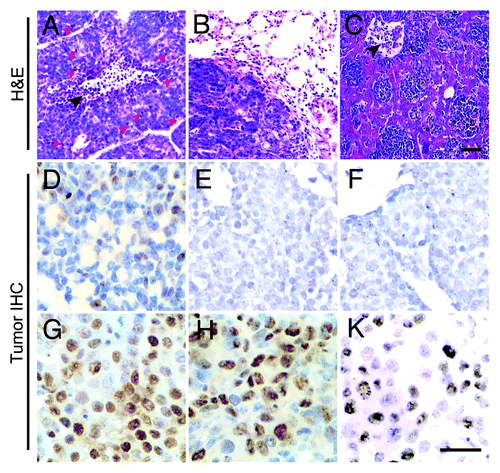
Breast cancer from 10 TA2 mice had the same histological type. Tumors were mostly composed of small round cells with small cytoplasms. The center of the solid tumor nest had masses of undifferentiated or poorly differentiated cells, and many cells were undergoing mitosis. Some tumor cells, especially at the periphery of tumor nests, may differentiate into acinar, glandular, and papillary patterns. A necrotic lesion in the center of a tumor nest was found (). show lung and liver metastases. IHC indicates that TA2 breast cancer cells were negative for ER, PR, and hEGFR-2 (). A strong p53 expression was detected in approximately 60% of the tumor cells. In addition, the expression ratio of cyclin D1 and proliferating cell nuclear antigen (PCNA) reached 40% to 50% in the tumors (). These results reveal that TA2 breast cancer has a high proliferative activity.
Figure 3. Morphological characteristics of TA2 spontaneous breast cancer. (A) TA2 spontaneous breast cancer is mostly composed of small round cells with few cytoplasms, and they form various tumor nests separated by well-developed stroma. The center of the solid tumor nest show necrosis (black arrow), and several cells are undergoing mitosis (red arrows). (B) TA2 spontaneous breast cancer metastasis in the lung. (C) TA2 spontaneous breast cancer metastasis in the liver. Tumors nest in the sinusoidal vessels of the liver; the black arrow indicates a necrotic area. (D) IHC for ER. Several tumor cells are positive for ER staining. (E) IHC for PR. No tumor cell expressing PR is found. (F) IHC for hEGFR-2. TA2 spontaneous breast cancer is negative for hEGFR-2. (G) IHC for p53. Mild to moderate p53 expression is detected in the tumor cells. (H) IHC for cyclin D1. Cyclin D1 expression is high in the neoplastic epithelial cells, indicating the high proliferative activity of breast cancer. (I) IHC for PCNA. Approximately 50% of the breast cancer cells express PCNA, indicating the high proliferation rate of TA2 breast cancer. Rulers are 100 μm.
sCLU expression during TA2 spontaneous breast cancer progression
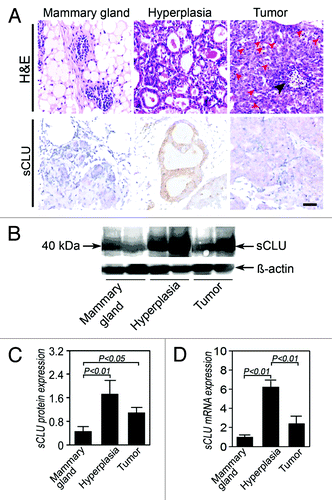
To investigate the role of sCLU in the oncogenesis of TN breast cancer, we collected mammary glands from virgin TA2 and mammary tissues from mice with spontaneous tumors identified as hyperplastic via histology (). IHC, western blot, and real-time PCR were performed to detect the sCLU expression in the three groups. IHC for CLU (M-18) shows that the CLU expression is localized in the cytoplasm, and western blot for CLU identified a 40 kDa protein (), which was identified as sCLU and not nCLU. sCLU expression in the hyperplastic mammary epithelium and breast cancer cells was significantly stronger than that in normal mammary glands. Remarkably, more sCLU proteins were found in the hyperplastic mammary glands than in spontaneous breast cancer cells (). The expression pattern of sCLU mRNA is similar to that of the sCLU protein among the three groups (). The differences are statistically significant.
