Abstract
Vasculogenic mimicry (VM), a process involving the formation of a tubular structure by highly invasive and genetically dysregulated tumor cells, can supplement the function of blood vessels to transport nutrients and oxygen to maintain the growth of tumor cells in many malignant tumors. We aimed to explore the existence of VM and its clinical significance in medulloblastoma in this study. VM was identified in 9 out of 41 (22%) medulloblastoma tissues. Immunohistochemical studies revealed that the presence of VM was associated with the expression of MMP-2, MMP-14, EphA2 and laminin 5γ2. Tumor tissues with VM were associated with lower microvessel density (MVD), which was indirect evidence of the blood supply function of VM. Survival analysis and log-rank tests showed that patients with VM had shorter overall survival time than those without VM. Multivariate analysis and the Cox proportional hazards model identified VM as independent prognostic factor for overall survival. Our results confirmed the existence of VM for the first time and revealed that VM is a strong independent prognostic factor for survival in patients with medulloblastoma.
Introduction
Medulloblastoma is the most common primary malignant brain tumor in children and young adults. Over the past three decades, many medulloblastoma patients have successfully been cured by multimodal treatments, including surgery, postsurgical radiotherapy and chemotherapy. However, these treatments also resulted in chronic suffering for patients. Cognitive dysfunction and neurological problems were two of the most common complications. Moreover, evidence indicates that the prognosis for medulloblastoma is still poor in a significant proportion of patients.Citation1 Thus, to develop more effective and harmless treatments, we need to explore medulloblastoma in detail.
Angiogenesis has been considered to be the most important way for tumors to sustain growth. Without angiogenesis, tumors cannot grow beyond 2–3 mm3.Citation2 Current research on vasculogenic mimicry (VM) has reinforced this viewpoint. In 1999, Maniotis et al. reported a new blood supply system independent of endothelial vessels in malignant melanoma, named VM. VM is defined as a fluid-conducting channels formed by highly invasive and genetically dysregulated melanoma cells. Endothelial cells are not present in VM channels as detected by light microscopy, immunohistochemistry and transmission electron microscopy.Citation3 Studies revealed that VM exists in many malignant tumors,Citation4-Citation8 and VM is a prognostic factor for poorer clinical outcome.Citation9-Citation13 Research on angiogenesis inhibitors such as Anginex, TNP-470, and endostatin revealed that they could abrogate new vessels formed by human vascular endothelial cells in vitro. Interestingly, these inhibitors did not affect tumor cell VM formation under the same conditions and even induced the formation of VM.Citation14 Medulloblastoma is abundant in vessels, but results from anti-angiogenic treatments are far from satisfactory.Citation15 These data suggest that VM functions as a supplementary blood supply system for the growth of tumors and contributes to the failure of anti-angiogenic treatments aimed at depriving tumors of blood supply.
This is the first study demonstrating the presence of VM and its clinical significance in medulloblastoma. These findings will provide additional information that will hopefully lead to improvement in targeted therapies for medulloblastoma.
Results
Clinical characteristics of the patients
and summarize the clinical and pathological characteristics of 41 medulloblastoma patients.
