Abstract
It has been reported that activating transcription factor 4 (ATF4) increases the processes of tumor growth, metastasis and drug resistance. However, the role played by ATF4 in chemoresistance of hepatocellular carcinoma (HCC) remains unknown. Clarification of this role of ATF4 in HCC could greatly benefit the efficacy of clinical treatment of HCC. In this study, we found that ATF4 was overexpressed in about 50.7% of HCC tissues. In fact knockdown of ATF4 significantly increased the cytotoxicity of cisplatin in both in vitro and in vivo assays, while overexpression of this molecule dramatically decreased the sensitivity of HCC cell lines to cisplatin. Additionally, we found that synthesis of glutathione was significantly reduced in HCC cell lines subjected to ATF4 knockdown. Taken together, these results demonstrate that ATF4 can increase resistance to cisplatin in HCC by increased biosynthesis of glutathione, and that this may be a potent novel target for the future development of anti-HCC drugs.
Keywords: :
Introduction
Hepatocellular carcinoma (HCC) is the fifth most frequent neoplasm worldwide, and the third most common cause of death from cancers. HCC has a distinct geographic distribution worldwide.Citation1 The highest rates of 1,020 per 100,000 population for HCC are found in Southeast Asian and tropical Africa.Citation2 A lower incidence has been found in Japan, the Middle East, and Mediterranean countries.Citation3,Citation4 Western countries, such as Australia, South America, and India report the lowest rates of 13 per 100,000 population for HCC.Citation3,Citation5 Early diagnosis of HCC may improve the prognosis of this cancer. Unfortunately, most HCCs are usually diagnosed at later stages, and the main therapeutic methods are surgical treatment and chemotherapy.
Human hepatocellular carcinoma has been reported with high chemo-resistance to chemotherapeutic agents, such as cisplatin, 5-fluorouracil (5-FU) and doxorubicin.Citation6-Citation8 The mechanisms of resistance to chemotherapeutic agents include decreased agents accumulation,Citation9 increased levels of cellular glutathione,Citation10 accelerated DNA repair,Citation11 and decreased induction of apoptosis.Citation12,Citation13 In addition to above mechanisms, some transcription factors also regulate cisplatin sensitivity.Citation14
In this regard, activating transcription factor 4 (ATF4) is a member of the cyclic adenosine monophosphate responsive element-binding (CREB) protein family where a stress responsive gene involved in multiple intracellular stress pathwaysCitation15 is expressed at much greater levels in many primary human tumors than in normal tissues. ATF4 protein expression is induced during hypoxia and facilitates tumor growth in several tumor xenograft models.Citation16-Citation18 In previous studies, ATF4 was shown to induce the expression of VEGF and E-selection and to be involved in metastasis of several types of tumors.Citation19 Kohno et al. also found that ATF4 might increase the multidrug resistance through a glutathione-dependent redox system in multiple human cell lines.Citation20,Citation21
Taken together, these findings and considerations led us to examine whether the level of ATF4 is also critical in regulating sensitivity to cisplatin in HCC. We could demonstrate here that ATF4 protein is strongly overexpressed in HCC tissues compared with normal liver samples, and causes resistance to cisplatin by promoting biosynthesis of glutathione.
Results
ATF4 is overexpressed in HCC but not normal liver tissues
To examine the expression of ATF4 in human HCC and normal liver tissues, we performed immunohistochemical staining on a tissue microarray which containing specimen cores of Grade I HCC (n = 4), Grade II (n = 44), Grade III (n = 17), Grade IV (n = 2) and normal liver tissue (n = 10). We found that ATF4 was strongly expressed in 50.7% of the total 67 HCC samples analyzed but not apparent in the normal liver tissues ( and ). However, the expression of ATF4 did not reveal any difference among different grades of HCC. Representative images of immunohistochemcal staining of HCC tissues and normal liver tissues are shown in .
Figure 1. overexpression of ATF4 in HCC tissues. Hepatocellular carcinoma (HCC) and normal liver tissues were analyzed for ATF4 expression by immunostaining. (A and B) normal liver tissues. (C–E), hepatocellular carcinoma tissues. (C and D) The expression of ATF4 is evident. (E and F) The expression of ATF4 is moderate. (G and H) The expression of ATF4 is negative. Representative pictures for HCC and normal tissues are shown. Scale bar equals 100 μm.