Figure 4. sCLU expression during TA2 spontaneous breast cancer progression. (A) H&E staining shows the morphology of a normal mammary gland, hyperplastic mammary glands, and breast cancer cells. Compared with the normal mammary gland, the number of cells and cell layers increase in the hyperplastic gland, and the cell and nucleus sizes also increase. IHC shows that the positive signal is localized in the cytoplasm, indicating that the protein is sCLU and not nCLU. A strong sCLU expression is detected in the mammary hyperplasia, whereas several mammary gland endothelial cells express sCLU. The sCLU expression in tumor cells is moderate. Ruler is 100 μm. (B) Western blot for clusterin (M-18). The expression of the 40 kDa sCLU is highest in the hyperplastic mammary epithelium, whereas the mammary epithelium of normal TA2 mice shows the lowest pre-sCLU expression. (C) Quantification of sCLU protein expression in the three groups (n = 4). The sCLU protein level in the normal mammary epithelium is significantly lower than that in the other groups. (D) sCLU mRNA expression among the three groups (n = 6). The hyperplastic mammary epithelium from TA2 mice with spontaneous breast cancer shows the highest sCLU mRNA expression among the three groups, whereas sCLU is the lowest in the mammary gland of normal TA2 mice. Spontaneous breast cancer expresses a middle level sCLU expression. Significant differences are found. The error bar means standard deviation (SD).
Discussion
Recently, an increasing number of researchers focus on TN breast cancer because of its unique pathological and clinical features. An improved descriptive analysis has provided greater insight into the epidemiology, morphology, genotype background, clinical behavior, and outcome of TN breast cancer. However, the molecular mechanism involved in this unique subtype is still unclear. We investigated the role of sCLU, an apoptosis chaperone, in human and mouse TN breast cancers for potential use as a therapeutic target for TN breast cancer treatment.
Among the 384 breast cancer cases, 15.8% were ER-negative, PR-negative, and HER-2-negative. This incidence in Tianjin is slightly higher than that involving Asians living in the US, and lower than the cases in South China.Citation6,Citation8,Citation9 The TN breast cancer size is larger than that of non-TN cases, which suggests that TN breast cancer cells proliferate rapidly. This has been proven by the high occurrence of mitosis and high-level p53 expression. Moreover, a big tumor size was associated with a high rate of prechemotherapy treatment before surgery in the TN group. A review of the breast cancer subgroups shows that chemotherapy of adjuvant and neoadjuvant settings is highly effective in the TN breast cancer patients.Citation27 In the current study, prechemotherapy improved the 10-y overall survival in the TN subgroup from 35–90%. However, they did not benefit from more frequent chemotherapy after surgery. TN breast cancer can develop refractory to chemotherapy after several cycles. These results suggest that a neoadjuvant treatment with suitable chemotherapy is more efficient than adjuvant chemotherapy for TN breast cancer.
Previously, the relationship between sCLU overexpression and metastasis and poor survival in breast cancer was established,Citation20 which is consistent with those of other clinical studies.Citation23,Citation24 sCLU can inhibit cell apoptosis and protect cells from stress, and its expression is closely associated with tumor initiation and progression. In breast cancer, CLU expression at both the protein and RNA levels has been correlated with metastasis to the lymph nodes as well as to ER-negative and PR-negative subtypes;Citation22,Citation28 however, its function in different breast cancer subtypes has not been investigated.
In the current study, we compared the sCLU expression between a human TN breast cancer group and a non-TN group. The results reveal that sCLU-positive cases accounted for 75.4% in the TN group, which is remarkably higher than that in the non-TN group. Furthermore, sCLU expression in the TN subgroup was proven related to a larger tumor size, an axillary node status, and a higher clinical stage upon diagnosis, which are associated with tumor initiation and growth in situ. This result supports the argument that sCLU in TN breast cancer is linked to a malignant phenotype. However, no significant difference in relapse and distant metastasis between the sCLU-positive and sCLU-negative cases was observed. Moreover, sCLU expression had no significant effect on the overall survival of TN breast cancer women patients. These observations suggest that sCLU is involved in the initiation of TN breast cancer but is not associated with its distant metastasis.