Table 1. Clinical data of medulloblastoma patients
Table 2. The association of clinicopothological data and VM in medulloblastoma patients
VM exists in medulloblastoma
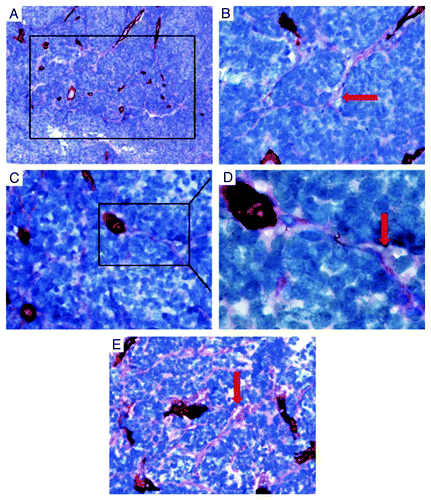
We used the CD34 antigen as a hallmark to identify endothelial cells, and we used periodic acid–Schiff (PAS) staining to highlight the basement membrane of tumor blood vessels and the PAS-positive patterns in medulloblastoma tissue sections. Tumor blood vessels positive for CD34 showed brown staining on the luminal surface and a PAS-positive reaction in the basement membrane. CD34-positive blood vessels played a dominant role in most cases. However some channels had characteristics clearly different from blood vessels in 9 out of 41 cases. The CD34-negative PAS-positive channels were found in 5 out of 25 classic medulloblastomas, 2 out of 5 desmoplastic medulloblastomas and 2 out of 4 large cell/anaplastic (LCA) medulloblastomas. The PAS-positive patterns, also described as a fluid-conducting ECM meshwork lined with tumor cells, coexisted with endothelial cell-lined blood vessels and had direct connections with them. The PAS-positive patterns extended directly from CD34-positive vessels, branched out into several smaller PAS-positive patterns which interconnected into a cluster of back-to-back looping patterns. At high magnification, translucent red blood cells were found spreading along the PAS-positive patterns (). In the adjacent tissue sections, we stained with eosin after PAS-CD34 dual staining and red blood cells were highlighted. We confirmed red blood cells spreading along PAS-positive patterns. In some regions, the patterns were splayed open, showing hollow channels containing red blood cells (). These characteristics strongly supported the belief that the PAS-positive patterns were a part of the medulloblastoma microcirculation.
Figure 1. Representative microphotographs of endothelial cell-lined blood vessels and VM in medulloblastoma. (A) PAS-positive patterns were in direct communication with CD34 positive endothelial cell-lined blood vessels (brown stainings). The PAS-positive patterns extended directly from CD34-positive vessels and branched out into smaller PAS-positive patterns that interconnected with a cluster of back-to-back looping patterns (Boxed area). (B) In high magnification field, translucent red blood cells were found spreading along the PAS-positive patterns (red arrow). (C) Tumor cells delimited by cross-linking PAS-positive patterns. Boxed area was illustrated in oil immersion objective in D. (D) In some regions, PAS-positive patterns showed hollow channels filled with translucent red blood cells (Red arrow). (E) Patterns in medulloblastoma were stained with CD34 and PAS dual staining in combination with eosin staining to highlight red blood cells. PAS-positive patterns splayed open and filled with red blood cells, without hints of endothelial cells in certain areas (red arrow). Tissue stains: CD34 and PAS dual staining with hematoxylin counterstaining (A, B, C, D) and in combination with eosin (E). Original magnifications, × 200 (A), × 400 (B), × 400 (C), × 1,000 (D), × 400(E).
VM is associated with the expression of MMP-2, MMP-14, EphA2 and laminin 5γ2 in medulloblastoma
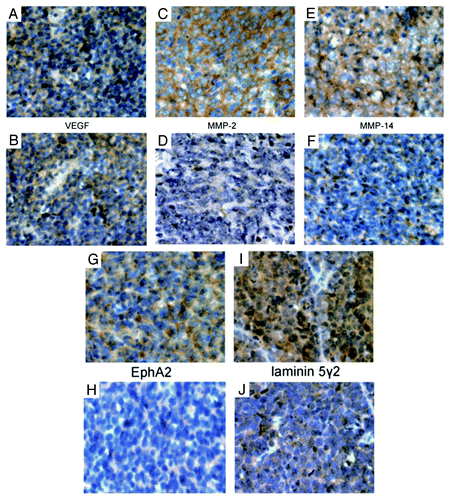
The results of immunohistochemical studies for VEGF, MMP-2, MMP-14, EphA2, and laminin 5γ2 were summarized in and . The expression of MMP-2 (p = 0.001), MMP-14 (p = 0.039), laminin 5γ2 (p = 0.005) and EphA2 (0.036) were significantly higher in the VM-positive group than in the VM-negative group, whereas the expression of VEGF (p = 0.518) showed no difference between these two groups.
Figure 2. Immunohistochemical studies of the expression of VEGF, MMP-2, MMP-14, EphA2 and laminin 5γ2 in medulloblastoma. A, C, E, G, and I are representative micrographs from VM-positive tissue sections. B, D, F, H and J are representative micrographs from VM-negative tissue sections. (A and B) Expression of VEGF in medulloblastoma. (C and D) Expression of MMP-2 in medulloblastoma. (E and F) Expression of MMP-14 in medulloblastoma. (G and H) Expression of EphA2 in medulloblastoma. (H and I) Expression of laminin 5γ2 in medulloblastoma. Original magnifications, × 400 (A–J).