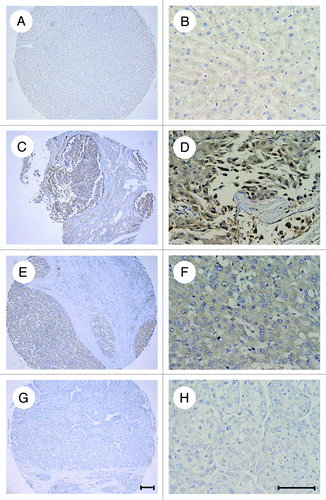
Table 1. Immunohistochemical analysis of tissue microarray of HCC
Overexpression of ATF4 causes cisplatin resistance in HCC cell lines
Previous studies reported that ATF4 was overexpressed in several cancer cell lines.Citation20,Citation22 Here, we first confirmed by western blot assay that ATF4 is also overexpressed in HCC cell lines (HepG2 and SMMC7721), testing with (Data not shown). It was also reported that ATF4-overexpression in the A549 cell line can increase its resistance to cisplatin.Citation21 Thus We were curious to determine whether ATF4 also increases chemoresistance in HCC cell lines. To answer this question, we first established two HCC cell lines, which overexpress ATF4, i.e., [HepG2/pcDNA3.1(-)-ATF4 and SMMC7721/pcDNA3.1(-)-ATF4) as well as two control cell lines (HepG2/pcDNA3.1(-), SMMC7721/pcDNA3.1(-)] (). We then incubated these cultured cells with cisplatin at different concentrations, and calculated half-maximal inhibitory concentration (IC50) for each group determined by the WST-1 assay. We found ATF4 overexpression significantly increased IC50 about 2- to 4-fold, while knockdown of ATF4 decreased the IC50 to about half a fold in HCC cell lines ( and ), which is consistent with other cancer cell lines.Citation21 To test the cisplatin induced apoptosis, both ATF4-overexpressing and control HCC cells were either treated or untreated with cisplatin, and there subjected to PI and AnnexinV staining and further flow cytometry analysis. Our results suggest that HCC cell lines overexpressing ATF4 can survive better after cisplatin-induced apoptosis (). Together, these data indicate that ATF4 plays an important role in determining cisplatin resistance in HCC cell lines.
Figure 2. Overexpression of ATF4 results in chemoresistance to cisplatin in HCC cell lines. (A and B) western blot analysis of ATF4 expression in HepG2 and SMMC7721 stable transfectants. (C and D) Overexpression of ATF4 confers cisplatin tolerance of HepG2 and SMMC7721 cell lines. ATF4-overexpressing cells and control cells were exposed to various concentrations of cisplatin. After 72 h incubation, cell survival was analyzed with a WST-1 assay. (E and F) Cisplatin-induced apoptosis is inhibited in cells overexpressing ATF4. HepG2/pcDNA3.1(-)-ATF4 and HepG2/pcDNA3.1(-) cells were treated with cisplatin (25 μg/ml) for 24 h, and SMMC7721 /pcDNA3.1(-)-ATF4 and SMMC7721 /pcDNA3.1(-) cells were treated with cisplatin (2.5 μg/ml) for 24 h. The cells were stained with AnnexinV-FITC and PI and analyzed by flow cytometry. All error bars indicate S.D., *p < 0.05, **p < 0.01.
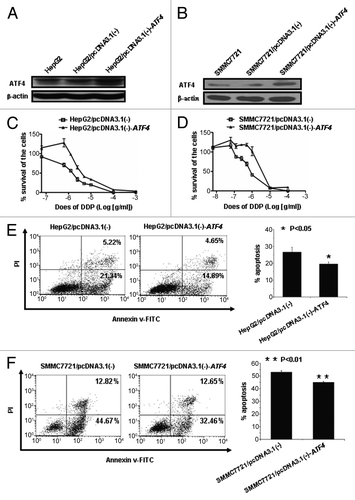
Table 2. Drug sensitivity (half-maximal inhibitory concentration [IC50]) and relative resistance of ATF4-overexpressing cell lines and konckdown of ATF4 cell line
Knockdown of ATF4 increases sensitivity to cisplatin in HCC cell lines
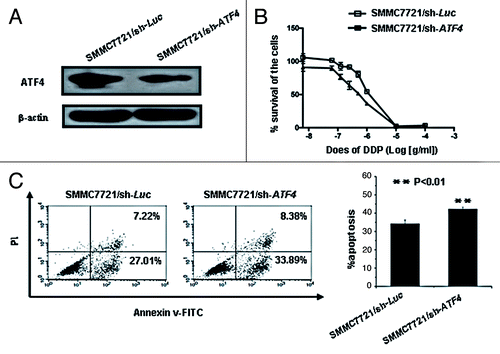
To further investigate the roles of ATF4 in cisplatin resistance of HCC by an alternative approach, we established the stable ATF4 knockdown HCC cell line (SMMC7721/sh-ATF4) and a control cell line (SMMC7721/sh-Luc) (). When treated with different concentration of cisplatin, the ATF4 knockdown SMMC7721/sh-ATF4 cell line was more sensitive to cell death than the control SMMC7721/sh-Luc cell line, at least based on the IC50 analysis for each cell line using WST-1 assay ( and ). When we analyzed the rate of apoptosis after cisplatin incubation, ATF4 knockdown SMMC7721/sh-ATF4 cell lines also showed increased sensitivity to cisplatin induced apoptosis, as shown . These data again suggest that ATF4 can increase resistance to cisplatin in HCC.