Retrospective studies on human breast cancer and cell experiments in vitro indirectly suggest that CLU may be involved in TN breast cancer oncogenesis.Citation22,Citation24,Citation29 However, because of the difficulty in acquiring human mammary glands, no evidence was obtained to show the dynamic change in CLU in TN breast cancer onset in patients. The TA2 mouse is a beneficial animal model for TN breast cancer. In the current study, we found that spontaneous breast cancer in TA2 mice shared similar characteristics with human TN breast cancer. Over 80% of TA2 mice developed breast cancer without any chemical stimuli. These tumors show a TN genotype, poor differentiation, pushing margins, high mitotic index, high proliferation rate, and high incidence of lung, liver, and spleen metastases, which are consistent with human TN breast cancer data. As previously mentioned, sCLU is associated with TA2 breast cancer. Hence, a TA2 mouse with spontaneous breast cancer is a good model to study the role of sCLU expression changes in the development of TN breast cancer.
In the present study, we collected normal mammary glands, hyperplastic mammary epithelium cells, and spontaneous breast cancer cells of TA2 to analyze the sCLU protein expression and CLU mRNA level. The results indicate that the level of sCLU expression is high in spontaneous breast cancer but low or absent in normal mammary glands. Remarkably, sCLU expression reached a peak in mammary epithelial hyperplasia, confirming that sCLU is upregulated in TN breast cancer. Given the similarity between TA2 and human TN breast cancer, sCLU may act as an important promoter in human TN breast cancer progression. Human TN breast cancer and TA2 breast cancer both expression high level of p53, cyclin D1 and proliferation-associated protein. p53 activation has been proved to be essential for mitochondrial apoptosis and growth retardation, respectively, in sCLU-depleted human osteosarcoma cells.Citation30 The expression of clusterin in human non-small cell lung cancer was positively correlated with the expression of p53, but was negatively related to the expression of Bax.Citation31 These suggested that sCLU might inhibit cell apoptosis by suppressing p53 activity in TN breast cancer initiation.
Given that sCLU is directly or indirectly responsible for the resistance to anti-estrogen therapy and chemotherapy in prostate and breast cancers, an antisense oligonucleotide (ASO) directed against CLU mRNA and anti-CLU monoclonal antibodies have been developed and approved for clinical trials.Citation32-Citation34 Despite the promising results obtained in vitro in MCF-7 breast cancer cells and in vivo in MCF-7-bearing athymic mice,Citation32 the recently published results of the phase II clinical trials are disappointing. The combination of CLU ASO (OGX-011) and docetaxel did not improve the response of metastatic breast cancer to docetaxel.Citation35-Citation37 Our results suggest that an anti-CLU treatment will be more efficient for TN breast cancer than for other breast cancer subtypes, and can be beneficial to the clinical trial design of OGX-11 for breast cancer treatment.
Materials and Methods
Patient samples
All human studies were approved by the Tianjin Cancer Hospital Ethics Committee. The patients involved were informed of the aims, methods, and other details of the medical research. Each signed a statement approving the use of their clinical and pathological information and samples for research. All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. For the present study, 384 breast cancer patients with detailed pathologic and clinical information were gathered for the identification of CLU expression in human TN breast cancer. All patients underwent surgery and chemotherapy at Tianjin General Hospital from 1993 to 1994. The median age of the patients was 50.0 y (27–78 y). The patients had invasive breast cancer, and 217 cases showed axillary node metastasis. The primary tumor diameter in 16 cases was less than 2 cm, and that in 117 cases was over 5 cm. Eight cases were in clinical stage I and 259 cases in stage II. The patients were treated with a combination of cyclophosphamide (CTX), methotrexate (MTX), and 5-fluorouracil (5-Fu). On day 1 and 8, CTX (600 mg/m2), MTX (40 mg/m2), and 5-Fu (500 mg/m2) were intravenously administered. One cycle lasted for four weeks. A total of 119 patients were treated for one cycle before surgery. After surgery, all patients underwent a treatment consisting of a CTX, MTX, and 5-Fu combination. The follow-up ranged from the surgical time to May 2005.