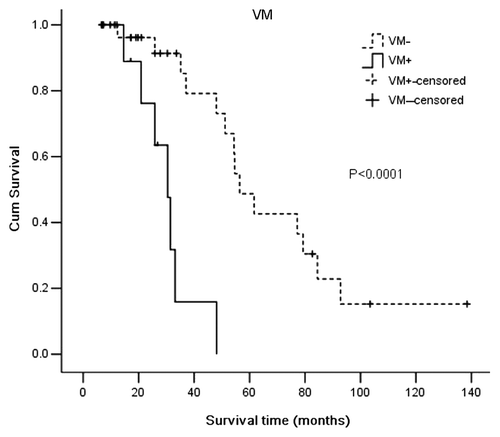
Table 3. Expression of VEGF, MMP-2, MMP-14, EphA2 and laminin 5γ2 in medulloblastoma tissues
No association was found between VM and clinical and pathological data
The presence of VM in medulloblastoma was not associated with age (p = 0.187), gender (p = 0.819), Karnofsky Performance Scale (KPS) score (0.224), hydrocephalus (0.580), histological classification (0.286), tumor location (p = 0.724), tumor size (p = 0.530), tumor metastasis (p = 0.479), and the extent of tumor resection (p = 0.819) ().
VM-positive medulloblastoma has lower microvessel density
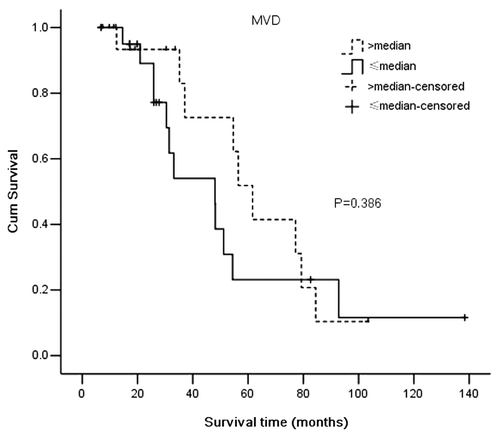
We compared the MVD counts between VM positive and VM negative medulloblastoma. The results demonstrated that VM positive medulloblastomas had lower levels of MVD (38.12 ± 5.64) when compared with VM negative medulloblastomas (58.53 ± 11.53), (p = 0.000) ().
Table 4. Correlation of MVD with VM in medulloblastoma
VM is a prognostic factor for shorter survival time in medulloblastoma
We used Kaplan–Meier survival analysis to compare the survival times between VM-positive (total 9, censored 2) and VM-negative patients (total 32, censored 18) or between below-median MVD (total 21, censored 9) and above-median MVD (total 20, censored 11). The results demonstrated that the VM-positive group had a shorter survival time (median 30.47 mo, 95%CI, 24.00-36.94 mo) when compared with the VM-negative group (median 56.43 mo, 95%CI 46.98–65.89 mo) (p < 0.0001) (). There was no significant correlation between MVD and overall survival time of the medulloblastoma patients (p = 0.386) (). Univariate analysis showed that survival time was significantly correlated with VM (p = 0.0001), metastasis (p = 0.044) and KPS score (p = 0.01). Patient age, gender, hydrocephalus, tumor histological classification, tumor size, tumor location and extent of resection were not significant variables. Multivariate analysis and the Cox proportional hazards model identified VM and metastasis as independent prognostic factors for overall survival time.