Figure 3. Knockdown of ATF4 increases the cytotoxicity of cisplatin in the SMMC7721 cell line. (A) Western blot analysis of ATF4 expression in SMMC7721 stable transfectants. (B) Knockdown of ATF4 confers cisplatin sensitivity on SMMC7721 cell line. ATF4 knockdown cells (SMMC7721/sh-ATF4) and control cells (SMMC7721/sh-Luc) were exposed to various concentrations of cisplatin. After 72 h incubation, cell survival was analyzed with a WST-1 assay. (C) Cisplatin-induced apoptosis is increased in ATF4 knockdown cells. SMMC7721/sh-ATF4 and SMMC7721/sh-Luc cells were treated with cisplatin (25 μg/ml) for 24 h, these cells were then stained with AnnexinV-FITC and PI and analyzed by flow cytometry. All error bars are indicated as SD.
Intracellular glutathione production determines ATF4-induced chemoresistance in HCC cell lines
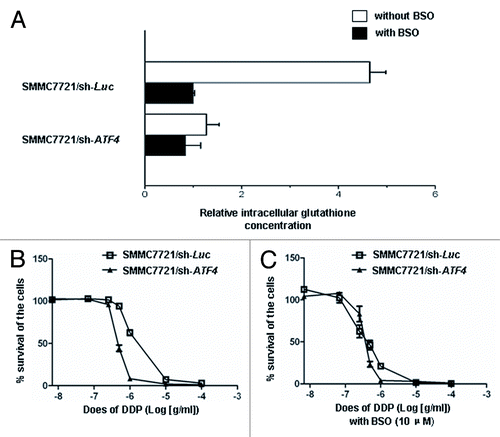
Since ATF4 is involved in chemoresistance to ciplatin in HCC, we next asked how ATF4 regulates this process by using ATF4−/− cells, which a previous study also reported to promote biosynthesis of glutathione.Citation23 Moreover, lung cancer cells overexpressing ATF4 can also increase the level of glutathione.Citation21 In concordance, we also found that the glutathione levels of ATF4 knockdown HCC cells were reduced to 21.7% of the control cells (). This decrease was inhabited by treating HCC cells with g-glutamylcysteine synthesis inhibitor: BSO (10μM), which can overcome the resistance to cisplatin caused by ATF4 (). Consequently, our results demonstrate that ATF4 also promotes the biosynthesis of intracellular glutathione, which then detoxify cisplatin.
Figure 4. ATF4 increases resistance to cisplatin in HCC by promoting intracellular glutathione production. (A) Intracellular glutathione levels were measured in the ATF4 knockdown cell line (SMMC7721/sh-ATF4) and control cell line (SMMC7721/sh-Luc) with or without 10 μM BSO treatment for 72 h. Each glutathione concentration value indicates the relative level in SMMC7721/sh-Luc with 10 μM BSO. (B and C) BSO overcomes the drug resistance to cisplatin in ATF4 knockdown cells. ATF4 knockdown cell line (SMMC7721/sh-ATF4) and control cell line (SMMC7721/sh-Luc) were each pretreated with or without 10 μM BSO for 24 h, and then exposed to various concentrations of cisplatin with or without 10 μM BSO. After 72 h incubation, cell survival was analyzed with the WST-1 assay. Cell survival in the absence of drugs was referred as 100%. All values are the mean of at least three independent experiments. All error bars are indicated as SD.
Knockdown of ATF4 increases chemosensitivity to cisplatin in NOD/SCID xenograft models
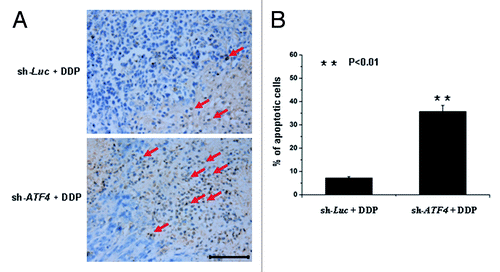
To test the in vivo efficacy of cisplatin on ATF4 knockdown transfectants both the SMMC7721/sh-ATF4 cells and the SMMC7721/sh-Luc cells were each transplanted s.c. into NOD/SCID mice. Two weeks after implantation, we supplied the mice with doxycycline in their drinking water. One week after this supplying, mice were treated weekly with cisplatin (5 mg/kg). Tumor growth was then monitored twice a week over a period of 45 d (). Control tumors reached a volume of 3cm3, whereas ATF4 knockdown tumors displayed an average volume of less than 1cm3 (). Furthermore, the tumors from the two different groups were peeled, harvested and subjected to TUNEL analyses. The fraction of TUNEL-positive cells was found to be significantly higher in tumor subjected to ATF4 knockdown than in control tumors (). Taken together, these data suggest that the knockdown of ATF4 increases chemosensitivity to cisplatin in an in vivo animal model by promoting cisplatin-induced apoptosis.