Tissue microarray and counting methods
Formalin-fixed, paraffin-embedded tissues from the patients were analyzed after staining with standard hematoxylin and eosin (H&E), and specific tissues were then chosen to prepare a tissue microarray with 1 mm diameter cores (1.5 mm between cores). Immunohistochemical staining (IHC) was performed on the tissue microarray sections using a standard protocol. All mouse anti-human monoclonal antibodies, including anti-p53 (MAB-0239), CK5/6 (MAB-0276), ER (MAB-0062), PR (MAB-0236), c-erbB-2/ hEGFR-2 (MAB-0198), and secondary anti-mouse antibodies (KIT-9701), were purchased from Maixin Biotechnology Co., Ltd. Goat polyclonal anti-CLU antibody (M-18, sc-6420, dilution 1:100) was purchased from Santa Cruz Biotechnology. Protein expression was quantified according to the method described by Sun et al.Citation20 For stain intensity, 0 denotes no stain, 1 denotes weak positive, 2 means moderate, and 3 means strong. The number of positive cells in 100 tumor cells per field was visually evaluated and scored as follows: 0 for < 10% positive cells, 1 for < 25%, 2 for < 50%, and 3 for > 50%. The sum (staining index) of the stain intensity and positive cell scores was used to determine the final result for each sample. The sample is defined as positive when the staining index is above 1.
Animals and groups
The protocols in the animal experiments were approved by the Tianjin Medical University Ethics Committee. All steps were taken to protect animal welfare and to prevent animals from suffering. Tumor masses and mammary glands from 10 TA2 mice with spontaneous breast cancer were collected after they were sacrificed. The average time for tumor development was 254.25 d. Mammary glands from 10 female virgin TA2 mice aged approximately 220 d were collected as the control. Breast cancer cells, hyperplastic mammary glands, and normal mammary epithelium were identified by morphology and were used in IHC, real-time polymerase chain reaction (PCR), and western blot.
Animals and groups
The protocols in the animal experiments were approved by the Tianjin Medical University Ethics Committee (permit number 20081279). All steps have been taken to protect the animal welfare and prevent suffering. Tumor masses and mammary glands from 10 TA2 mice with spontaneous breast cancer were collected, after they were sacrificed. The average time for tumor development was 254.25 d. Mammary glands from 10 female virgin TA2 mice aging approximate 220 d were collected as the control. Breast cancer, hyperplasic mammary glands and normal mammary epithelium were identified by morphology and used in IHC, real-time PCR and western blot.
Western blot
CLU isoform protein expression was assessed by western blot. Four samples were collected from every group. All agents were purchased from Santa Cruz Biotechnology. Total protein was obtained using a lysis buffer (1% SDS, 10 Mm Tris-Hcl, pH 7.6, 20 μg/ml aprotinin, 20 μg/ml leupeptin and 1 mM AEBSF) and the protein concentration was measured with Bradford method. Twenty micrograms of protein were separated on an 8% SDS-PAGE gel and blotted onto a PVDF membrane. After blocking with 5% fat-free milk in TBS-Tween overnight, the membrane was incubated with goat polyclonal anti-CLU M-18 IgG antibody (1:1,000) for 2 h at room temperature. After washing thrice TBS-Tween, the membrane was labeled with horseradish peroxidase-conjugated anti-goat IgG (1:1,000) for 1 h at room temperature. Blots were developed with a DAB kit. β-actin (sc-1616) was used as an internal control. The bands for samples were analyzed with a gel imaging system (Kodak). The gray-scale ratio of CLU to β-actin in every sample was considered as the relative protein level of CLU.
Immunohistochemical staining
Five-micrometer-thick paraffin-embedded sections were made and stained immunohistochemically (SP method). Rabbit polyclonal antibodies, including p53 (FL-393), cyclin D1 (H-295), PCNA (FL-261), ER (MC-20), PR (C-20), mouse monoclonal Neu antibody (F-11) and secondary anti mouse antibody (KIT-9701) were purchased from Zhongshan Goldenbridg Biotechnology Co. Anti-CLU (M-18, dilution 1:100) was from Santa Cruz Biotechnology. Sections were deparaffinized with xylen and rehydraded through graded alcohols. Endogenous peroxidase was blocked with 3% hydrogen peroxide at room temperature for 10 min. The sections were pretreated in a microwave oven in citrate buffer for 20 min. The slides were incubated with primary antibodies overnight at 4°C, washed with PBS, and incubated with the biotinylated secondary antibody and preformed avidin-biotinylated peroxidase complex by turns (consistent with western blot). The color was developed with DAB. Finally, the sections were counterstained with hematoxylin. PBS was used in place of primary antibody to serve as a negative control.