Discussion
The vascularization of tumors is heterogeneous. Angiogenesis (the tumor vasculature arising from sprouting of endothelial cells from local vessels) and vasculogenesis (vasculature formed by colonization of circulating endothelial progenitor cells from the bone marrow) were once two well-accepted paradigms for the formation of tumor vascularization. The blood vessels formed by angiogenesis and vasculogenesis are both initiated and regulated by angiogenic factors secreted by tumor cells.Citation16 However, these observations have been challenged over the last decade by the discovery of VM in a series of malignant tumors. Until now, the underlying molecular mechanisms for VM were not completely understood. Previous research on melanoma, breast cancer, ovarian cancer suggest that the plasticity of cancer cells enables them to mimic the phenotypes and functions of endothelial cells, thus leading to the formation of patterned matrix type of VM-patterned networks of interconnected loops of PAS-positive extracellular matrix that may be solid or hollow, with no involvement of endothelial cells.Citation17-Citation19 The plasticity of highly malignant tumor cells has some similarities to the stemness of cancer stem cells. Recently, reports reveal that glioblastoma stem-like cells (CSC) can differentiate into functional endothelial cells, which harbor the same genomic alterations as cancer cells.Citation20,Citation21 And there is evidence suggesting that glioblastoma stem-like cells are capable of forming blood vessels de novo-tubular type of VM.Citation5 Together, these observations strongly suggest that the plasticity of highly malignant tumor cells and cancer stem-like cell may mimic the role of normal stem cells and facilitate the formation of vascular network to sustain the blood supply of fast-growing tumor.Citation22
Morphologically, VM channels are patterned networks of solid or hollow interconnected loops of PAS-positive extracellular matrix, lined externally by tumor cells. Functionally, they interconnect with endothelial blood vessels and conduct plasma and red blood cells. Previous studies in melanoma provided considerable evidence that the looping patterned matrix conducts fluid in vitro and in vivo. Melanoma cells cultured in three-dimensional matrix gel formed looping patterns which can transmit fluid after direct microinjection and by passive absorption.Citation23 In a xenograft mouse model of uveal melanoma, intravenous tracer material can perfuse to a looping patterned matrix.Citation24 Further studies in patients with uveal melanoma using laser scanning confocal microscopy revealed similar results.Citation25 Based on three morphological manifestations, we believe that the PAS-positive patterns in medulloblastoma are a part of the tumor microcirculation, but not blood leaks or hemorrhage. First, the patterned matrix was in direct communication with blood vessels. Second, in certain regions, these patterns branched out into smaller patterns with hollow channels filled with red blood cells. Third, using the CD34 and PAS dual staining in combination with eosin staining to highlight the red blood cells in tissue sections, we found that red blood cells spread along the PAS-positive patterns.
In this article, immunohistochemical studies revealed significant increases in the expression of MMP-2, MMP-14, EphA2 and laminin 5γ2 in VM-positive tissues compared with VM-negative tissues, especially in those VM lesions. These components play significant roles in the formation of VM. Studies in melanoma indicated that cooperative interactions between laminin 5 γ2-chain and MMP-2 and MMP-14 are required for the formation of VM. EphA2 can activate phosphatidylinositol 3-kinase (PI3K), which regulates the activity of MMP-14. Activated MMP-14 leads to the activation of pro-MMP-2 to an active MMP2 proteinase. Active MMP-2 can cleave laminin 5 γ2-chain into promigratory γ2′ and γ2x fragments, which can lead to the formation of VM.Citation26,Citation27 Our results provide evidence that VM may be associated with the degradation and remodeling of the extracellular matrix in medulloblastoma. Research on VEGF reveals that it is a stimulator of endothelial cell proliferation, and VEGF can induce the formation of VM through a VEGFR-1dependent manner, increasing the expression of VM associated proteins in melanoma.Citation28 In our research, the expression of VEGF had no significant correlation with VM in medulloblastoma. It is possible that VM in medulloblastoma might be regulated through a pathway distinct from VEGF.