Figure 5. Knockdown of ATF4 increases chemoresistance to cisplatin in vivo. SMMC7721 cells were stably transduced with sh-ATF4 or sh-Luc constructs. 3 × 106 transfectant cells were injected subcutaneously into the right flanks of the immunodeficient NOD/SCID mice. One week after inoculation, the NOD/SCID mice were treated with cisplatin. (A) Tumor volumes were measured over a period of 45 d using calipers (n = 5 for each cell type). (B) The tumors from the two groups are shown.
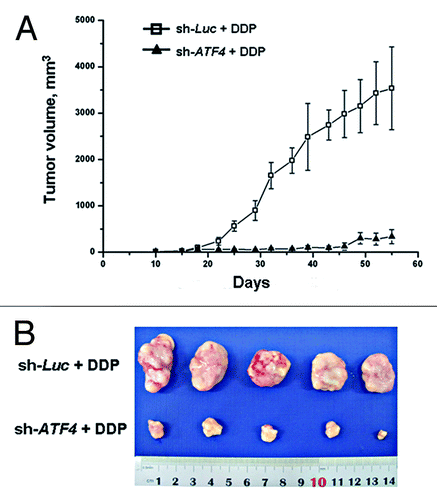
Figure 6. ATF4 protects HCC cells from apoptosis induced by cisplatin in xenograft models. (A) Tumors from xenograft models were sectioned and stained with immunohistochemistry using a TUNEL assay kit. Representative areas are shown in the figure. Scale bar equals 100 μm. (B) The ratio of TUNEL-positive cells to total cells were expressed as percentage. Data are shown as the mean ± SD from the calculation of five random fields at × 400 magnification. **p < 0.01.
Discussion
Previously, Kimitoshi Kohno et al. reported that ATF4 was involved in multidrug resistance in human lung cancer, prostate cancer and breast cancer cell lines.Citation20,Citation21 Takushi Namba et al. also found that ATF4 can decrease cisplatin-induced apoptosis via upregulating ORP150.Citation24 Interestingly, a study conducted by Reshma Shringarpure et al. has reported that Bortezomib induced a significant upregulation of ATF4 in sensitive SUDHL- 6 cells but not in resistant in SUDHL- 4 cells,Citation25 which is opposite to the results of Kimitoshi Kohno et al. Thus, the role of ATF4 in chemoresistance needs to be clarified in certain cancer types.
Since chemoresistance is a major obstacle to successful chemotherapy of HCC. We first examined here the expression of ATF4 in HCC tissues and HCC cell lines. We found that ATF4 was overexpressed in more than 50% HCC samples and also highly expressed in HepG2 and SMMC7721 cell lines. We also discovered that the expression of ATF4 was upregulated in cell lines treated with cisplatin (data not shown). To investigate the function of the transcription factor ATF4 in chemoresistance of HCC, ATF4-overexpressing stable HCC cell lines were established. Overexpression of ATF4 in HepG2 and SMMC7721 led to resistance to cisplatin and decreased cisplatin-associated apoptosis. Both ATF4 overexpressing HCC stable cell lines showed increased resistance to cisplatin, with a 2- to 4-fold higher IC50 than control cell lines. To further confirm this finding, we derived a ATF4 knockdown SMMC7721 cell line, and found that ATF4 knockdown sensitized this cell line to ciplatin-induced apoptosis. The knockdown of ATF4 SMMC7721 resulted in an IC50 value of 0.749 μg/ml cisplatin as compared with an IC50 value of 1.732 μg/ml for control SMMC7721 cells. Taken together, ATF4 may thus play an intriguing positive role in determent cisplatin resistance in HCC.