Real-time PCR
Total RNA was isolated from frozen samples using TRIzol reagent (Invitrogen) according to the instruction. Six samples were collected from every group. cDNA was synthesized through reverse transcription. Real-time PCR analysis was performed using the Gene AMP PCR System 5700 Sequence Detector. The cDNA was used as the template to be amplified in a 25 μl reaction mixture (Premix Ex Taq, TaKaRa Biotechnology Co., Ltd.). β-actin was the internal control. CT value was determined and 2-ΔΔCT where ΔΔCT = (CT target-CT β-actin) sample - (CT target-CT β-actin) control was defined as the relative quantity of the amplified fragment. Every sample was tested in triplicate and the mean value was used. The primers used for pre-sCLU and β-actin and the best annealing temperature were listed in .
Table 1. Primers used in this study
Statistical methods
SPSS 11.0 was used to evaluate the data. The test was used to assess the difference in CLU expression and the pathological and clinical factors between TN and non-TN groups. Survival was analyzed using the Kaplan–Meier analysis. The standard two-tailed Student’s t-test was performed to compare the differences of the CLU protein and mRNA expression in TA2 mice. The significance level was defined as p < 0.05.
| Abbreviations: | ||
| ER | = | estrogen receptor |
| PR | = | progesterone receptor |
| hEGFR-2 | = | human epidermal growth factor receptor 2 |
| TN | = | triple-negative |
| CLU | = | clusterin |
| APOJ | = | apolipoprotein J |
| SGP2 | = | sulfated glycoprotein 2 |
| CLI | = | complement lysis inhibitor |
| sCLU | = | secreted clusterin |
| nCLU | = | nuclear clusterin |
| ASO | = | antisense oligonucleotide |
Acknowledgments
This study was supported by the Key Project of National Nature Science Foundation of China (No. 30830049), the National Nature Science Foundation of China (No. 30770828), the co-operation of China-Sweden (No. 09ZCZDSF04400), the Tianjin Natural Science Foundation (No. 09JCYBJC12100), and the 973 Program from the Ministry of Science and Technology of China (No. 2011CB933104). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- von Minckwitz G, Untch M, Nüesch E, Loibl S, Kaufmann M, Kümmel S, et al. Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat 2011; 125:145 - 56; http://dx.doi.org/10.1007/s10549-010-1228-x; PMID: 21042932
- Tischkowitz M, Brunet J-S, Bégin LR, Huntsman DG, Cheang MCU, Akslen LA, et al. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer 2007; 7:134; http://dx.doi.org/10.1186/1471-2407-7-134; PMID: 17650314
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer 2007; 109:1721 - 8; http://dx.doi.org/10.1002/cncr.22618; PMID: 17387718
- Takei H, Kurosumi M, Yoshida T, Hayashi Y, Higuchi T, Uchida S, et al. Neoadjuvant endocrine therapy of breast cancer: which patients would benefit and what are the advantages?. Breast Cancer 2011; 18:85 - 91; http://dx.doi.org/10.1007/s12282-010-0239-0; PMID: 21104350
- Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 1998; 58:2825 - 31; PMID: 9661897
- Pal SK, Childs BH, Pegram M. Triple negative breast cancer: unmet medical needs. Breast Cancer Res Treat 2011; 125:627 - 36; http://dx.doi.org/10.1007/s10549-010-1293-1; PMID: 21161370
- Lund MJB, Butler EN, Bumpers HL, Okoli J, Rizzo M, Hatchett N, et al. High prevalence of triple-negative tumors in an urban cancer center. Cancer 2008; 113:608 - 15; http://dx.doi.org/10.1002/cncr.23569; PMID: 18484596
- Yuan Z-Y, Wang S-S, Gao Y, Su Z-Y, Luo W-B, Guan Z-Z. Clinical characteristics and prognosis of triple-negative breast cancer: a report of 305 cases. Ai Zheng 2008; 27:561 - 5; PMID: 18570725