We have confirmed the existence of VM in medulloblastoma. Moreover, we explored whether the presence of VM has any association with clinicopathological data and its prognostic significance in medulloblastoma. In this retrospective study of 41 patients with medulloblastoma, we found that VM had no association with clinicopathological characteristics. Notably VM had no association with histological classification, which was inconsistent with previous studies in glioma, renal cell carcinoma, and mesothelial sarcoma.Citation9,Citation10,Citation12 Among all the subtypes of medulloblastoma, the LCA variant is notable for its high aggressiveness and relatively poorer clinical outcome.Citation1 However, it might be unconvincing to draw the above conclusion as there are only four examples of the LCA subtype in this study. The true association has yet to be conclusively determined. Metastases in this study were found in 10 patients: 8 with spinal cord metastases and 2 with extracranial metastases. Although the generation of VM by highly aggressive tumor cells facilitates tumor metastasis,Citation22 there was no association between VM and metastasis in our study. Our observation needs to be further studied in larger amount of samples. The MVD counts in the VM-positive group were significantly less than in the VM-negative group, which is indirect evidence of the blood supply function of VM. A previous study revealed that MVD has no prognostic significance in medulloblastoma.Citation29 Our observations in medulloblastoma lead us to the same conclusion.
Observations in a series of malignant tumors reveal that VM might serve as an unfavorable prognostic factor. However, there has been no research on the prognostic significance of VM in medulloblastoma prior to our study. Although VM had no association with patients’ clinicopathological characteristics in this study, Kaplan-Meier survival analyses and log-rank tests revealed that medulloblastomas with VM have a poorer clinical outcome compared with those without VM. Univariate analysis and multivariate analysis showed that VM is an independent prognostic factor for overall survival time in patients with medulloblastoma. Our observation in medulloblastoma is consistent with previous studies. It is noteworthy that while VM correlates with the MVD, MVD does not correlate with overall survival, yet VM does. It is not surprising that, although we found no correlation between VM and pathological classifications or between VM and metastasis which was considered as inconclusive results in this study, VM is indeed associated with more malignant biological behaviors such as invasiveness and metastasis that directly affect patient clinical outcome.Citation3 Moreover, currently widespread used anti-angiogenic drugs may have no effect on VM,Citation14 even inducing VM formation when blood vessels are destroyed leaving a hypoxic environment.Citation30 Therefore, newly developed drugs based on anti-angiogenic strategies must take both anti-angiogenic and anti-VM treatment into serious consideration.
In conclusion, this pilot study provided direct evidence that VM, a supplementary microcirculation for angiogenesis, exists in medulloblastoma and is an adverse predictor of clinical outcome.
Materials and methods
Tissue samples
A total of 41 paraffin-embedded medulloblastoma tissues between 1999 and 2010 were obtained from the Department of Pathology, Zhujiang Hospital, Southern Medical University. All tissue samples were collected from patients who did not undergo therapy before the surgical operation to remove the tumor. Tumor sections were reviewed by two neuropathologists to verify the diagnosis of medulloblastoma in accordance with the 2007 World Health Organization (WHO) classification of central nervous system (CNS) tumors. According to the 2007 WHO classification of CNS tumors, medulloblastomas are separated into the classic tumor and four main histological subtypes:desmoplastic/nodular (D/N), medulloblastoma with extensive nodularity (MBEN), anaplastic medulloblastoma and large cell medulloblastoma. D/N medulloblastoma and MBEN share fundamental clinical and pathological features as do anaplastic medulloblastoma and large cell medulloblastoma.Citation1,Citation31 We divided the 41 patients into three groups: classic medulloblastoma, desmoplastic medulloblastoma that contained D/N and MBEN medulloblastomas and LCA medulloblastoma that contained anaplastic medulloblastoma and large cell medulloblastoma. Detailed clinical and pathological data were collected for all samples, including age, gender, KPS score, hydrocephalus, histological classification, size, location, metastasis, total or partial resection. Research Ethics Committee approval has been obtained for the use of all human tissues in this study.
CD34-PAS dual staining
For CD34-PAS dual staining paraffin sections were cut at 5 μm. Slides were deparaffinized twice with xylene and subsequently hydrated against a concentration gradient in ethanol solutions. Antigen retrieval was performed using citrate buffer (pH6.0) at 96°C for 20 min. For demonstration of endothelial cells in medulloblastoma, the slides were incubated with mouse monoclonal anti-human CD34 antibody (Santa Cruz Biotechnology, catalog: sc-52312, dilution 1:100) at 4°C over night. After washing with PBS, the slides were treated with PowerVision Two-Step Histostaining Reagent (Zhongshan Goldenbridge Biotechnology, catalog: PV-6002) for 30 min at 37°C. The chromogen used to highlight a positive reaction was 3,3′-diaminebenzidine tetrahydrochloride (DAB), resulting in a brown product. To highlight the matrix-associated vascular channels of medulloblastoma, slides were stained following the PAS staining procedures before counterstaining with Mayer’s hematoxylin. Finally, the slides were covered with a permanent mounting medium. To highlight the red blood cells in PAS positive patterns, the adjacent sections were stained with eosin after the above procedures.