In general, cisplatin induces DNA damage, which activates the tumor suppressor gene products p53 and p73, and results in cell cycle arrest and apoptosis. Expression of both p53 and p73 can increase the sensitivity of cisplatin in multiple cancer cell lines.Citation26 Except for this mechanism, C.Thomas et al. also reported that enhanced cisplatin cytotoxicity can be induced by disturbing the nucleotide excision repair pathway in ovarian cancer cell lines.Citation27 Yuji Murata et al. also found that inhibition of the NF-κB pathway can increase cisplatin sensitivity in ovarian cancer models.Citation28 Furthermore, intracellular cisplatin can be inactivted by glutathione (GSH), which has also been proposed as a major mechanism responsible for cisplatin resistance. In addition to these mechanisms, more and more researches demostrated that transcription factors were also involved in cisplatin sensitivity. Thus, Kimitoshi Kohno et al. have reported that ATF4 was upregulated in cisplatin-resistant lung cancer cell lines, and Clock and ATF4 function together increased multidrug resistance through glutathione-dependent redox system in various cancer cell lines.Citation20,Citation21
Although various mechanisms of ATF4-induced chemoresistance in some tumor cell lines were reported, the mechanism in HCC remained far unclear. Consequently, the second aim of our current study was to explore the mechanisms of drug resistance regulated by ATF4. We indeed found impaired glutathione biosynthesis in ATF4 knockdown SMMC7721 cells, and depletion of the level of glutathione by BSO was able to reverse the resistance in control SMMC7721 cells. Their findings were consistent with those of another the study that the level of glutathione in the ATF4−/− cells was lower than that of control cells.Citation23 Thus, glutathione can either extinguish DNA-Pt mono-adducts before they transform into cytotoxic DNA cross-links or take a complex with cisplatin,Citation29,Citation30 and MRP1 can export the drug conjugates out of cells.Citation31 Based on our results indicating that knockdown of ATF4 enhanced cisplatin sensitivity in cultured SMMC7721 cells, we further studied this effect in vivo in a xenograft model. Indeed, in this model, we also found that knockdown of ATF4 decreased cisplatin-induced apoptosis in vivo. Some previous studies have also found ATF4 can promote tumor growth.Citation18,Citation19 In this study, to more closely examine ATF4 may be responsible for this ATF4-dependent in vivo growth, we next used xenograft model of tumorigenicity, and we found ATF4 suppressed tumor growth slightly (Fig. S2.).
In summary, we represent here a novel mechanism of chemoresistance in HCC, which functions through transcriptional regulation by ATF4. Results of our study suggest that by targeting ATF4 through overexpression or knockdown techniques, it is possible to alter the levels of glutathione and thereby eventually change the sensitivity to cispatin by HCC. Together, there data suggest that the inhibitor of ATF4 has strong potential as a novel chemotherapeutic target for HCC treatment.
Materials and methods
Cell culture
Human hepatocellular carcinoma cell lines, HepG2 and SMMC7721, were obtained from the Chinese Academy of Sciences. HepG2 and SMMC7721 cells were maintained in DMEM (Invitrogen) with 10% fetal bovine serum, and were propagated in a 5% CO2 atmosphere at 37°C.
Plasmid construction
To construct a mammalian overexpression plasmid, the cDNA of ATF4 was cloned by reverse transcription PCR using total RNA from SMMC7721 cells, and adopting the following primer pairs (NheI/Hind III): sence 5′-CTAGCTAGCATGGGAGAGAAGATGGTAGC-3′ and antisence 5′-CCCAAGC- TTCTAAGGAGGATCGTAAGGT-3′. (The NheI/HindIII). Digested amplified fragments were ligated into pcDNA3.1(-) vector (Invitrogen).
The constructed small hairpin RNA (shRNA) vector pSingle-tTS-shRNA was purchased from Clontech (Clontech Laboratories), we designed two shRNA sequences targeting ATF4 using shRNA Target Finder and Design Tool available at http://bioinfo.clontech.com/rnaidesigner/sirnaSequenceDesign.do and the third one target sequence form the reference of Fung H et al.Citation32 In addition, their efficacies in extinguishing ATF4 expression were evaluated by RT-PCR and Western Blot(Fig. S1). At last, we chose the third one, and shRNA of ATF4 has the following sequences: sense 5′-TCGAGGTCCCTCAGTGCA-GTGCATAAAGGATTCAAGAGATCCTTTATGCACTGAGGGATTTTTTA-3′ and antisense 5′-AGCTTAAAAAATCCCTCAGTGCATAAAGGATCTCTTGAATCCT-TTATGCACTGAGGGACC-3′. Meanwhile, we also designed shRNA sequences for firefly luciferase as the scramble (negative) control. These sequences are sense 5′-TCGAGGAGACAAGGGTACGGACTAATTCAAGAGATTAGTCCGTACCCTTGTCTTTTTTTA-3′ and antisense 5′-AGCTTAAAAAAAGACAAGGGTACGGACTAATCTCTTGAATTAGTCCGTACCCTTGTCTCC-3′. To obtain the knockdown vector, the shRNA Oligonucleotides were digested by XhoI/HindIII, and the XhoI/HindIII. The digested fragments were then ligated into the pSingle-tTS-shRNA vector.