- Yin W-J, Lu J-S, Di G-H, Lin Y-P, Zhou L-H, Liu G-Y, et al. Clinicopathological features of the triple-negative tumors in Chinese breast cancer patients. Breast Cancer Res Treat 2009; 115:325 - 33; http://dx.doi.org/10.1007/s10549-008-0096-0; PMID: 18563552
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010; 363:1938 - 48; http://dx.doi.org/10.1056/NEJMra1001389; PMID: 21067385
- De Laurentiis M, Cianniello D, Caputo R, Stanzione B, Arpino G, Cinieri S, et al. Treatment of triple negative breast cancer (TNBC): current options and future perspectives. Cancer Treat Rev 2010; 36:Suppl 3 S80 - 6; http://dx.doi.org/10.1016/S0305-7372(10)70025-6; PMID: 21129616
- Santana-Davila R, Perez EA. Treatment options for patients with triple-negative breast cancer. J Hematol Oncol. 2010; 3:42; http://dx.doi.org/10.1186/1756-8722-3-42; PMID: 20979652
- Rosenberg ME, Silkensen J. Clusterin: physiologic and pathophysiologic considerations. Int J Biochem Cell Biol 1995; 27:633 - 45; http://dx.doi.org/10.1016/1357-2725(95)00027-M; PMID: 7648419
- Sallman DA, Chen X, Zhong B, Gilvary DL, Zhou J, Wei S, et al. Clusterin mediates TRAIL resistance in prostate tumor cells. Mol Cancer Ther 2007; 6:2938 - 47; http://dx.doi.org/10.1158/1535-7163.MCT-07-0345; PMID: 18025278
- Caccamo AE, Scaltriti M, Caporali A, D’Arca D, Corti A, Corvetta D, et al. Ca2+ depletion induces nuclear clusterin, a novel effector of apoptosis in immortalized human prostate cells. Cell Death Differ 2005; 12:101 - 4; http://dx.doi.org/10.1038/sj.cdd.4401491; PMID: 15499376
- Lakins JN, Poon S, Easterbrook-Smith SB, Carver JA, Tenniswood MPR, Wilson MR. Evidence that clusterin has discrete chaperone and ligand binding sites. Biochemistry 2002; 41:282 - 91; http://dx.doi.org/10.1021/bi0157666; PMID: 11772027
- Zellweger T, Miyake H, July LV, Akbari M, Kiyama S, Gleave ME. Chemosensitization of human renal cell cancer using antisense oligonucleotides targeting the antiapoptotic gene clusterin. Neoplasia 2001; 3:360 - 7; http://dx.doi.org/10.1038/sj.neo.7900174; PMID: 11571636
- Hara I, Miyake H, Gleave ME, Kamidono S. Introduction of clusterin gene into human renal cell carcinoma cells enhances their resistance to cytotoxic chemotherapy through inhibition of apoptosis both in vitro and in vivo. Jpn J Cancer Res 2001; 92:1220 - 4; http://dx.doi.org/10.1111/j.1349-7006.2001.tb02143.x; PMID: 11714447
- Rizzi F, Bettuzzi S. Targeting Clusterin in prostate cancer. J Physiol Pharmacol 2008; 59:Suppl 9 265 - 74; PMID: 19261985
- Zhang S, Zhang D, Zhu Y, Guo H, Zhao X, Sun B. Clusterin expression and univariate analysis of overall survival in human breast cancer. Technol Cancer Res Treat 2006; 5:573 - 8; PMID: 17121433
- Shannan B, Seifert M, Leskov K, Willis J, Boothman D, Tilgen W, et al. Challenge and promise: roles for clusterin in pathogenesis, progression and therapy of cancer. Cell Death Differ 2006; 13:12 - 9; http://dx.doi.org/10.1038/sj.cdd.4401779; PMID: 16179938
- Redondo M, Villar E, Torres-Muñoz J, Tellez T, Morell M, Petito CK. Overexpression of clusterin in human breast carcinoma. Am J Pathol 2000; 157:393 - 9; http://dx.doi.org/10.1016/S0002-9440(10)64552-X; PMID: 10934144
- Krüger S, Ola V, Fischer D, Feller AC, Friedrich M. Prognostic significance of clusterin immunoreactivity in breast cancer. Neoplasma 2007; 54:46 - 50; PMID: 17203891