Immunohistochemistry
To determine the expression of VEGR, MMP-2, MMP-14, EphA2 and laminin 5γ2 in medulloblastoma by immunohistochemistry, tissue sections were prepared as previously described. Slides were then incubated over night at 4°C with a monoclonal antibody against VEGF (Santa Cruz Biotechnology, catalog: sc-80437, dilution 1:50); EphA2 (Millipore, catalog: 05-480, dilution 1:100), MMP-2 (Abcam, catalog: ab2462, dilution 1:100), MMP-14 (Abcam, catalog: ab53712, dilution 1:100), or a polyclonal antibody against laminin 5γ2 (Abcam, catalog: ab96327, dilution 1:100). Appropriate positive and negative controls for each antibody were included. The staining index (SI) was used to evaluate the expression level of each marker based on the varying degrees of staining intensity and percentage of positively stained cells in the specimens. Therefore, a combined intensity and percentage positive scoring method was used. Ten visual fields of each tissue section were selected randomly under the microscope at 400 × magnification, and 100 cells in each visual field were counted. The average percentage of positively stained cells in 10 visual fields of each section was converted into a score as follows: 0 for < 10% positive cells, 1 for < 25% positive cells, 2 for < 50% positive cells, 3 for > 50% positive cells. The score of intensity of each section was graded as follows: Strong intensity staining was scored as 3, moderate as 2, weak as 1, and negative as 0. The staining index was determined by multiplying the average percentage of positive cells and the score of intensity. The assessment of immunohistochemistry results was completed by two independent investigators blinded to the clinicopathological data. Discrepancies were resolved by consensus.Citation9
Microvessel density
Microvessel density counts were completed according to the method described by Foote et al.Citation32 This portion of the study was completed by two additional independent observers unaware of the clinical outcome of patients. Tumor sections were examined under 200 × magnification, and the average number of microvessels in 10 randomly selected visual fields represented the MVD of the tumor. Any endothelial cell or endothelial cell cluster positive for CD34 and clearly separate from the adjacent cluster was considered to be a single countable microvessel. The average MVD for the 41 specimens was calculated and patients were grouped according to whether their MVD was lower (below-median MVD) or higher (above-median MVD) than median. The overall survival times for these two groups were compared, and the association between VM and MVD was determined.
Statistical analysis
Statistical analyses were performed using SPSS 13.0 for Windows (SPSS Inc.). A p value of less than 0.05 was defined as statistically significant. The differential expression levels of VEGF, MMP-2, MMP-14, laminin 5γ2,EphA2 in the VM-positive and VM-negative groups were determined by the Mann-Whitney test. MVD counts in the VM-positive and VM-negative groups were also compared by the Mann-Whitney test. Associations between VM and clinical and pathological data were determined by the Pearson’s χ2 test. The survival analysis was calculated by the Kaplan-Meier method and the differences between groups were compared using the log-rank test. Univariate analysis was performed to identify prognostic variables for overall survival. Multivariate analysis was performed to identify the independent prognostic factors for survival using the Cox regression hazard model. Data on surviving patients and patients without follow-up information were considered censored data in the analysis.