Generation of stable transfectants
HepG2 and SMMC7721 cells were seeded into 6-well cell culture plates at a concentration of 2 × 105. On the following day, they were transfected with either 4μg of pcDNA3 (-), pcDNA3(-)-ATF4, pSingle-tTS-shLUC or pSingle-tTS-shATF4 expression plasmid using 10μl of Lipofectamine 2000 reagent (Invitrogen, CA, USA) according to the manufacturer’s instructions. After 6 h incubation, the cells were transferred to fresh medium. After 24 h, cells were selected in selection medium containing 500 μg/ml of geneticin and incubated for 2 weeks. Several transfectants were selected. These transfectants, referred to as HepG2/pcDNA3.1(-), HepG2/pcDNA3.1(-)-ATF4, SMMC7721/pcDNA3.1(-), SMMC7721/pcDNA3.1(-)-ATF4, SMMC7721/sh-LUC and SMMC7721/sh-ATF4 were maintained in the presence of 500 μg/ml of geneticin.
Analysis of Cytotoxicity with the WST-1 assay
For use of the water-soluble tetrazolium salt (WST)-1 assay (Beyotime Institute of Biotechnology), 2 × 103 HepG2/pcDNA3.1(-)-ATF4 and HepG2/pcDNA3.1(-) cells, or 3 × 103 SMMC7721/pcDNA3.1(-)-ATF4 and SMMC7721/pcDNA3.1(-) cells, or 3 × 103 SMMC7721/ sh-Luc and SMMC7721/ sh-ATF4 cells were seeded in 96-well plates. On the following day, the concentrations of the drugs indicated were applied. Cell viability was analyzed by the addition of 10 μl WST-1 solution. After 2 h incubation with WST-1, colorimetric changes were quantified by measuring the absorbance in a spectrophotometer at 450 nm. For the WST-1 assay with buthionine-sulfoximin (BSO), 10 μM BSO were treated once ATF4 knockdown cells were seeded, and on the following day the indicated concentrations of the cisplatin were applied.
Apoptosis assays
Cell apoptosis was assessed by flow cytometry after staining use the Annexin V-FITC Apoptosis Detection Kit (Keygen Biotechnology). Cells were harvested by centrifugation for 5 min at 2,000 rpm and aliquots containing 1–5 × 105 were resuspended in 500 μl Binding Buffer, followed by 10 min incubation in 5 μl of Annexin V-FITC and 5 μl of PI. Samples were analyzed by flow cytometer. Three independent assays were conducted in such experiments and the mean values were expressed as mean ± SD (Standard Deviation).
Quantification of intracellular glutathione
5 × 105 SMMC7721/sh-Luc and SMMC7721/ sh-ATF4 cultured cells were treated either with or without 10 μM BSO for 72 h, collected, and washed twice with phosphate-buffered saline. Then, 10 μM HCl was added, and the cells lysed by freezing and thawing twice. Next, we added 20 μl of 5% sulfosalicylic acid (SSA), and centrifuged at 8,000 x g for 10 min. The glutathione concentration of the cell supernatants was measured with the Total Glutathione Quantification Kit (Dojindo Molecular Technologies) according to the manufacturer’s instructions.
Western blots
Total cell protein were prepared by lysis of cells with the radioimmunoprecipitation assay (RIPA) buffer and protein concentrations were determined by the bicinchoninic acid (BCA) protein assay reagent (Pierce). For western blot analysis, 30 μg of each sample was added. Antibody against ATF4 and secondary antibody were both purchased from Santa Cruz Biotechnology (Santa Cruz Biotechnology). Secondary antibodies, coupled to horseradish peroxidase were visualized by ECL (Millipore). All western blots were repeated at least three times.
Immunohistochemistry
Staining of ATF4 was performed on HCC Tissue Microarrays (LV808) (Chaoying Biotechnology) with a polyclonal antibody against ATF4 at 1:100 dilution using the Immunocruz staining system (Santa Cruz Biotechnology) according to the manufacturer’s protocol. For analysis of apoptosis, an ApopTag Plus peroxidase in situ apoptosis kit (Millipore) was used according to the manufacturer’s instructions. For determination of TUNEL expression in tissue sections, we counted the number of apoptotic events in six random fields at × 400 magnification and then divided that number by the total number of cells per field.
Tumor xenograft experiments
SMMC7721/sh-Luc and SMMC7721/sh-ATF4 transfectants were used for the cisplatin sensitivity study in xenograft models. Cells were resuspended in DMEM medium at 106cells/200μl, and injected subcutaneously into the right flank of NOD/SCID mice. These animals were treated with 0.2 mg/ml doxycycline in a 0.5% sucrose solution, which induce the Dox-inducible pSingle-tTS-shRNA system to work, and refreshed once every 4 d. Three weeks after inoculation, mice were treated i.p., four times in seven weeks, with cisplatin at 5 mg/kg body weight. Untreated control mice received only physiological saline. Volume of tumors was measured with microcalipers twice a week and tumor tissues were peeled and fixed in 10% formaldehyde solution and embedded in paraffin. Paraffin sections (5 μm) were used for TUNEL analysis.