- Flanagan L, Whyte L, Chatterjee N, Tenniswood M. Effects of clusterin over-expression on metastatic progression and therapy in breast cancer. BMC Cancer 2010; 10:107; http://dx.doi.org/10.1186/1471-2407-10-107; PMID: 20307318
- Sutton D, Kim S, Shuai X, Leskov K, Marques JT, Williams BRG, et al. Efficient suppression of secretory clusterin levels by polymer-siRNA nanocomplexes enhances ionizing radiation lethality in human MCF-7 breast cancer cells in vitro. Int J Nanomedicine 2006; 1:155 - 62; http://dx.doi.org/10.2147/nano.2006.1.2.155; PMID: 17722531
- Sun B, Zhang S, Zhang D, Li Y, Zhao X, Luo Y, et al. Identification of metastasis-related proteins and their clinical relevance to triple-negative human breast cancer. Clin Cancer Res 2008; 14:7050 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-08-0520; PMID: 18981002
- Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011; 121; http://dx.doi.org/10.1172/JCI45014; PMID: 21633166
- Rizzi F, Bettuzzi S. The clusterin paradigm in prostate and breast carcinogenesis. Endocr Relat Cancer 2010; 17:R1 - 17; http://dx.doi.org/10.1677/ERC-09-0140; PMID: 19903745
- Cappelletti V, Gariboldi M, De Cecco L, Toffanin S, Reid JF, Lusa L, et al. Patterns and changes in gene expression following neo-adjuvant anti-estrogen treatment in estrogen receptor-positive breast cancer. Endocr Relat Cancer 2008; 15:439 - 49; http://dx.doi.org/10.1677/ERC-07-0274; PMID: 18508997
- Trougakos IP, Lourda M, Antonelou MH, Kletsas D, Gorgoulis VG, Papassideri IS, et al. Intracellular clusterin inhibits mitochondrial apoptosis by suppressing p53-activating stress signals and stabilizing the cytosolic Ku70-Bax protein complex. Clin Cancer Res 2009; 15:48 - 59; http://dx.doi.org/10.1158/1078-0432.CCR-08-1805; PMID: 19118032
- Niu L, Zang J, Cai L, Li C, Cao H. Relationship of clusterin expression with Bax and p53 expression in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2007; 10:284 - 7; PMID: 21122294
- So A, Sinnemann S, Huntsman D, Fazli L, Gleave M. Knockdown of the cytoprotective chaperone, clusterin, chemosensitizes human breast cancer cells both in vitro and in vivo. Mol Cancer Ther 2005; 4:1837 - 49; http://dx.doi.org/10.1158/1535-7163.MCT-05-0178; PMID: 16373699
- Chi KN, Hotte SJ, Yu EY, Tu D, Eigl BJ, Tannock I, et al. Randomized phase II study of docetaxel and prednisone with or without OGX-011 in patients with metastatic castration-resistant prostate cancer. J Clin Oncol 2010; 28:4247 - 54; http://dx.doi.org/10.1200/JCO.2009.26.8771; PMID: 20733135
- Chi KN, Eisenhauer E, Fazli L, Jones EC, Goldenberg SL, Powers J, et al. A phase I pharmacokinetic and pharmacodynamic study of OGX-011, a 2’-methoxyethyl antisense oligonucleotide to clusterin, in patients with localized prostate cancer. J Natl Cancer Inst 2005; 97:1287 - 96; http://dx.doi.org/10.1093/jnci/dji252; PMID: 16145049
- Chi KN, Siu LL, Hirte H, Hotte SJ, Knox J, Kollmansberger C, et al. A phase I study of OGX-011, a 2’-methoxyethyl phosphorothioate antisense to clusterin, in combination with docetaxel in patients with advanced cancer. Clin Cancer Res 2008; 14:833 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-07-1310; PMID: 18245546
- Chia S, Dent S, Ellard S, Ellis PM, Vandenberg T, Gelmon K, et al. Phase II trial of OGX-011 in combination with docetaxel in metastatic breast cancer. Clin Cancer Res 2009; 15:708 - 13; http://dx.doi.org/10.1158/1078-0432.CCR-08-1159; PMID: 19147778
- Zoubeidi A, Chi K, Gleave M. Targeting the cytoprotective chaperone, clusterin, for treatment of advanced cancer. Clin Cancer Res 2010; 16:1088 - 93; http://dx.doi.org/10.1158/1078-0432.CCR-09-2917; PMID: 20145158