| Abbreviations: | ||
| VM | = | vasculogenic mimicry |
| MVD | = | microvessel density |
| KPS | = | Karnofsky Performance Scale |
| D/N | = | desmoplastic/nodular |
| MBEN | = | medulloblastoma with extensive nodularity |
| LCA | = | large cell/anaplastic |
| SI | = | staining index |
| PI3K | = | phosphatidylinositol 3-kinase |
Acknowledgments
We express our gratitude to Professor Shengli An (Department of Biostatistics, Southern Medical University) for the analysis of statistical data and Mr. Xuan Lu (Department of Pathology, Zhujiang Hospital, Southern Medical University) for the sectioning of tissues. This project was supported by grants from the National Science Foundation of China (No. 30901174), and from the Science and Technology Planning Project of Guangdong (No. 2008B030301152).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
References
- Ellison DW. Childhood medulloblastoma: novel approaches to the classification of a heterogeneous disease. Acta Neuropathol 2010; 120:305 - 16; http://dx.doi.org/10.1007/s00401-010-0726-6; PMID: 20652577
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1:27 - 31; http://dx.doi.org/10.1038/nm0195-27; PMID: 7584949
- Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe'Er J, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 1999; 155:739 - 52; http://dx.doi.org/10.1016/S0002-9440(10)65173-5; PMID: 10487832
- Shih IM. Trophoblastic vasculogenic mimicry in gestational choriocarcinoma. Mod Pathol 2011; 24:646 - 52; http://dx.doi.org/10.1038/modpathol.2010.231; PMID: 21217646
- El HS, Boisselier B, Peglion F, Rousseau A, Colin C, Idbaih A, et al. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain 2010; 133:973 - 82; http://dx.doi.org/10.1093/brain/awq044; PMID: 20375132
- van der Schaft DW, Hillen F, Pauwels P, Kirschmann DA, Castermans K, Egbrink MG, et al. Tumor cell plasticity in Ewing sarcoma, an alternative circulatory system stimulated by hypoxia. Cancer Res 2005; 65:11520 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-05-2468; PMID: 16357161
- Yue WY, Chen ZP. Does vasculogenic mimicry exist in astrocytoma?. J Histochem Cytochem 2005; 53:997 - 1002; http://dx.doi.org/10.1369/jhc.4A6521.2005; PMID: 15923371
- Shirakawa K, Wakasugi H, Heike Y, Watanabe I, Yamada S, Saito K, et al. Vasculogenic mimicry and pseudo-comedo formation in breast cancer. Int J Cancer 2002; 99:821 - 8; http://dx.doi.org/10.1002/ijc.10423; PMID: 12115483
- Liu XM, Zhang QP, Mu YG, Zhang XH, Sai K, Pang JC, et al. Clinical significance of vasculogenic mimicry in human gliomas. J Neurooncol 2011; In press http://dx.doi.org/10.1007/s11060-011-0578-5; PMID: 21533525
- Vartanian AA, Stepanova EV, Gutorov SL, Solomko E, Grigorieva IN, Sokolova IN, et al. Prognostic significance of periodic acid-Schiff-positive patterns in clear cell renal cell carcinoma. Can J Urol 2009; 16:4726 - 32; PMID: 19671223
- Sun B, Qie S, Zhang S, Sun T, Zhao X, Gao S, et al. Role and mechanism of vasculogenic mimicry in gastrointestinal stromal tumors. Hum Pathol 2008; 39:444 - 51; http://dx.doi.org/10.1016/j.humpath.2007.07.018; PMID: 18261629
- Sun B, Zhang S, Zhao X, Zhang W, Hao X. Vasculogenic mimicry is associated with poor survival in patients with mesothelial sarcomas and alveolar rhabdomyosarcomas. Int J Oncol 2004; 25:1609 - 14; PMID: 15547697
- Sood AK, Fletcher MS, Zahn CM, Gruman LM, Coffin JE, Seftor EA, et al. The clinical significance of tumor cell-lined vasculature in ovarian carcinoma: implications for anti-vasculogenic therapy. Cancer Biol Ther 2002; 1:661 - 4; PMID: 12642690
- van der Schaft DW, Seftor RE, Seftor EA, Hess AR, Gruman LM, Kirschmann DA, et al. Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells. J Natl Cancer Inst 2004; 96:1473 - 7; http://dx.doi.org/10.1093/jnci/djh267; PMID: 15467037