Statistical analysis
All statistical analyses were performed by Student’s t-test or chi-square test, and p < 0.05 was considered significant. All data were expressed as the mean ± SD.
Additional material
Download Zip (4.4 MB)Acknowledgments
This project was supported by grants from Tianjin Municipal Science and Technology Commission (08ZCGYSH04700) to CHL, from National Science and Technology Infrastructure Program(2009BAI86B05) to YPT, and from National Development Plan of High Technology 863 (2007AA021010) and 973 (2007CB914804) to RX.
Reference
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55:74 - 108; http://dx.doi.org/10.3322/canjclin.55.2.74; PMID: 15761078
- De Flora S, Bonanni P. The prevention of infection-associated cancers. Carcinogenesis 2011; 32:787 - 95; http://dx.doi.org/10.1093/carcin/bgr054; PMID: 21436188
- Leong TY, Leong AS. Epidemiology and carcinogenesis of hepatocellular carcinoma. HPB (Oxford) 2005; 7:5 - 15; http://dx.doi.org/10.1080/13651820410024021; PMID: 18333156
- Rampone B, Schiavone B, Martino A, Viviano C, Confuorto G. Current management strategy of hepatocellular carcinoma. World J Gastroenterol 2009; 15:3210 - 6; http://dx.doi.org/10.3748/wjg.15.3210; PMID: 19598295
- Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: A population-based case-control study. Hepatology 2011; 54:463 - 71; http://dx.doi.org/10.1002/hep.24397; PMID: 21538440
- Han KH, Seong J, Kim JK, Ahn SH, Lee Y, Chon CY. Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer 2008; 113:995 - 1003; http://dx.doi.org/10.1002/cncr.23684; PMID: 18615601
- Morise Z, Sugioka A, Fujita J, Hoshimoto S, Kato T, Ikeda M. S-1 plus cisplatin combination therapy for the patients with primary liver carcinomas. Hepatogastroenterology 2007; 54:2315 - 8; PMID: 18265655
- Ye CG, Wu WK, Yeung JH, Li HT, Li ZJ, Wong CC, et al. Indomethacin and SC236 enhance the cytotoxicity of doxorubicin in human hepatocellular carcinoma cells via inhibiting P-glycoprotein and MRP1 expression. Cancer Lett 2011; 304:90 - 6; http://dx.doi.org/10.1016/j.canlet.2011.01.025; PMID: 21377266
- Fujii R, Mutoh M, Niwa K, Yamada K, Aikou T, Nakagawa M, et al. Active efflux system for cisplatin in cisplatin-resistant human KB cells. Jpn J Cancer Res 1994; 85:426 - 33; http://dx.doi.org/10.1111/j.1349-7006.1994.tb02376.x; PMID: 8200854
- Lai GM, Ozols RF, Young RC, Hamilton TC. Effect of glutathione on DNA repair in cisplatin-resistant human ovarian cancer cell lines. J Natl Cancer Inst 1989; 81:535 - 9; http://dx.doi.org/10.1093/jnci/81.7.535; PMID: 2493524
- Chaney SG, Sancar A. DNA repair: enzymatic mechanisms and relevance to drug response. J Natl Cancer Inst 1996; 88:1346 - 60; http://dx.doi.org/10.1093/jnci/88.19.1346; PMID: 8827012
- Sedletska Y, Giraud-Panis MJ, Malinge JM. Cisplatin is a DNA-damaging antitumour compound triggering multifactorial biochemical responses in cancer cells: importance of apoptotic pathways. Curr Med Chem Anticancer Agents 2005; 5:251 - 65; http://dx.doi.org/10.2174/1568011053765967; PMID: 15992353
- Zhu AX, El-Khoueiry A, Llovet JM. Accomplishments in 2008 in the management of hepatobiliary cancers. Gastrointest Cancer Res 2009; 3:Supplement 2 S28 - 36; PMID: 20011562
- Wang Q, Zheng XL, Yang L, Shi F, Gao LB, Zhong YJ, et al. Reactive oxygen species-mediated apoptosis contributes to chemosensitization effect of saikosaponins on cisplatin-induced cytotoxicity in cancer cells. J Exp Clin Cancer Res 2010; 29:159; http://dx.doi.org/10.1186/1756-9966-29-159; PMID: 21143894
- Rutkowski DT, Kaufman RJ. All roads lead to ATF4. Dev Cell 2003; 4:442 - 4; http://dx.doi.org/10.1016/S1534-5807(03)00100-X; PMID: 12689582
- Armstrong JL, Flockhart R, Veal GJ, Lovat PE, Redfern CP. Regulation of endoplasmic reticulum stress-induced cell death by ATF4 in neuroectodermal tumor cells. J Biol Chem 2010; 285:6091 - 100; http://dx.doi.org/10.1074/jbc.M109.014092; PMID: 20022965