- Grizzi F, Weber C, Di Ieva A.. Anti-angiogenic strategies in medulloblastoma: reality or mystery?. Pediatr Res 2008; In press
- Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med 2003; 9:702 - 12; http://dx.doi.org/10.1038/nm0603-702; PMID: 12778169
- Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer 2003; 3:411 - 21; http://dx.doi.org/10.1038/nrc1092; PMID: 12778131
- Hendrix MJ, Seftor EA, Kirschmann DA, Seftor RE. Molecular biology of breast cancer metastasis. Molecular expression of vascular markers by aggressive breast cancer cells. Breast Cancer Res 2000; 2:417 - 22; http://dx.doi.org/10.1186/bcr88; PMID: 11250735
- Sood AK, Seftor EA, Fletcher MS, Gardner LM, Heidger PM, Buller RE, et al. Molecular determinants of ovarian cancer plasticity. Am J Pathol 2001; 158:1279 - 88; http://dx.doi.org/10.1016/S0002-9440(10)64079-5; PMID: 11290546
- Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010; 468:824 - 8; http://dx.doi.org/10.1038/nature09557; PMID: 21102434
- Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature 2010; 468:829 - 33; http://dx.doi.org/10.1038/nature09624; PMID: 21102433
- Yao XH, Ping YF, Bian XW. Contribution of cancer stem cells to tumor vasculogenic mimicry. Protein Cell 2011; 2:266 - 72; http://dx.doi.org/10.1007/s13238-011-1041-2; PMID: 21533771
- Folberg R, Maniotis A. Vasculogenic mimicry. APMIS 2004; 112:508 - 25; http://dx.doi.org/10.1111/j.1600-0463.2004.apm11207-0810.x; PMID: 15563313
- Clarijs R, Otte-Holler I, Ruiter DJ, de Waal RM. Presence of a fluid-conducting meshwork in xenografted cutaneous and primary human uveal melanoma. Invest Ophthalmol Vis Sci 2002; 43:912 - 8; PMID: 11923228
- Frenkel S, Barzel I, Levy J, Lin AY, Bartsch DU, Majumdar D, et al. Demonstrating circulation in vasculogenic mimicry patterns of uveal melanoma by confocal indocyanine green angiography. Eye (Lond) 2008; 22:948 - 52; http://dx.doi.org/10.1038/sj.eye.6702783; PMID: 17363922
- Hess AR, Seftor EA, Seftor RE, Hendrix MJ. Phosphoinositide 3-kinase regulates membrane Type 1-matrix metalloproteinase (MMP) and MMP-2 activity during melanoma cell vasculogenic mimicry. Cancer Res 2003; 63:4757 - 62; PMID: 12941789
- Seftor RE, Seftor EA, Koshikawa N, Meltzer PS, Gardner LM, Bilban M, et al. Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res 2001; 61:6322 - 7; PMID: 11522618
- Vartanian A, Stepanova E, Grigorieva I, Solomko E, Baryshnikov A, Lichinitser M. VEGFR1 and PKCalpha signaling control melanoma vasculogenic mimicry in a VEGFR2 kinase-independent manner. Melanoma Res 2011; 21:91 - 8; http://dx.doi.org/10.1097/CMR.0b013e328343a237; PMID: 21389833
- Tural S, Gercek A, Konya D, Ozgen S, Toplamoglu H, Ozek MM. Microvessel density and vascular endothelial growth factor expression as predictors of childrens' survival from cerebellar medulloblastoma. J Clin Neurosci 2009; 16:1199 - 202; http://dx.doi.org/10.1016/j.jocn.2008.10.026; PMID: 19524442
- Seftor EA, Meltzer PS, Schatteman GC, Gruman LM, Hess AR, Kirschmann DA, et al. Expression of multiple molecular phenotypes by aggressive melanoma tumor cells: role in vasculogenic mimicry. Crit Rev Oncol Hematol 2002; 44:17 - 27; http://dx.doi.org/10.1016/S1040-8428(01)00199-8; PMID: 12398997
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114:97 - 109; http://dx.doi.org/10.1007/s00401-007-0243-4; PMID: 17618441
- Foote RL, Weidner N, Harris J, Hammond E, Lewis JE, Vuong T, et al. Evaluation of tumor angiogenesis measured with microvessel density (MVD) as a prognostic indicator in nasopharyngeal carcinoma: results of RTOG 9505. Int J Radiat Oncol Biol Phys 2005; 61:745 - 53; http://dx.doi.org/10.1016/j.ijrobp.2004.07.694; PMID: 15708253