- Ameri K, Hammond EM, Culmsee C, Raida M, Katschinski DM, Wenger RH, et al. Induction of activating transcription factor 3 by anoxia is independent of p53 and the hypoxic HIF signalling pathway. Oncogene 2007; 26:284 - 9; http://dx.doi.org/10.1038/sj.onc.1209781; PMID: 16847457
- Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J 2005; 24:3470 - 81; http://dx.doi.org/10.1038/sj.emboj.7600777; PMID: 16148948
- Chakraborty G, Jain S, Kundu GC. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res 2008; 68:152 - 61; http://dx.doi.org/10.1158/0008-5472.CAN-07-2126; PMID: 18172307
- Tanabe M, Izumi H, Ise T, Higuchi S, Yamori T, Yasumoto K, et al. Activating transcription factor 4 increases the cisplatin resistance of human cancer cell lines. Cancer Res 2003; 63:8592 - 5; PMID: 14695168
- Igarashi T, Izumi H, Uchiumi T, Nishio K, Arao T, Tanabe M, et al. Clock and ATF4 transcription system regulates drug resistance in human cancer cell lines. Oncogene 2007; 26:4749 - 60; http://dx.doi.org/10.1038/sj.onc.1210289; PMID: 17297441
- Ameri K, Lewis CE, Raida M, Sowter H, Hai T, Harris AL. Anoxic induction of ATF-4 through HIF-1-independent pathways of protein stabilization in human cancer cells. Blood 2004; 103:1876 - 82; http://dx.doi.org/10.1182/blood-2003-06-1859; PMID: 14604972
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 2003; 11:619 - 33; http://dx.doi.org/10.1016/S1097-2765(03)00105-9; PMID: 12667446
- Namba T, Hoshino T, Tanaka K, Tsutsumi S, Ishihara T, Mima S, et al. Up-regulation of 150-kDa oxygen-regulated protein by celecoxib in human gastric carcinoma cells. Mol Pharmacol 2007; 71:860 - 70; http://dx.doi.org/10.1124/mol.106.027698; PMID: 17167033
- Shringarpure R, Catley L, Bhole D, Burger R, Podar K, Tai YT, et al. Gene expression analysis of B-lymphoma cells resistant and sensitive to bortezomib. Br J Haematol 2006; 134:145 - 56; http://dx.doi.org/10.1111/j.1365-2141.2006.06132.x; PMID: 16846475
- Niedner H, Christen R, Lin X, Kondo A, Howell SB. Identification of genes that mediate sensitivity to cisplatin. Mol Pharmacol 2001; 60:1153 - 60; PMID: 11723219
- Selvakumaran M, Pisarcik DA, Bao R, Yeung AT, Hamilton TC. Enhanced cisplatin cytotoxicity by disturbing the nucleotide excision repair pathway in ovarian cancer cell lines. Cancer Res 2003; 63:1311 - 6; PMID: 12649192
- Mabuchi S, Ohmichi M, Nishio Y, Hayasaka T, Kimura A, Ohta T, et al. Inhibition of NFkappaB increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J Biol Chem 2004; 279:23477 - 85; http://dx.doi.org/10.1074/jbc.M313709200; PMID: 15026414
- Garrido N, Pérez-Martos A, Faro M, Lou-Bonafonte JM, Fernández-Silva P, López-Pérez MJ, et al. Cisplatin-mediated impairment of mitochondrial DNA metabolism inversely correlates with glutathione levels. Biochem J 2008; 414:93 - 102; http://dx.doi.org/10.1042/BJ20071615; PMID: 18426391
- Choy CS, Cheah KP, Chiou HY, Li JS, Liu YH, Yong SF, et al. Induction of hepatotoxicity by sanguinarine is associated with oxidation of protein thiols and disturbance of mitochondrial respiration. J Appl Toxicol 2008; 28:945 - 56; http://dx.doi.org/10.1002/jat.1360; PMID: 18548746
- Grant CE, Gao M, DeGorter MK, Cole SP, Deeley RG. Structural determinants of substrate specificity differences between human multidrug resistance protein (MRP) 1 (ABCC1) and MRP3 (ABCC3). Drug Metab Dispos 2008; 36:2571 - 81; http://dx.doi.org/10.1124/dmd.108.022491; PMID: 18775981
- Fung H, Liu P, Demple B. ATF4-dependent oxidative induction of the DNA repair enzyme Ape1 counteracts arsenite cytotoxicity and suppresses arsenite-mediated mutagenesis. Mol Cell Biol 2007; 27:8834 - 47; http://dx.doi.org/10.1128/MCB.00974-07; PMID: 17938202